High Thermoelectric Power Generation by SWCNT/PPy Core Shell Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SWCNT/PPy Nanocomposites
2.3. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, C.; Chen, G. Conducting polymer/carbon particle thermoelectric composites: Emerging green energy materials. Compos. Sci. Technol. 2016, 124, 52–70. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N. Impact of the doping method on conductivity and thermopower in semiconducting polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Krause, B.; Liguoro, A.; Pötschke, P. Blend Structure and n-Type Thermoelectric Performance of PA6/SAN and PA6/PMMA Blends Filled with Singlewalled Carbon Nanotubes. Nanomaterials 2021, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Li, P.; Liu, Y.; He, C. Recent Advances in Polyaniline-Based Thermoelectric Composites. CCS Chem. 2021, 3, 2547–2560. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Tian, Z. Thermoelectric properties of crystalline and amorphous polypyrrole: A computational study. Appl. Therm. Eng. 2017, 111, 1441–1447. [Google Scholar] [CrossRef]
- Tonga, M.; Wei, L.; Lahti, P.M. Enhanced thermoelectric properties of PEDOT:PSS composites by functionalized single wall carbon nanotubes. Int. J. Energ. Res. 2020, 44, 9149–9156. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, C.; Xu, J.; Lu, B.; Song, H.; Shi, H.; Yao, Y.; Zhang, L. An effective substrate for the enhancement of thermoelectric properties in PEDOT: PSS. J. Polym. Sci. B Polym. Phys. 2014, 52, 737–742. [Google Scholar] [CrossRef]
- Xu, K.; Gao, C.; Chen, G.; Qiu, D. Direct evidence for effect of molecular orientation on thermoelectric performance of organic polymer materials by infrared dichroism. Org. Electron. 2016, 31, 41–47. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Enhanced thermoelectric performance by self-assembled layered morphology of polypyrrole nanowire/single-walled carbon nanotube composites. Compos. Sci. Technol. 2016, 129, 130–136. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Wang, H.; Li, X. Template-directed in situ polymerization preparation of nanocomposites of PEDOT: PSS-coated multi-walled carbon nanotubes with enhanced thermoelectric property. Chem. Asian J. 2015, 10, 149–153. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Wang, H.; Zhai, W. Enhanced thermoelectric property by the construction of a nanocomposite 3D interconnected architecture consisting of graphene nanolayers sandwiched by polypyrrole nanowires. J. Mater. Chem. C. 2015, 3, 1649–1654. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Jia, R.; Xu, J.; Shen, S.Z. Flexible thermoelectric composite films of polypyrrole nanotubes coated paper. Coatings 2017, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, Y.; Pei, W.-B.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Culebras, M.; Uriol, B.; Gómez, C.M.; Cantarero, A. Controlling the thermoelectric properties of polymers: Application to PEDOT and polypyrrole. Phys. Chem. Chem. Phys. 2015, 17, 15140–15145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-D.; Xiao, H.-M.; Fu, S.-Y. Preparation and characterization of novel polypyrrole-nanotube/polyaniline free-standing composite films via facile solvent-evaporation method. Compos. Sci. Technol. 2012, 72, 1812–1817. [Google Scholar] [CrossRef]
- Song, H.; Cai, K.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Baghdadi, N.; Zoromba, M.S.; Abdel-Aziz, M.H.; Al-Hossainy, A.F.; Bassyouni, M.; Salah, N. One-Dimensional Nanocomposites Based on Polypyrrole-Carbon Nanotubes and Their Thermoelectric Performance. Polymers 2021, 13, 278. [Google Scholar] [CrossRef]
- Liang, L.; Gao, C.; Chen, G.; Guo, C.-Y. Large-area, stretchable, super flexible and mechanically stable thermoelectric films of polymer/carbon nanotube composites. J. Mater. Chem. C 2016, 4, 526–532. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jin, C.; Zhang, T.; Yin, Q. Preparation of polypyrrole/graphene nanosheets composites with enhanced thermoelectric properties. RSC Adv. 2014, 4, 46187–46193. [Google Scholar] [CrossRef]
- Han, S.; Zhai, W.; Chen, G.; Wang, X. Morphology and thermoelectric properties of graphene nanosheets enwrapped with polypyrrole. RSC Adv. 2014, 4, 29281–29285. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Abdel-Aziz, M.H.; Bassyouni, M.; Abusorrah, A.M.; Attar, A.; Baghdadi, N.; Salah, N. Polypyrrole sheets composed of nanoparticles as a promising room temperature thermo-electric material. Phys. E 2021, 134, 114889. [Google Scholar] [CrossRef]
- Almasoudi, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Bassyouni, M.; Alshahrie, A.; Abusorrah, A.M.; Salah, N. Optimization preparation of one-dimensional polypyrrole nanotubes for enhanced thermoelectric performance. Polymer 2021, 225, 123950. [Google Scholar] [CrossRef]
- Mei, H.; Cheng, Y. Research progress of electrical properties based on carbon nanotubes; interconnection. Ferroelectrics 2020, 564, 1–18. [Google Scholar] [CrossRef]
- Salah, N.; Alhebshi, N.A.; Salah, Y.N.; Alshareef, H.N.; Koumoto, K. Thermoelectric properties of oil fly ash-derived carbon nanotubes coated with polypyrrole. J. Appl. Phys. 2020, 128, 235104. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Z.; He, N. Hierarchical nanostructured polypyrrole/graphene composites as supercapacitor electrode. RSC Adv. 2015, 5, 15096–15102. [Google Scholar] [CrossRef]
- Šetka, M.; Calavia, R.; Vojkůvka, L.; Liobet, E.; Drbohlavova, J.; Vallejos, S. Raman and XPS studies of ammonia sensitive polypyrrole nanorods and nanoparticles. Sci. Rep. 2019, 9, 8465. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Zheng, Y.; Dai, L.; Zhang, M.; Yang, J.; Chen, Y.; Chen, X.; Zhou, J. A Facile Synthesis of Polypyrrole/Carbon Nanotube Composites with Ultrathin, Uniform and Thickness-Tunable Polypyrrole Shells. Nanoscale Res. Lett. 2011, 6, 431. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-C.; Lin, M.-P.; Chen, S.-W.; Lee, P.-H.; Li, J.-H.; Su, W.-F. Surface-enhanced Raman scattering substrate based on a Ag coated monolayer array of SiO2 spheres for organic dye detection. RSC Adv. 2014, 4, 10043–10050. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and characterization of polypyrrole (PPy) thin films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Rafique, S.; Sharif, R.; Rashid, I.; Chani, S. Facile fabrication of novel silver-polypyrrole-multiwall carbon nanotubes nanocomposite for replacement of platinum in dye-sensitized solar cell. AIP Adv. 2016, 6, 085018. [Google Scholar] [CrossRef] [Green Version]
- Márquez-Herrera, A.; Ovando-Medina, V.M.; Castillo-Reyes, B.E.; Zapata-Torres, M.; Meléndez-Lira, M.; González-Castañeda, J. Facile synthesis of SrCO3-Sr(OH)2/PPy nanocomposite with enhanced photocatalytic activity under visible light. Materials 2016, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Bagai, R.; Christopher, J.; Kapur, G.S. Evaluating industrial grade functionalized multiwalled carbon nanotubes by X-ray photoelectron spectroscopy. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 240–246. [Google Scholar] [CrossRef]
- Du, Y.; Niu, H.; Li, J.; Dou, Y.; Shen, S.Z.; Jia, R.; Xu, J. Morphologies tuning of polypyrrole and thermoelectric properties of polypyrrole nanowire/graphene composites. Polymers 2018, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cai, K.; Shen, S.; Dou, Y.; Shen, S.Z.; Jia, R.; Xu, J. Preparation and thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2014, 195, 132–136. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, J.; Song, H.; Pan, Y.; Song, Y.; Zhu, Y.; Yao, Y.; Huang, F.; Zuo, C. High performance polypyrrole/SWCNTs composite film as a promising organic thermoelectric material. RSC Adv. 2021, 11, 17704–17709. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sivasamy, M.; Murugan, K.; Venugopal, K.; Veluswamy, P. Pressure-driven thermoelectric properties of defect chalcopyrite structured ZnGa2Te4: Ab initio study. RSC Adv. 2022, 12, 12573–12582. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; He, J.; Li, H.; Biswas, K.; Androulakis, J.; Wu, C.-I.; Hogan, T.P.; Chung, D.-Y.; Dravid, V.P.; et al. High performance thermoelectrics from earth-abundant materials: Enhanced figure of merit in PbS by second phase nanostructures. J. Am. Chem. Soc. 2011, 133, 20476–20487. [Google Scholar] [CrossRef]
- Salah, N.; Baghdadi, N.; Alshahrie, A.; Saeed, A.; Ansari, A.R.; Memic, A.; Koumoto, K. Nanocomposites of CuO/SWCNT: Promising thermoelectric materials for mid-temperature thermoelectric generators. J. Eur. Ceram. Soc. 2019, 39, 3307–3314. [Google Scholar] [CrossRef]
- Xin, S.; Yang, N.; Gao, F.; Zhao, J.; Li, L.; Teng, C. Free-standing and flexible polypyrrole nanotube/reduced graphene oxide hybrid film with promising thermoelectric performance. Mater. Chem. Phys. 2018, 212, 440–445. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.; Lin, T. Single-walled carbon nanotube/polypyrrole thermoelectric composite materials. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Fan, W.; Zhang, Y.; Guo, C.-Y.; Chen, G. Toward high thermoelectric performance for polypyrrole composites by dynamic 3-phase interfacial electropolymerization and chemical doping of carbon nanotubes. Compos. Sci. Technol. 2019, 183, 107794. [Google Scholar] [CrossRef]
- Rahaman, M.; Aldalbahi, A.; Almoiqli, M.; Alzahly, S. Chemical and electrochemical synthesis of polypyrrole using carrageenan as a dopant: Polypyrrole/multi-walled carbon nanotube nanocomposites. Polymers 2018, 10, 632. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Jiang, S.; We, Y.; Chen, M.; Liu, X. Synthesis of stable aqueous dispersion of graphene/polyaniline composite mediated by polystyrene sulfonic acid. J. Polym. Sci. A Polym. Chem. 2012, 50, 4888–4894. [Google Scholar] [CrossRef]
- Neophytou, N.; Vargiamidis, V.; Foster, S.; Graziosi, P.; Oliveira, L.d.S.; Chakraborty, D.; Li, Z.; Thesberg, M.; Kosina, H.; Bennett, N.; et al. Hierarchically nanostructured thermoelectric materials: Challenges and opportunities for improved power factors. Eur. Phys. J. B 2020, 93, 213. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Liu, Y.; Wang, L.; Gao, C. Enhanced thermoelectric properties of polyaniline/polypyrrole/carbon nanotube ternary composites by treatment with a secondary dopant using ferric chloride. J. Mater. Chem. C 2020, 8, 528–535. [Google Scholar] [CrossRef]
- Angrist, S.W. Direct Energy Conversion, 3rd ed.; Allyn and Bacon: Boston, MA, USA, 1976. [Google Scholar]
- Madenci, E.; Guven, I. The Finite Element Method and Applications in Engineering Using ANSYS®; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Aljaghtham, M.; Celik, E. Design optimization of oil pan thermoelectric generator to recover waste heat from internal combustion engines. Energy 2020, 200, 117547. [Google Scholar] [CrossRef]
- Aljaghtham, M.; Celik, E. Numerical analysis of energy conversion efficiency and thermal reliability of novel, unileg segmented thermoelectric generation systems. Int. J. Energy Res. 2021, 45, 8810–8823. [Google Scholar] [CrossRef]
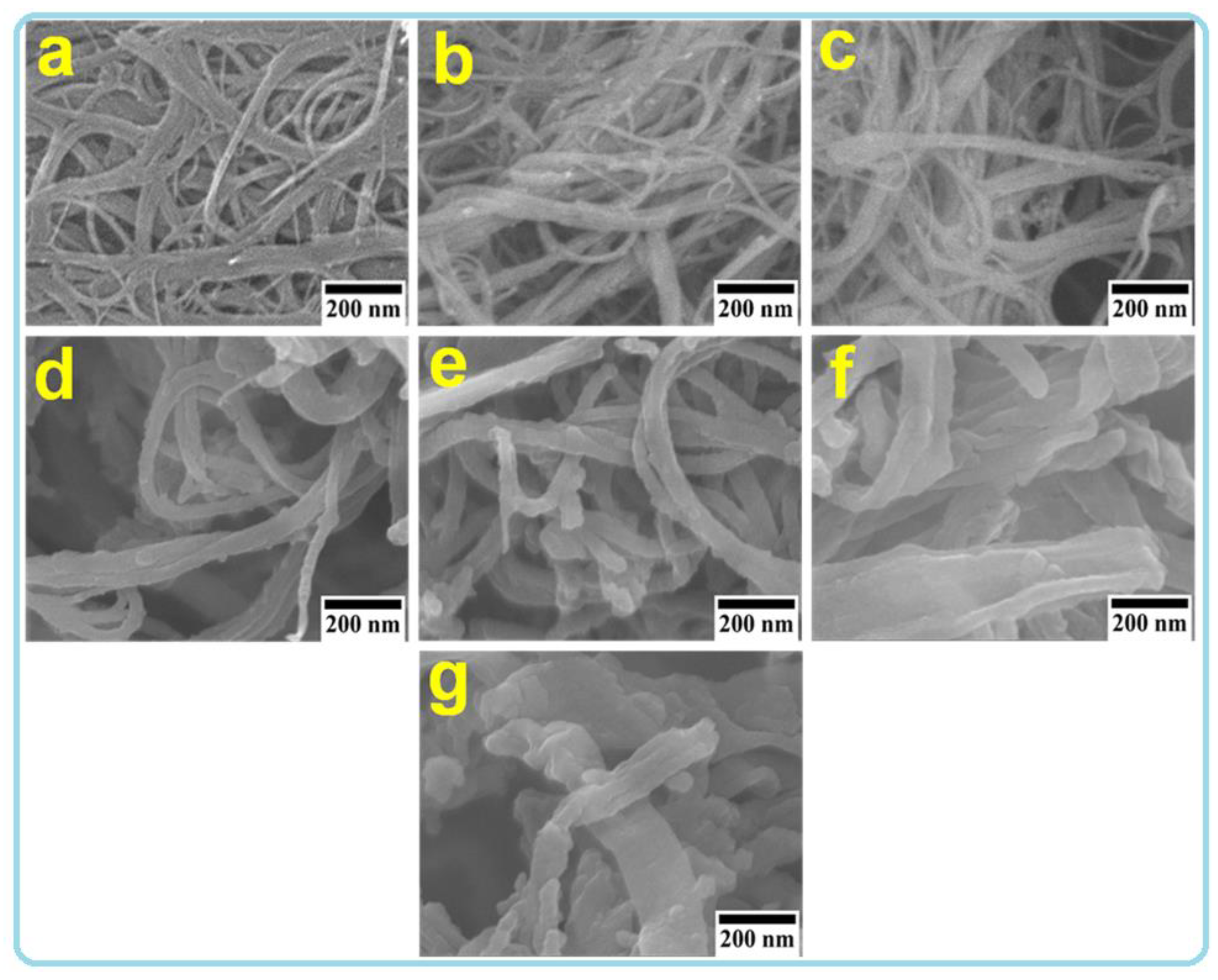

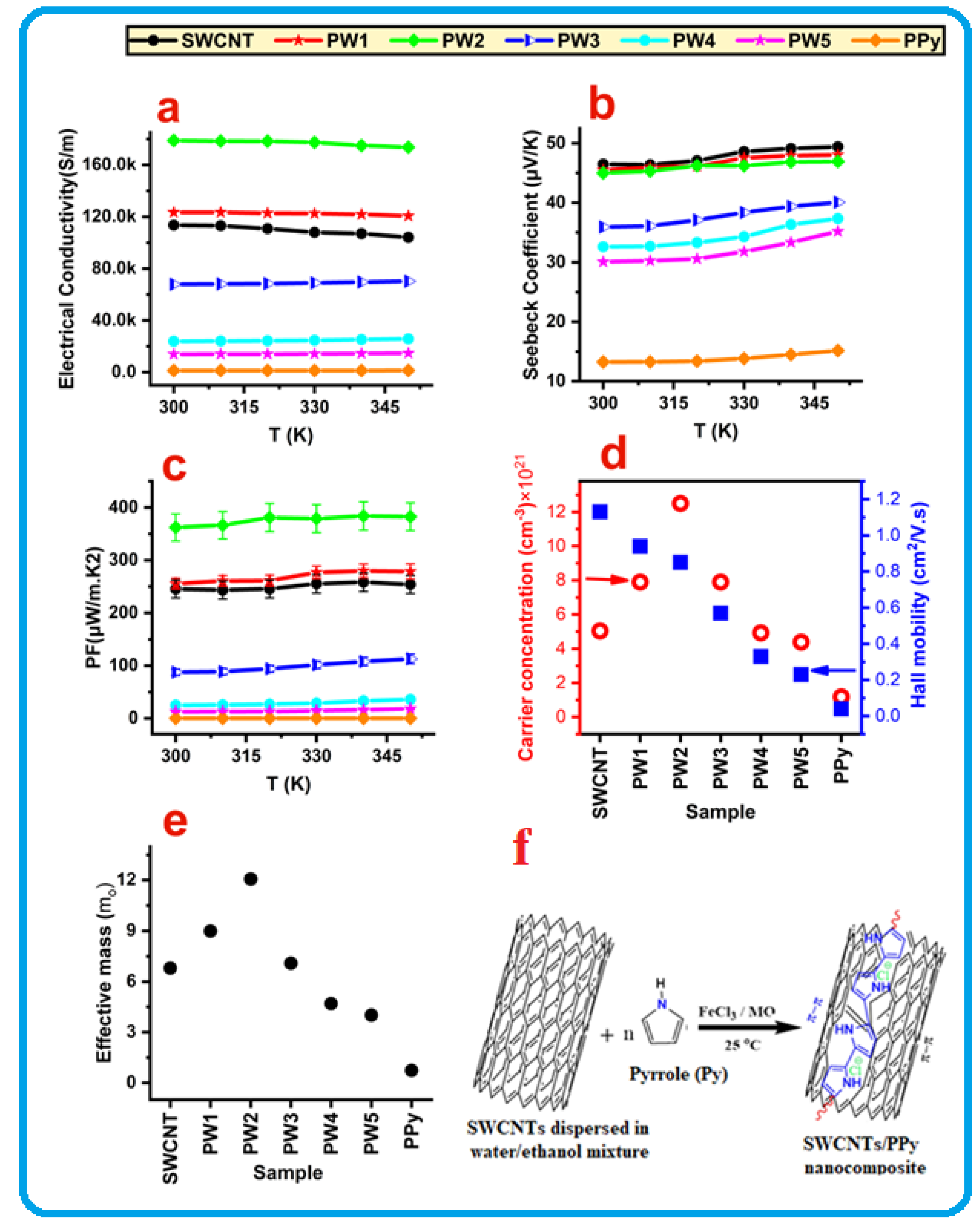

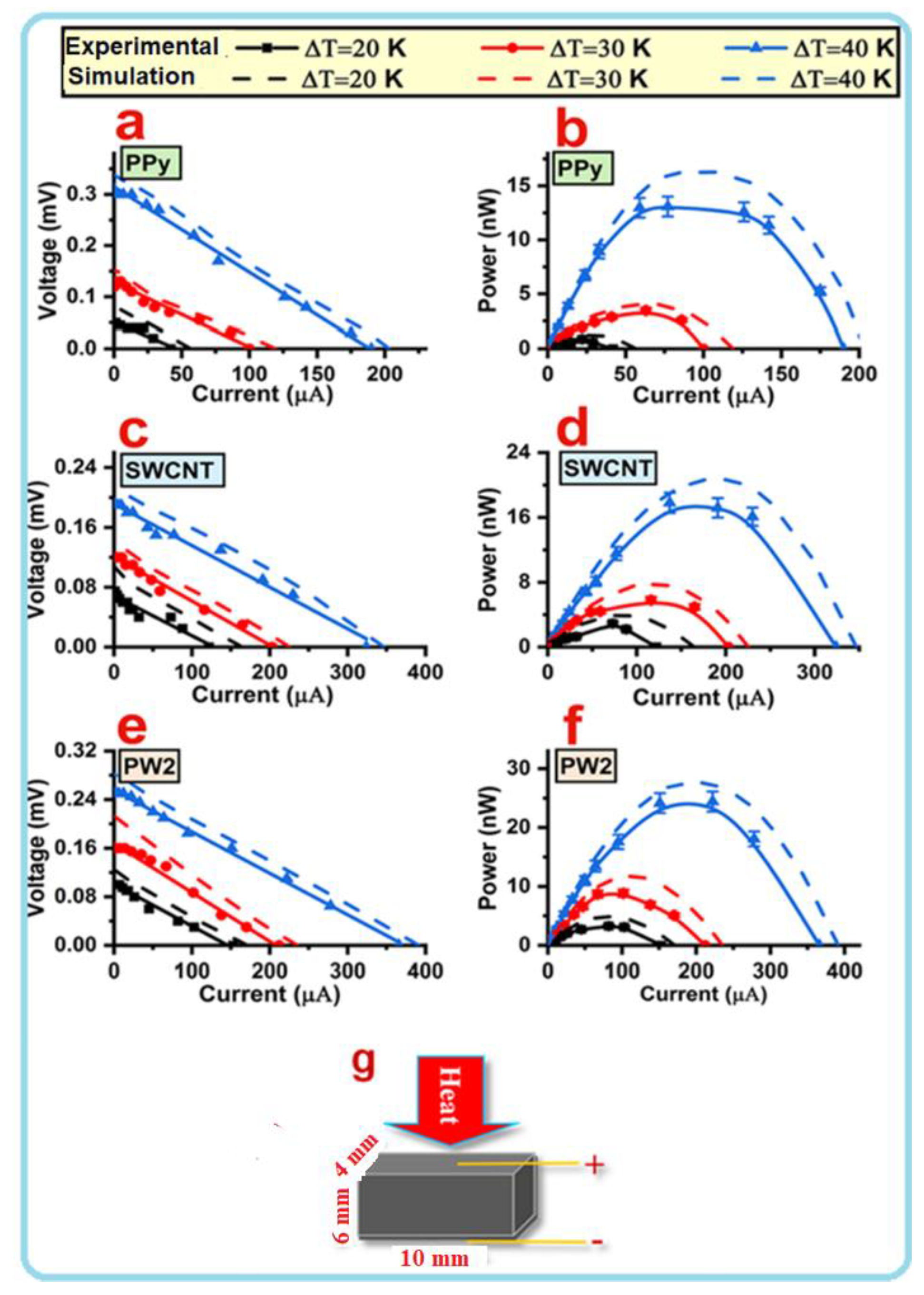
| Samples | SWCNT (mg) | MO (mg) | Py (mL) (In 200 mL DI) | FeCl3 (mg) (In 200 mL DI) |
|---|---|---|---|---|
| PW1 | 300 mg | 37.5 | 0.05 | 117 |
| PW2 | 75 | 0.1 | 234 | |
| PW3 | 375 | 0.5 | 1170 | |
| PW4 | 750 | 1 | 2340 | |
| PW5 | 1500 | 2 | 4680 |
| Nanocomposite Materials | σ (S/m) | S (μV/K) | P.F. (μW/mK2) | zT | Ref. |
|---|---|---|---|---|---|
| PPy/rGO | 4160 | 26.9 | 3.01 | - | [21] |
| PPy/GNs (PPy/graphene nanosheets) | 10,168 | 31.74 | 10.24 | 2.80 × 10−3 | [20] |
| PPy/rGO thin film | 8000 | 29 | 7.28 | - | [41] |
| PPy nanowire/rGO | 7500.1 | 33.8 | 8.56 ± 0.76 | - | [12] |
| PPy/SWCNT (60 wt %) | 39,900 | 22.2 | 19.7 ± 0.8 | - | [19] |
| PPy/MWCNT (20 wt %) | ~3150 | ~25.4 | 2.079 | [36] | |
| PPy/MWCNT (68 wt %) (at RT) | 3670 | 24.5 | 2.2 | - | [17] |
| PPy nanowire/SWCNT (60 wt %) (at RT) | ~30,000 | ~25 | 21.7 ± 0.8 | - | [10] |
| PPy/graphene (20 wt %) (at 380 K) | 3690 | 16.6 | 1.01 | [35] | |
| SWCNT/PPy (40 wt %) (at 398 K) | 10,699 | 22.59 | 5.46 | - | [42] |
| PPy/SWCNTs film (at RT) | 34,160 | 33.2 | 37.6 ± 2.3 | - | [37] |
| MWCNTs/PPy PPy/acid-doped SWCNT | 4000 385,000 | ~14 25 | 0.77 240.3 | 1 × 10−3 - | [18] [43] |
| SWCNT/PPy (at RT) SWCNT/PPy (at 350 K) | 178,835 173,637 | 45 47 | 362 382 | 90 × 10−3 110 × 10−3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasoudi, M.; Salah, N.; Alshahrie, A.; Saeed, A.; Aljaghtham, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Koumoto, K. High Thermoelectric Power Generation by SWCNT/PPy Core Shell Nanocomposites. Nanomaterials 2022, 12, 2582. https://doi.org/10.3390/nano12152582
Almasoudi M, Salah N, Alshahrie A, Saeed A, Aljaghtham M, Zoromba MS, Abdel-Aziz MH, Koumoto K. High Thermoelectric Power Generation by SWCNT/PPy Core Shell Nanocomposites. Nanomaterials. 2022; 12(15):2582. https://doi.org/10.3390/nano12152582
Chicago/Turabian StyleAlmasoudi, M., Numan Salah, Ahmed Alshahrie, Abdu Saeed, Mutabe Aljaghtham, M. Sh. Zoromba, M. H. Abdel-Aziz, and Kunihito Koumoto. 2022. "High Thermoelectric Power Generation by SWCNT/PPy Core Shell Nanocomposites" Nanomaterials 12, no. 15: 2582. https://doi.org/10.3390/nano12152582
APA StyleAlmasoudi, M., Salah, N., Alshahrie, A., Saeed, A., Aljaghtham, M., Zoromba, M. S., Abdel-Aziz, M. H., & Koumoto, K. (2022). High Thermoelectric Power Generation by SWCNT/PPy Core Shell Nanocomposites. Nanomaterials, 12(15), 2582. https://doi.org/10.3390/nano12152582










