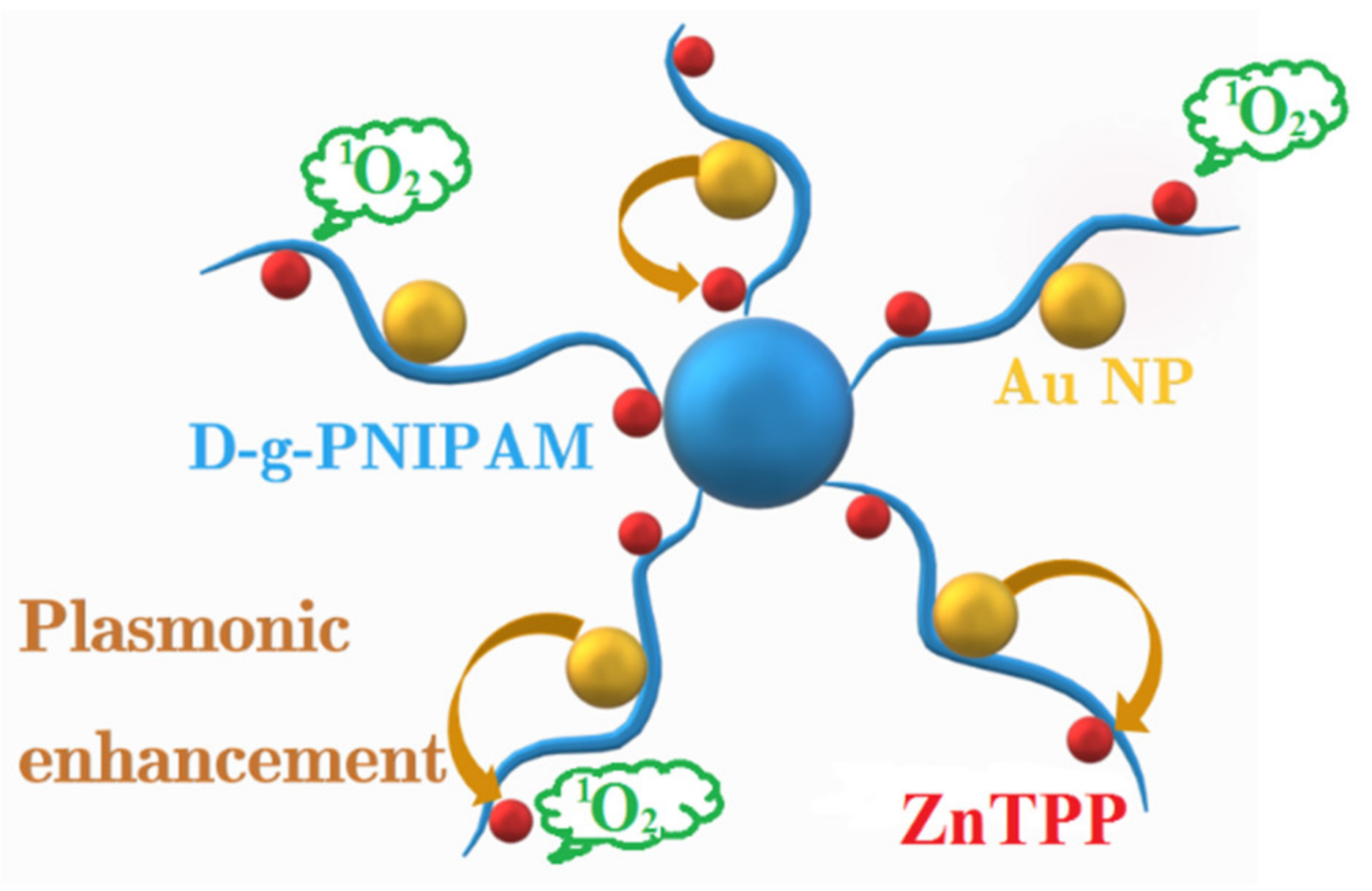
Thermoresponsive Zinc TetraPhenylPorphyrin Photosensitizer/Dextran Graft Poly(N-IsoPropylAcrylAmide) Copolymer/Au Nanoparticles Hybrid Nanosystem: Potential for Photodynamic Therapy Applications
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication of ZnTPP/D-g-PNIPAM and ZnTPP/D-g-PNIPAM/AuNPs Hybrid Nanosystems
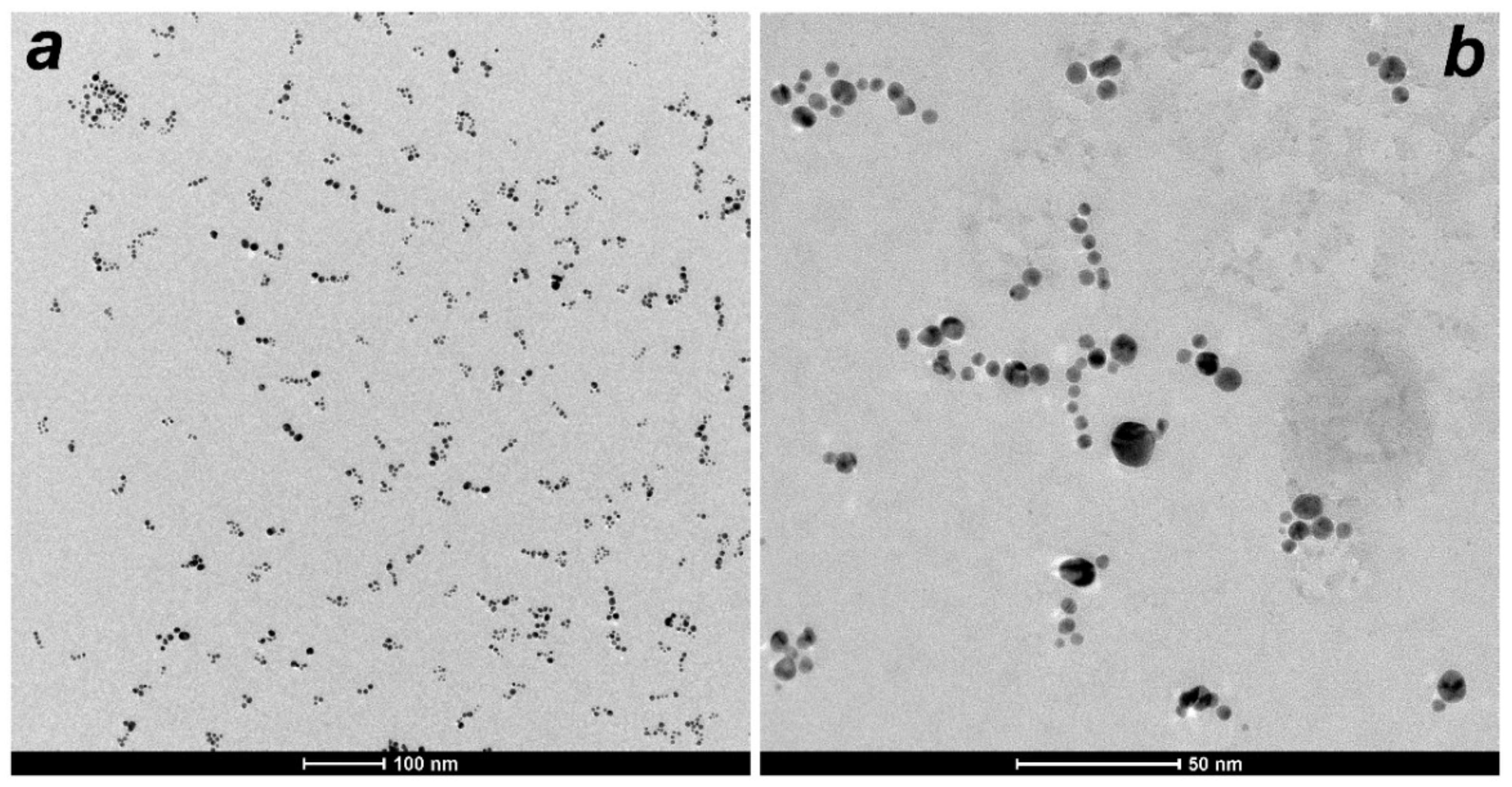
2.2. Morphology and Size Characterization by TEM and DLS
2.3. Absorption and Fluorescence Spectroscopy
2.4. Biological Experiments
3. Results and Discussion
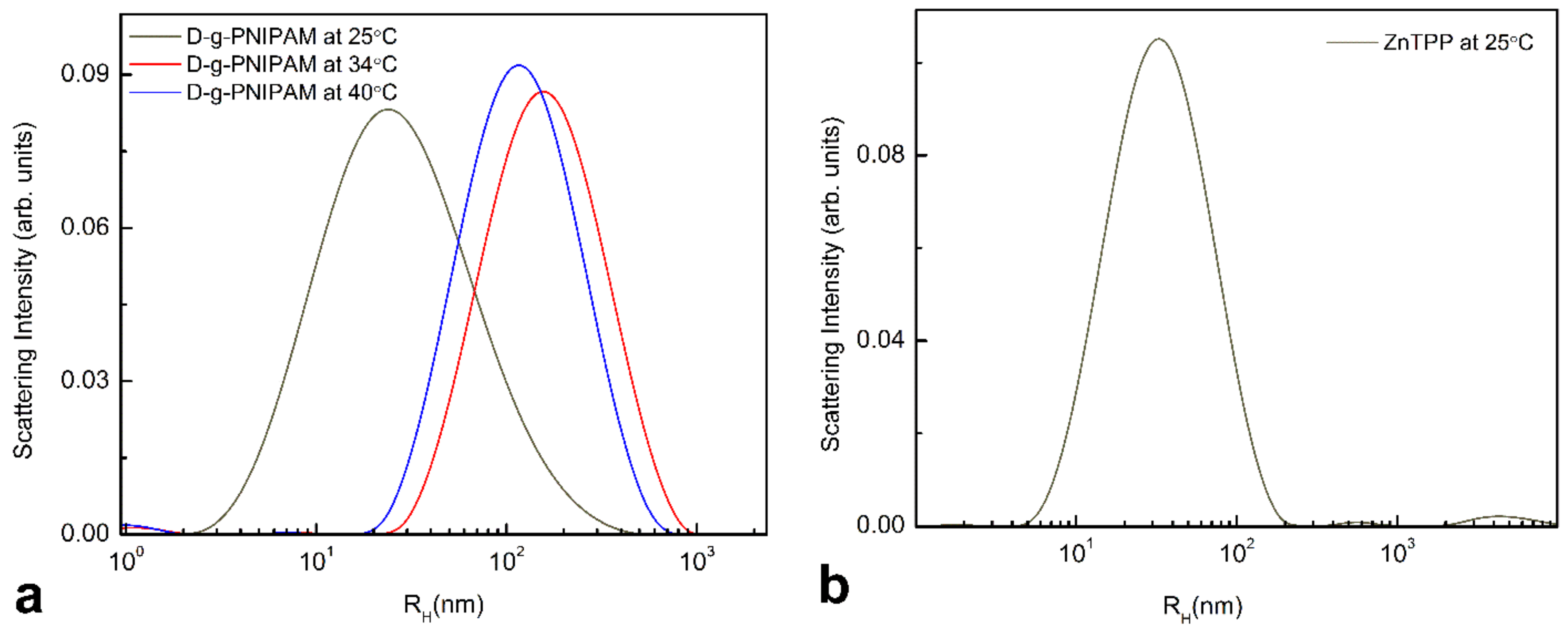
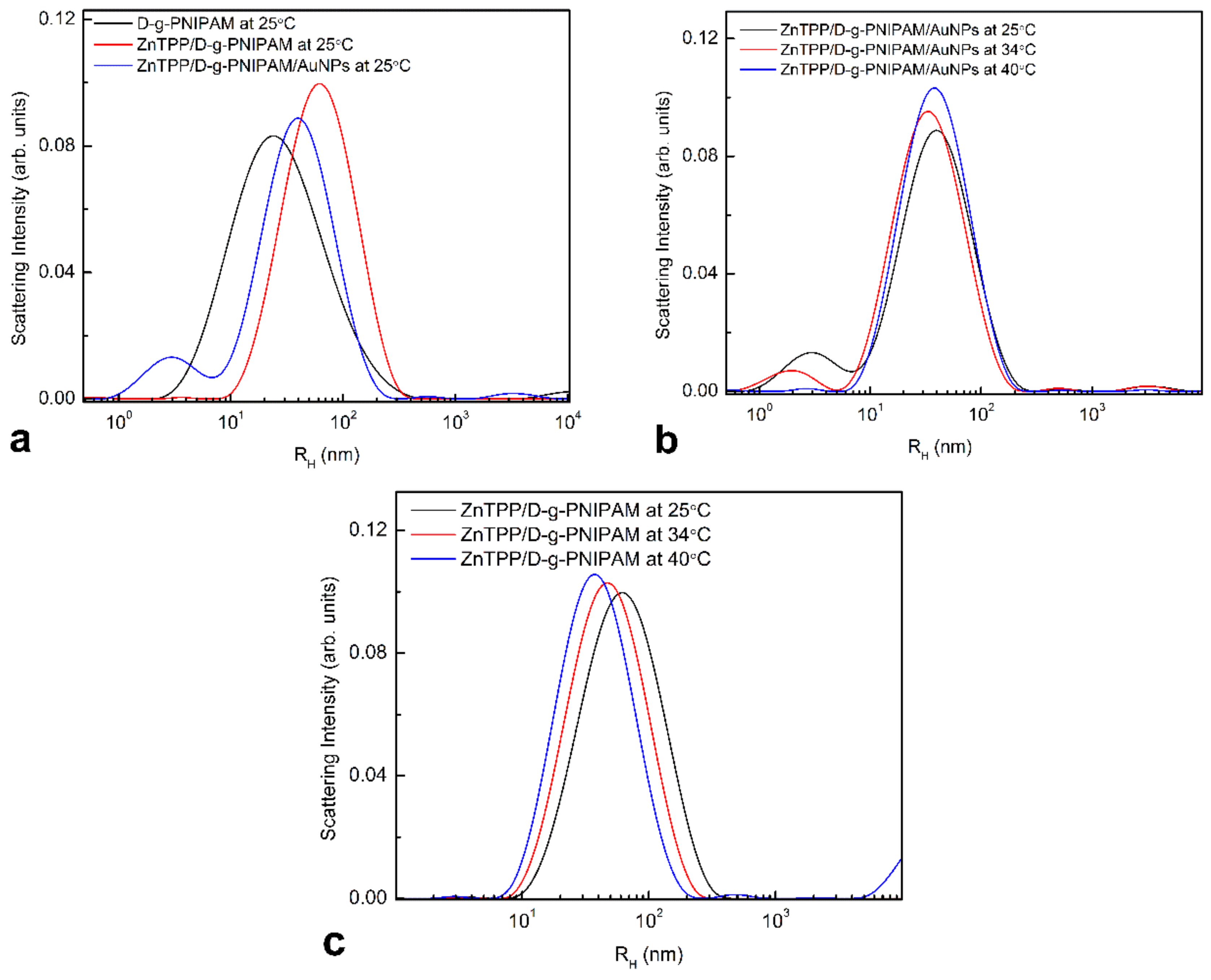
3.1. Morphology and Size Study
3.2. Absorption and Fluorescence Spectroscopy
3.2.1. Spectral Manifestations of ZnTPP Binding to D-g-PNIPAM and PNIPAM/AuNPs
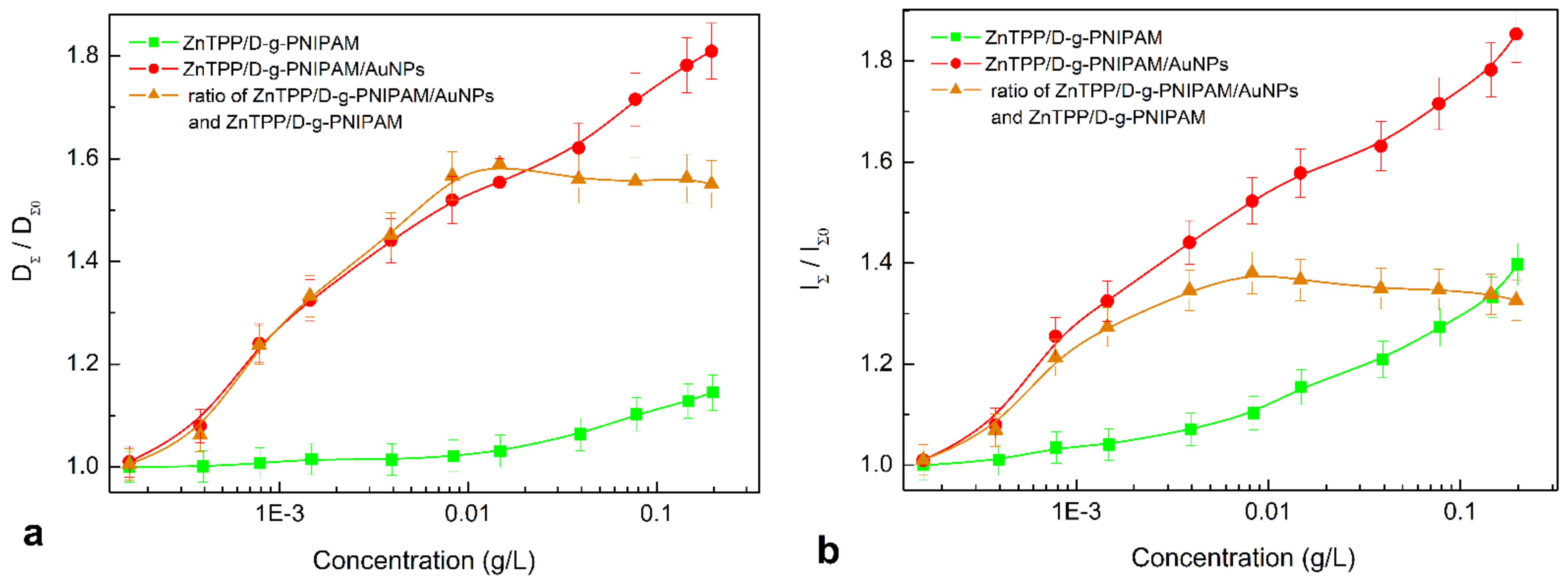
3.2.2. Absorption and Fluorescence of ZnTPP/D-g-PNIPAM and ZnTPP/D-g-PNIPAM/AuNPs Nanosystems: Concentration Effects
3.2.3. Thermally Induced Processes in ZnTPP/D-g-PNIPAM and ZnTPP/D-g-PNIPAM/AuNPs Nanohybrids
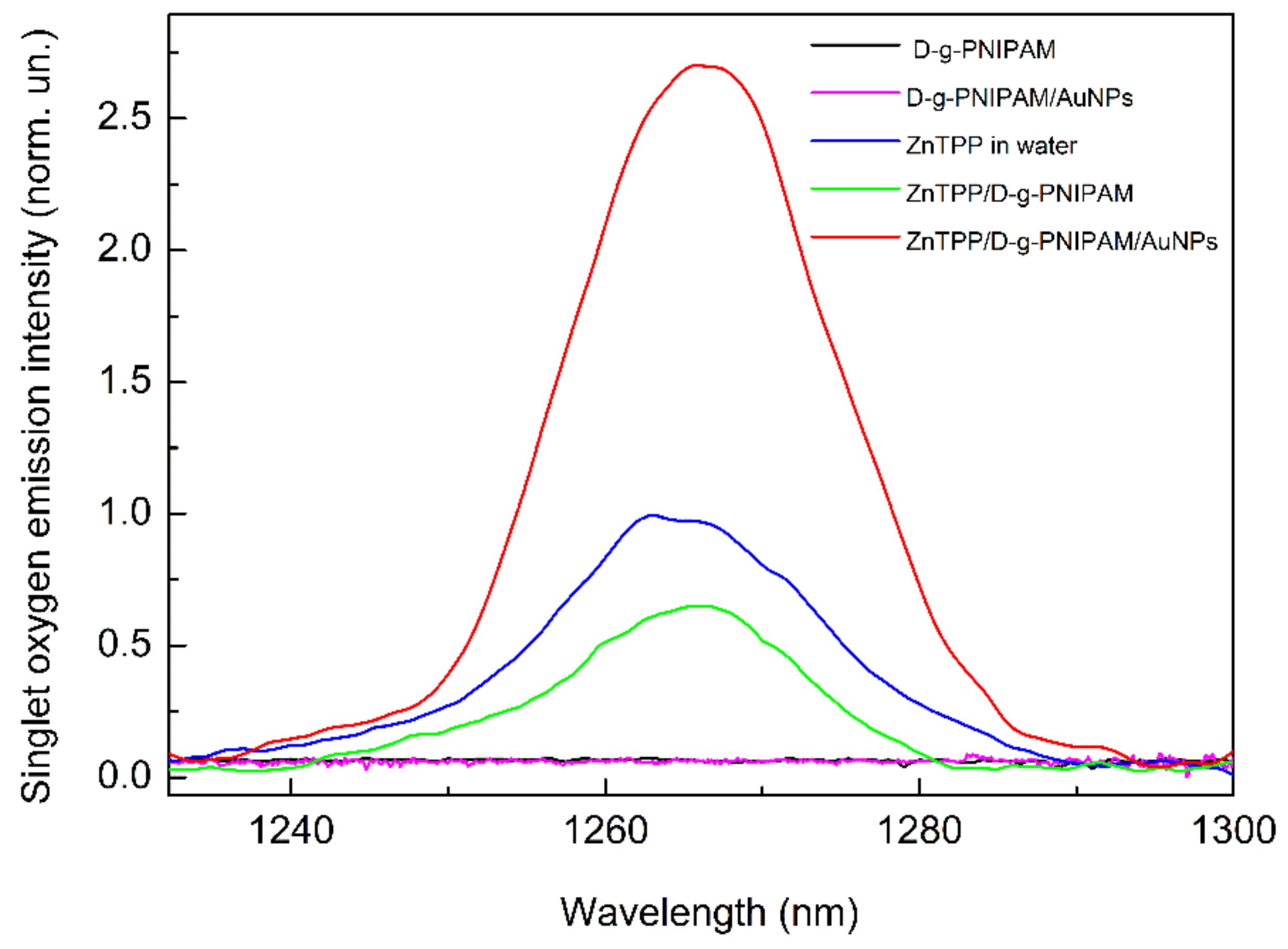
3.3. Singlet Oxygen Photogeneration Enhancement in ZnTPP/D-g-PNIPAM/AuNPs System
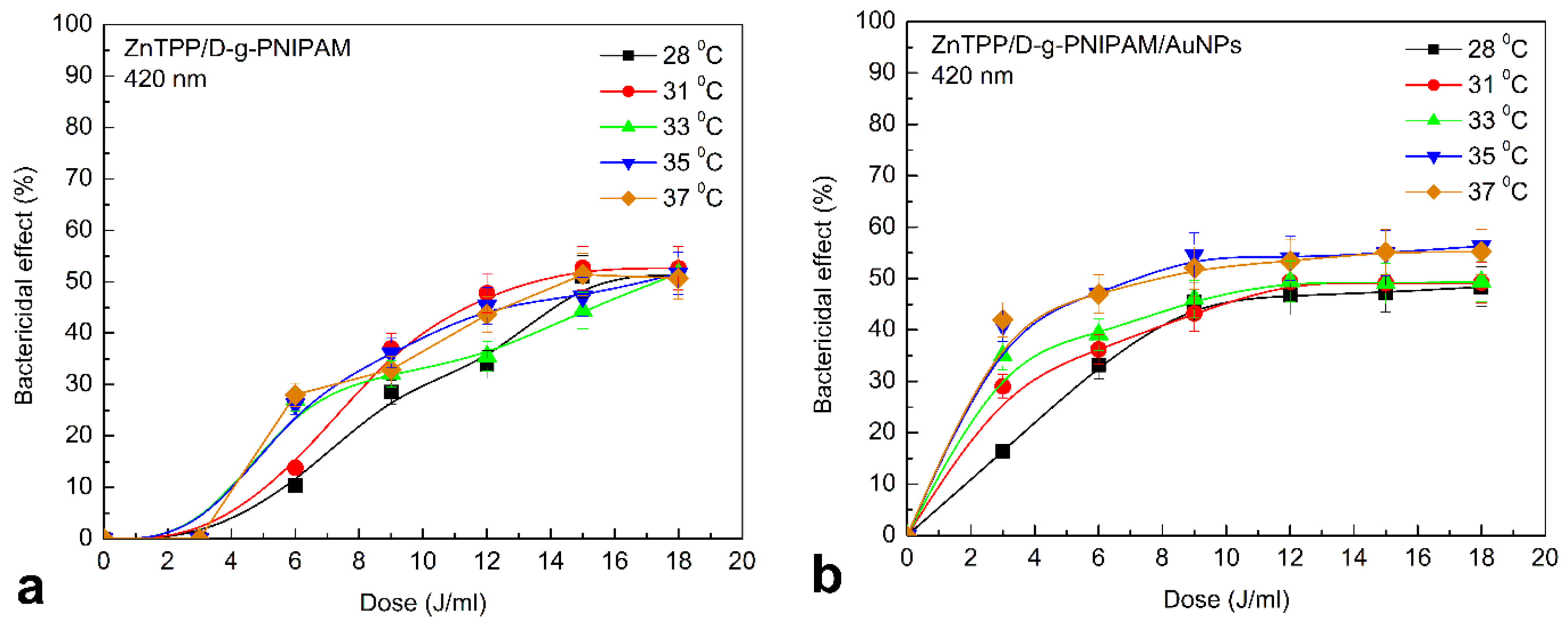
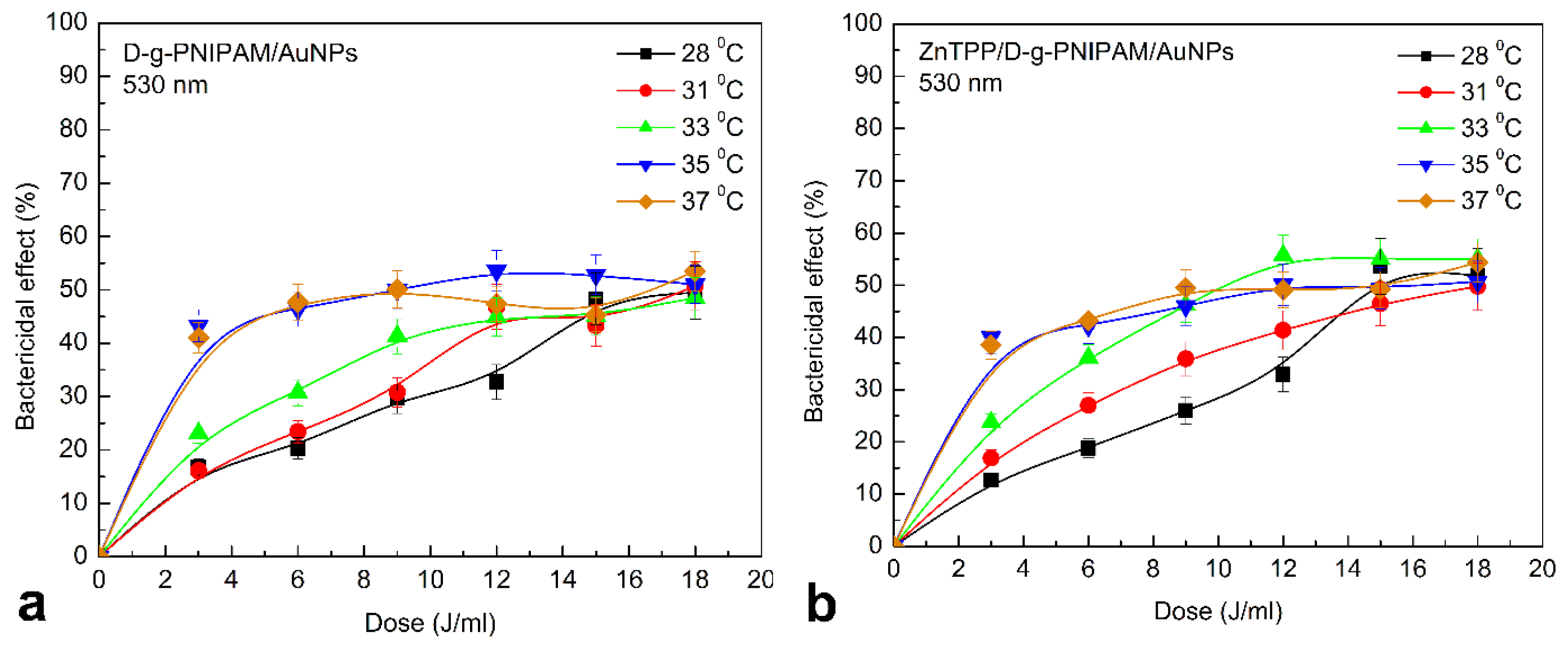
3.4. Photodynamic Antibacterial Activity In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 4, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S. Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Joglecar, M.; Trewyn, B.G. Polymer-based stimuli responsible nanosystems for biomedical applications. Biotechnol. J. 2013, 8, 931–945. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli responsible polymers and their application in nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogeles as drug delivery systems. Curr. Med. Chem. 2016, 20, 79–94. [Google Scholar] [CrossRef]
- Dal Lago, V.; França de Oliveira, L.; de Almeida Gonçalves, K.; Kobarg, J.; Cardoso, M.B. Size-selective silver nanoparticles: Future of biomedical devices with enhanced bactericidal properties. J. Mater. Chem. 2011, 21, 12267–12273. [Google Scholar] [CrossRef]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical applications of multifunctional polymeric nanocarriers: A review of current literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef]
- Kutsevol, N.V.; Chumachenko, V.A.; Harahuts, I.I.; Marinin, A.I. Aging process of gold nanoparticles synthesized in situ in aqueous solutions of polyacrylamides. In Chemical Engineering of Polymers: Production of Functional and Flexible Materials; Mukbanianym, O.V., Abadie, M.J., Tatrishvili, T., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 119–129. [Google Scholar]
- Bulavin, L.; Kutsevol, N.; Chumachenko, V.; Soloviov, D.; Kuklin, A.; Marynin, A. SAXS combined with UV-Vis spectroscopy and QELS: Accurate characterization of silver sols synthesized in polymer matrices. Nanoscale Res. Lett. 2016, 11, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumachenko, V.; Kutsevol, N.; Harahuts, Y.; Rawiso, M.; Marinin, A.; Bulavin, L. Star-like dextran-graft-PNiPAM copolymers. Eff. Intern. Mol. Struct. Phase Transit. J. Mol. Liq. 2017, 235, 77–82. [Google Scholar]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Chumachenko, V.A.; Shton, I.O.; Shishko, E.D.; Kutsevol, N.V.; Marinin, A.I.; Gamaleia, N.F. Branched copolymers dextran-graft-polyacrylamide as nanocarriers for delivery of gold nanoparticles and photosensitizers to tumor cells. In Nanophysics Nanophotonics Surface Studies and Applications, Springer Proceedings in Physics Series; Fesenko, O., Yatsenko, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 379–390. [Google Scholar]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart polymers and their applications as biomaterials. III Biomaterials. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R., Chiellini, E., Eds.; Academic Press: Cambridge, MA, USA, 2007; Volume 3, pp. 1–27. [Google Scholar]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Sedláček, O.; Černoch, P.; Kučka, J. Thermoresponsive polymers for nuclear medicine: Which polymer is the best? Langmuir 2016, 32, 6115–6122. [Google Scholar] [CrossRef]
- Ma, Y.M.; Wei, D.X.; Yao, H.; Wu, L.P.; Chen, G.Q. Synthesis, characterization and application of thermoresponsive polyhydroxyalkanoate-graft-poly (N-isopropylacrylamide). Biomacromolecules 2016, 17, 2680–2690. [Google Scholar] [CrossRef]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The role of backbone hydration of poly(N-isopropyl acrylamide) across the volume phase transition compared to its monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef] [Green Version]
- Bischofberger, I.; Trappe, V. New aspects in the phase behaviour of poly-N-isopropyl acrylamide: Systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST. Sci. Rep. 2015, 5, 15520. [Google Scholar] [CrossRef]
- De Oliveira, T.E.; Marques, C.M.; Netz, P.A. Molecular dynamics study of the LCST transition in aqueous poly(N-n-propylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 10100. [Google Scholar] [CrossRef]
- Lopez, V.C.; Hadgraft, J.; Snowden, M.J. The use of colloidal microgels as a (trans)dermal drug delivery system. Int. J. Pharm. 2005, 292, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.Y. Plasmon-enhanced light–matter interactions and applications. NPJ Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Baumberg, J.J.; Aizpurua, J.; Mikkelsen, M.H.; Smith, D.R. Extreme nanophotonics from ultrathin metallic gaps. Nat. Mater. 2019, 18, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Zhang, Y.; Aslan, K.; Previte, M.J.R.; Geddes, C.D. Metal-enhanced singlet oxygen generation: A consequence of plasmon enhanced triplet yields. J. Fluoresc. 2007, 17, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kutsevol, N.V.; Chumachenko, V.A.; Rawiso, M.; Shkodich, V.F.; Stoyanov, O.V. Star-like polymers dextran-polyacrylamide: The prospects of application for nanotechnology. J. Struct. Chem. 2015, 56, 1016–1023. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Naumenko, A.P.; Kutsevol, N.V.; Maskova, D.O.; Harahuts, I.I.; Chumachenko, V.A.; Marinin, A.I. Anomalous inverse hysteresis of phase transition in thermosensitive dextran-graft-PNIPAM copolymer/Au nanoparticles hybrid nanosystem. J. Phys. Chem. C 2018, 122, 8003–8010. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Naumenko, A.P.; Kutsevol, N.V.; Harahuts, I.I. Laser-driven structural transformations in dextran-graft-PNIPAM copolymer/Au nanoparticles hybrid nanosystem: The role of plasmon heating and attractive optical forces. RSC Adv. 2018, 8, 38400–38409. [Google Scholar] [CrossRef] [Green Version]
- Chumachenko, V.; Kutsevol, N.; Harahuts, I.; Soloviov, D.; Bulavin, L.; Yeshchenko, O.; Naumenko, A.; Nadtoka, O.; Marinin, A. Temperature driven transformation in dextran-graft-PNIPAM/embedded silver nanoparticle hybrid system. Int. J. Polym. Sci. 2019, 2019, 3765614. [Google Scholar] [CrossRef] [Green Version]
- Yeshchenko, O.A.; Bartenev, A.O.; Naumneko, A.P.; Kutsevol, N.V.; Harahuts, I.I.; Marinin, A.I. Laser-driven aggregation in dextran-graft-PNIPAM/silver nanoparticles hybrid nanosystem: Plasmonic effects. Ukr. J. Phys. 2020, 65, 254–267. [Google Scholar] [CrossRef] [Green Version]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhou, T.; Bai, R.; Xie, Y. Chemical approaches for the enhancement of porphyrin skeleton-based photodynamic therapy. J. Enzym. Inhib. Med. Chem. 2020, 35, 1080–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, H.J.; Wu, S.; Huang, H.; Si, L.P.; Liu, H.Y.; Shi, L.; Zhang, H.T. Synthesis, characterization, and photodynamic therapy activity of 5,10,15,20-tetrakis(carboxyl)porphyrin. Bioorganic Med. Chem. 2019, 27, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Elms, J.; Beckett, P.N.; Griffin, P.; Curran, A.D. Mechanisms of isocyanate sensitisation. An in vitro approach. Toxicol. Vitr. 2001, 15, 631. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutsevol, N.; Harahuts, Y.; Shton, I.; Borikun, T.; Storchai, D.; Lukianova, N.; Chekhun, V. In vitro study of toxicity of hybrid gold-polymer composites. Mol. Cryst. Liq. Cryst. 2018, 671, 1–8. [Google Scholar] [CrossRef]
- Milacic, V.; Dou, Q.P. The tumor proteasome as a novel target for gold(III) complexes: Implications for breast cancer therapy. Coord. Chem. Rev. 2009, 19, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Lammer, A.D.; Cook, M.E.; Sessler, J.L. Synthesis and anti-cancer activities of a water soluble gold(III) porphyrin. J. Porphyr. Phthalocyanines 2015, 19, 398–403. [Google Scholar] [CrossRef]
- Macia, N.; Kabanov, V.; Côté-Cyr, M.; Heyne, B. Roles of near and far fields in plasmon-enhanced singlet oxygen production. J. Phys. Chem. Lett. 2019, 10, 3654–3660. [Google Scholar] [CrossRef]
- Macia, N.; Kabanov, V.; Heyne, B. Rationalizing the plasmonic contributions to the enhancement of singlet oxygen production. J. Phys. Chem. C 2020, 124, 3768–3777. [Google Scholar] [CrossRef]
- Planas, O.; Macia, N.; Agut, M.; Nonell, S.; Heyne, B. Distance-dependent plasmon-enhanced singlet oxygen production and emission for bacterial inactivation. J. Am. Chem. Soc. 2016, 138, 2762–2768. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Daqiqeh Rezaei, S.; Tan, Y.N. Simulation guided design of silver nanostructures for plasmon-enhanced fluorescence singlet oxygen generation and SERS applications. Phys. Chem. Chem. Phys. 2020, 22, 5673–5687. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshchenko, O.A.; Kutsevol, N.V.; Naumenko, A.P. Light-induced heating of gold nanoparticles in colloidal solution: Dependence on detuning from surface plasmon resonance. Plasmonics 2016, 11, 345–350. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Kozachenko, V.V. Light-induced heating of dense 2D ensemble of gold nanoparticles: Dependence on detuning from surface plasmon resonance. J. Nanoparticle Res. 2015, 17, 296. [Google Scholar] [CrossRef]
- Harahuts, Y.I.; Pavlov, V.A.; Mokrinskaya, E.V.; Chuprina, N.G.; Davidenko, N.A.; Naumenko, A.P.; Bezugla, T.M.; Kutsevol, N.V. The study of Au sol synthesized in uncharged and charged star-like copolymers under light irradiation. Funct. Mater. 2019, 4, 723–728. [Google Scholar]
- Kutsevol, N.; Kuziv, Y.; Bezugla, T.; Virych, P.; Marynin, A.; Borikun, T.; Lukianova, N.; Virych, P.; Chekhun, V. Application of new multicomponent nanosystems for overcoming doxorubicin resistance in breast cancer therapy. Appl. Nanosci. 2022, 12, 427–437. [Google Scholar] [CrossRef]
- Millenbaugh, N.J.; Baskin, J.B.; DeSilva, M.N.; Elliot, W.R.; Glickman, R.D. Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int. J. Nanomed. 2015, 10, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Lim, Z.Z.J.; Li, J.E.J.; Ng, C.T.; Yung, L.Y.L.; Bay, B.H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Von Maltzahn, G.; Park, J.H.; Lin, K.Y.; Singh, N.; Schwöppe, C.; Mesters, R.; Berdel, W.E.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011, 10, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Liu, Z.; Wang, Y.; Xu, Y.; Zhang, W.; Mu, T. The non-negative truncated singular value decomposition for adaptive sampling of particle size distribution in dynamic light scattering inversion. J. Quant. Spectrosc. Radiat. Transf. 2020, 246, 106917. [Google Scholar] [CrossRef]
- Marsh, D.F.; Mink, L.M. Microscale synthesis and electronic absorption spectroscopy of tetraphenylporphyrin H2(TPP) and metalloporphyrins ZnII(TPP) and NiII(TPP). J. Chem. Educ. 1996, 73, 1181. [Google Scholar] [CrossRef]
- Strachan, J.P.; Gentemann, S.; Seth, J.; Kalsbeck, W.A.; Lindsey, J.S.; Holten, D.; Bocian, D.F. Effects of orbital ordering on electronic communication in multiporphyrin arrays. J. Am. Chem. Soc. 1997, 119, 11191–11201. [Google Scholar] [CrossRef]
- Harriman, A. Luminescence of porphyrins and metalloporphyrins. Part 1—Zinc(II) nickel(II) and manganese(II) porphyrins. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1980, 6, 1978–1985. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Day, P.N.; Pachter, R.; Tretiak, S.; Chernyak, V.; Mukamel, S. Analysis of absorption spectra of zinc porphyrin, zinc meso-tetraphenylporphyrin and halogenated derivatives. J. Phys. Chem. A 2002, 106, 10285–10293. [Google Scholar] [CrossRef] [Green Version]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Naumenko, A.P.; Marinin, A.I. Plasmonic enhancement of the antibacterial photodynamic efficiency of a zinc tetraphenylporphyrin photosensitizer/dextran graft polyacrylamide anionic copolymer/Au nanoparticles hybrid nanosystem. RSC Adv. 2022, 12, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Naumenko, A.P.; Marinin, A.I. Zinc tetraphenylporphyrin/dextran-graft-polyacrylamide/gold nanoparticles hybrid nanosystem for photodynamic therapy: Plasmonic enhancement effect. Nanomed. Res. J. 2022, 7, 173–188. [Google Scholar]
- Törmö, P.; Barnes, W.L. Strong coupling between surface plasmon polaritons and emitters: A Review. Rep. Prog. Phys. 2015, 78, 013901. [Google Scholar] [CrossRef]
- Rodarte, A.L.; Tao, A.R. Plasmon-exciton coupling between metallic nanoparticles and dye monomers. J. Phys. Chem. C 2017, 121, 3496–3502. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.L. Novotny, Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshchenko, O.A.; Khort, P.S.; Kutsevol, N.V.; Prokopets, V.M.; Kapush, O.; Dzhagan, V. Temperature driven plasmon-exciton coupling in thermoresponsive dextran-graft-PNIPAM/Au nanoparticle/CdTe quantum dots hybrid nanosystem. Plasmonics 2021, 16, 1137–1150. [Google Scholar] [CrossRef]
- Roller, E.M.; Argyropoulos, C.; Högele, A.; Liedl, T.; Pilo-Pais, M. Plasmon-exciton coupling using DNA templates. Nano Lett. 2016, 16, 5962–5966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolinnyi, A.I. Nanometric rulers based on plasmon coupling in pairs of gold nanoparticles. J. Phys. Chem. C 2015, 119, 4990–5001. [Google Scholar] [CrossRef]
- Su, Q.; Jiang, C.; Gou, D.; Long, Y. Surface plasmon-assisted fluorescence enhancing and quenching: From theory to application. ACS Appl. Bio Mater. 2021, 4, 4684–4705. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.; Hildebrandt, N. FRET—Förster Resonance Energy Transfer: From Theory to Applications; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ashraf, S.; Park, H.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Garcia-Pinel, B.; Ortega-Rodríguez, A.; Porras-Alcalá, C.; Cabeza, L.; Contreras-Cáceres, R.; Ortiz, R.; Díaz, A.; Moscoso, A.; Sarabia, F.; Prados, J.; et al. Magnetically active pNIPAM nanosystems as temperature-sensitive biocompatible structures for controlled drug delivery. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sedev, R.; Beattie, D.; Ralston, J. Light-induced aggregation of colloidal gold nanoparticles capped by thymine derivatives. Langmuir 2008, 24, 4506–4511. [Google Scholar] [CrossRef] [PubMed]
- Schmarsow, R.N.; dell’Erba, I.E.; Villaola, M.S.; Hoppe, C.E.; Zucchi, I.A.; Schroeder, W.F. Effect of light intensity on the aggregation behavior of primary particles during in situ photochemical synthesis of gold/polymer nanocomposites. Langmuir 2020, 36, 13759–13768. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Narasimha, S.; Roy, A.; Banerjee, S. Does shining light on gold colloids influence aggregation? Sci. Rep. 2014, 4, 5213. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.S.; Wei, S.C.; Rogowski, J.L.; Tsuji, J.M.; Chen, P.Z.; Lin, C.W.; Jones, L.; Gu, F.X. Interactions between bacterial surface and nanoparticles govern the performance of “chemical nose” biosensors. Biosens. Bioelectron. 2016, 15, 115–125. [Google Scholar] [CrossRef]
- Gold, B.; Smith, R.; Nguyen, Q.; Roberts, J.; Ling, Y.; Lopez Quezada, L.; Somersan, S.; Warrier, T.; Little, D.; Pingle, M.; et al. Novel cephalosporins selectively active on nonreplicating mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 6027–6044. [Google Scholar] [CrossRef]
- Kavitha, S.; Harikrishnan, A.; Jeevaratnam, K. Characterization and evaluation of antibacterial efficacy of a novel antibiotic-type compound from a probiotic strain Lactobacillus plantarum KJB23 against food-borne pathogens. LWT 2020, 118, 108759. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Marinin, A.I.; Cheng, L.; Nie, G. Thermoresponsive Zinc TetraPhenylPorphyrin Photosensitizer/Dextran Graft Poly(N-IsoPropylAcrylAmide) Copolymer/Au Nanoparticles Hybrid Nanosystem: Potential for Photodynamic Therapy Applications. Nanomaterials 2022, 12, 2655. https://doi.org/10.3390/nano12152655
Yeshchenko OA, Kutsevol NV, Tomchuk AV, Khort PS, Virych PA, Chumachenko VA, Kuziv YI, Marinin AI, Cheng L, Nie G. Thermoresponsive Zinc TetraPhenylPorphyrin Photosensitizer/Dextran Graft Poly(N-IsoPropylAcrylAmide) Copolymer/Au Nanoparticles Hybrid Nanosystem: Potential for Photodynamic Therapy Applications. Nanomaterials. 2022; 12(15):2655. https://doi.org/10.3390/nano12152655
Chicago/Turabian StyleYeshchenko, Oleg A., Nataliya V. Kutsevol, Anastasiya V. Tomchuk, Pavlo S. Khort, Pavlo A. Virych, Vasyl A. Chumachenko, Yulia I. Kuziv, Andrey I. Marinin, Lili Cheng, and Guochao Nie. 2022. "Thermoresponsive Zinc TetraPhenylPorphyrin Photosensitizer/Dextran Graft Poly(N-IsoPropylAcrylAmide) Copolymer/Au Nanoparticles Hybrid Nanosystem: Potential for Photodynamic Therapy Applications" Nanomaterials 12, no. 15: 2655. https://doi.org/10.3390/nano12152655
APA StyleYeshchenko, O. A., Kutsevol, N. V., Tomchuk, A. V., Khort, P. S., Virych, P. A., Chumachenko, V. A., Kuziv, Y. I., Marinin, A. I., Cheng, L., & Nie, G. (2022). Thermoresponsive Zinc TetraPhenylPorphyrin Photosensitizer/Dextran Graft Poly(N-IsoPropylAcrylAmide) Copolymer/Au Nanoparticles Hybrid Nanosystem: Potential for Photodynamic Therapy Applications. Nanomaterials, 12(15), 2655. https://doi.org/10.3390/nano12152655





