The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer
Abstract
:1. Introduction
2. Composition and Sources of Particulate Matter
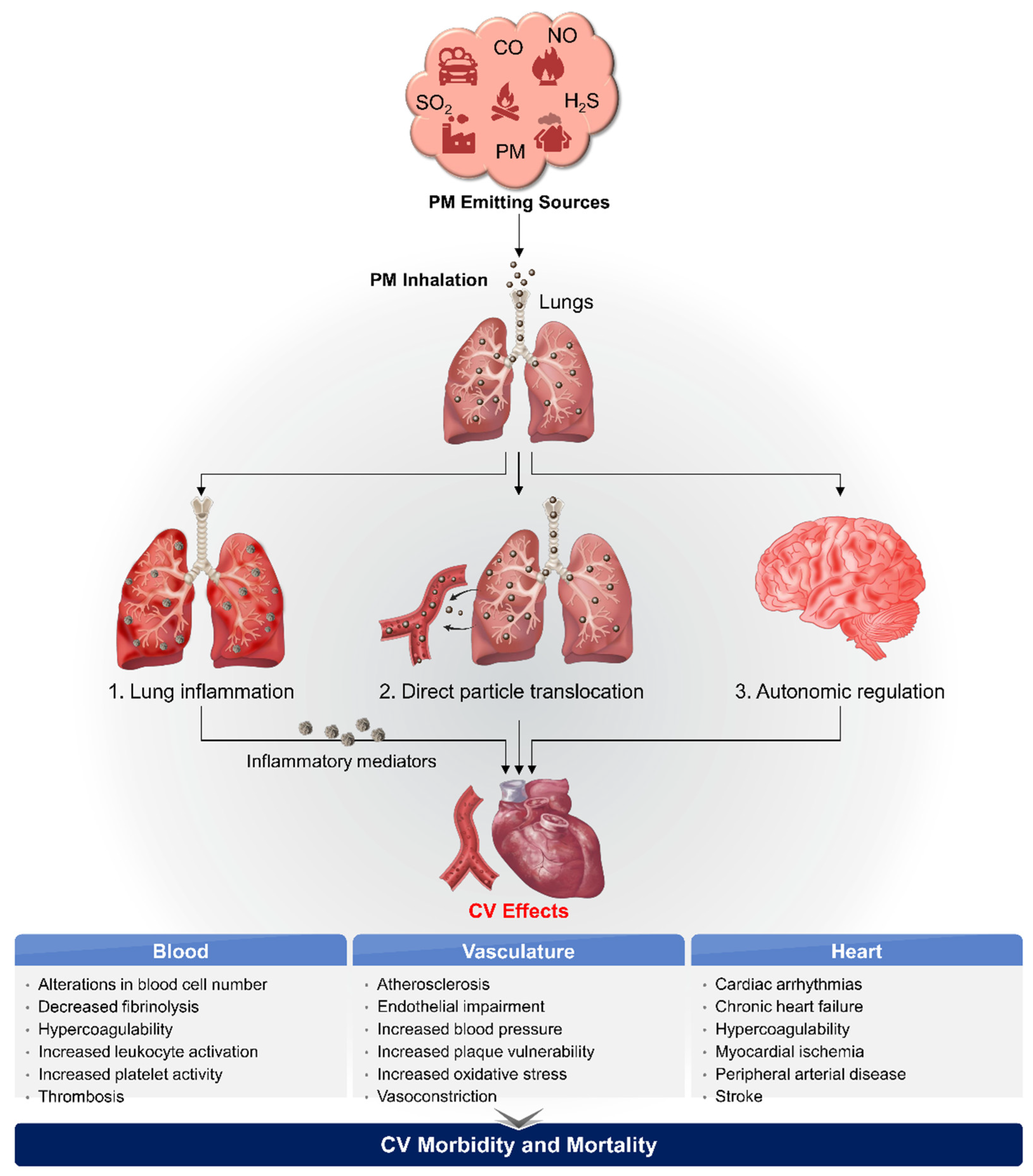
3. Biological Pathways Linking PM2.5 and CVD
3.1. Oxidative Stress and Systemic Inflammation
3.2. Direct Translocation into Systemic Circulation
3.3. Perturbation of the Autonomic Nervous System
4. PM2.5 and Mitochondrial Dysfunction
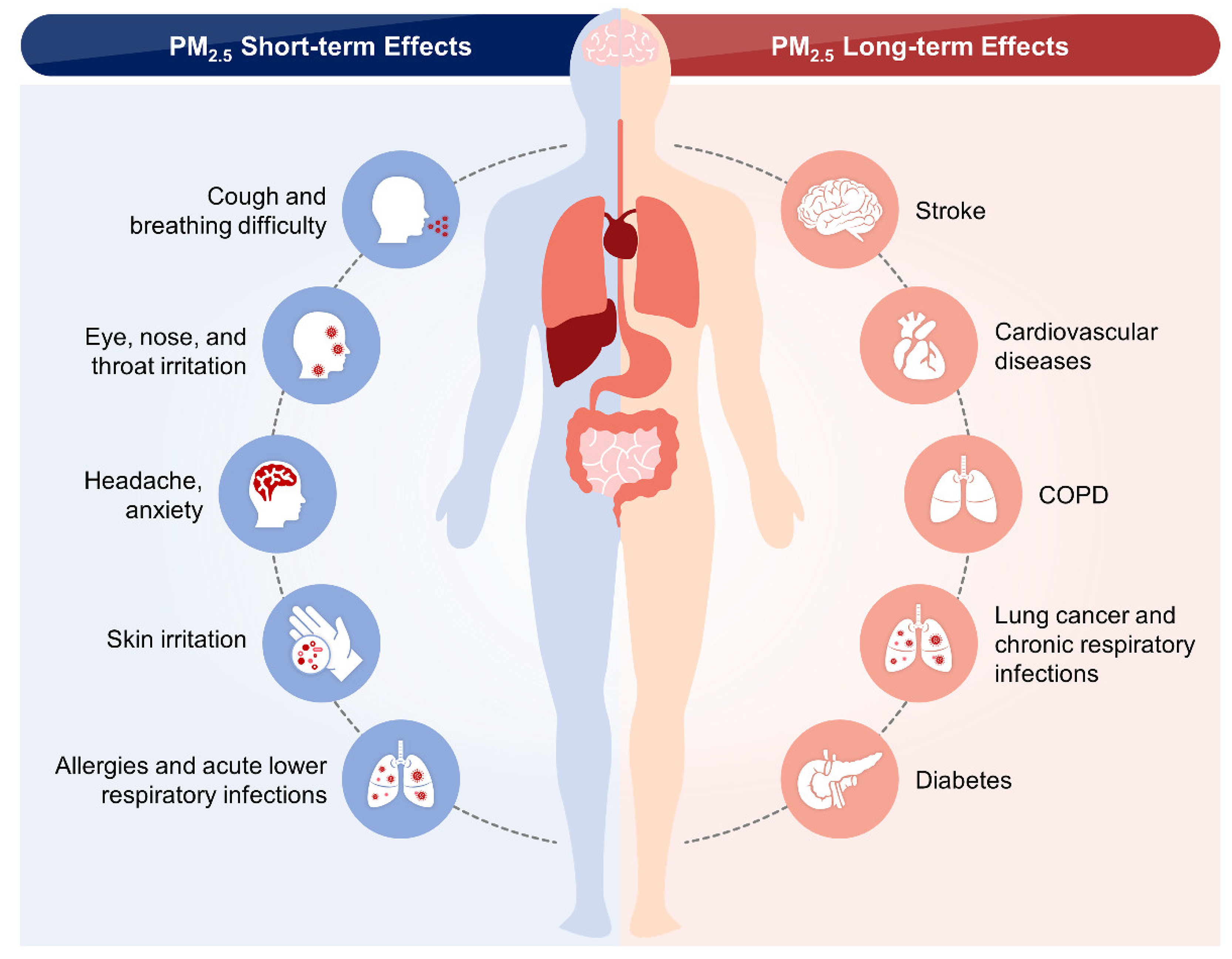
5. Epidemiological Studies on the Short- and Long-Term Effects of PM2.5 in the CVS
| Study and Year | Study Name | Study Period | Number of Participants | Age Range of Participants | Country, Region | PM2.5, μg/m3 (Mean or Range) | Outcome Types | |
|---|---|---|---|---|---|---|---|---|
| Short-term PM2.5 exposure studies | Achilleos et al. 2017 [101] | Meta-analysis (Pubmed and Web of Science) | 1996–July 2015 | 3851 records | All ages | Europe, U.S., West Pacific, Canada, and South America | 10 | Mortality: Cardiovascular disease (CVD) (0.80% (95% CI: 0.41, 1.20%). |
| Newell et al. 2017 [102] | Meta-analysis (PubMed, Web of Science, Embase, LILACS, Global Health, and Proquest) | Database inception–November 2016 | 85 records | ≥18 years | East Asia, Pacific region, Latin America, Caribbean, Europe, Central Asia, and (Middle East and North Africa) or Sub-Saharan Africa. | 10 | Mortality: CVD (0·47% (95% CI 0·34–0·61)). | |
| Chen et al. 2017 [103] | China’s Disease Surveillance Points system (DSPS) | January 2013–December 2015 | 272 Chinese cities | >5 years | China | 10 | Mortality: CVD (0.27% (95% posterior interval (PI), 0.18–0.36)), coronary heart disease (0.30% (95% PI, 0.19–0.40)), Stroke: 0.23% (95% PI, 0.13–0.34), cardiopulmonary disease (CPD) (17.55 (95% PI, 12.25–22.86)). | |
| Zhao et al. 2017 [104] | Meta-analysis (PubMed, and CNKI databases) | 2007–2017 | 30 records | All ages | China | 10 | Mortality: CVD (0.68%, 95% confidence interval (CI): 0.39–0.97%). | |
| Amsalu et al. 2019 [105] | Beijing Public Health Information Center | January 2013–December 2017 | 460,938 admissions | 18–64 years and ≥65 years | China, Beijing | 10 | Mortality: CVD (0.30, 95% CI: 0.20, 0.39%), CHD (0.34, 95% CI: 0.22 to 0.45%), Atrial Fibrillation (AF) (0.29, 95% CI, 0.03 to 0.55%). | |
| Tian et al. 2019 [106] | The urban employee basic medical insurance (UEBMI), urban resident basic medical insurance, and new rural cooperative medical scheme | January 2014–December 2017 | 8,834,533 hospital admissions | 18–64 years, 65–74 years, and ≥75 years | China | 10 | CVD (0.26% (95% CI 0.17% to 0.35%)), Ischaemic heart disease (IHD) (0.31% (0.22% to 0.40%)), heart failure (0.27% (0.04% to 0.51%)), heart rhythm disturbances (HRD) (0.29% (0.12% to 0.46%)), ischaemic stroke (IS) (0.29% (0.18% to 0.40%). | |
| Wyatt et al. 2020 [107] | US Renal Data System (RDS) | 2008–2014 | 361,568 patients | NA | U.S. | 10 | CVD (1.8%, 95% CI 0.4% to 3.2%), dysrhythmia, conduction disorder (4.8% (95% CI 2.3% to 7.4%)), and heart failure (3.7% (95% CI 1.4% to 6.0%). | |
| Qiu et al. 2020 [108] | Victim -crossover study of US New England Medicare participants | 2000–2012 | 532,154 individuals | >64 years | U.S. New England- (states of Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) | 10 | CVD, acute myocardial infarction (AMI) (4.3% (95% CI: 2.2%, 6.4%)), and congestive heart failure (CHF) (3.9% (2.4%, 5.5%)), IS (2.6% (0.4%, 4.7%)). | |
| Dahlquist et al. 2020 [109] | Victim-crossover study of Stockholm | 2012–2013 and 2016–2018 | 8899 individuals | 75 years | Sweden-Stockholm | 4.6 | Acute AF. | |
| Farhadi et al. 2020 [110] | Meta-analysis (PubMed, Scopus, Web of Science, and Embase) | January 2000–January 2018 | 26 records | NA | NA | 10 | MI (relative risk (RR) = 1.02; 95% CI 1.01–1.03). | |
| Ren et al. 2020 [111] | Victim-crossover study of Shenyang, China | January 2014–December 2017 | 157,144 patients | 0–30 years, 31–60 years, and >60 years | China-Shenyang Liaoning | 10 | CVD. | |
| Zhou et al. 2021 [112] | Taiyuan Center for Disease Control and Prevention | January 2013–October 2015 | 50,782 patients | >65 years | China-Taiyuan | 10 | Mortality: CVD (0.51% (95% CI: 0.08, 0.94)), IHD (1.01% (95% CI: 0.53, 1.50)), MI (1.08% (95% CI: 0.34, 1.83)). | |
| Yue et al. 2021 [113] | Meta-analysis (PubMed, Embase, the Cochrane library and Web of Science) | 2015–2020 | 18 records | <65 years and >65 years | China, Sweden, Korea, U.S., Italy, Canada, Iran, Israel, Denmark | 10 | AF (1.01(95% CI 1.00–1.02) and 1.07 (1.04–1.10)). | |
| Kuzma et al. 2021 [114] | Victim-crossover study of Bialystok and Katowice in Poland, Europe | 2008–2017 | 9046 patients | 64–69 years | Europe-Poland | 10 | Incidence: STEMI (OR = 1.041, 95% CI = 1.020–1.073; P < 0.001, lag-1). | |
| Chen et al. 2021 [115] | Meta-analysis (PubMed, Embase, and Web of Science) | 2006–2019 | 13 studies | <65 years and ≥65 years | North America, Europe, and Asia | 10 | AF (ER = 23.2%, 95% CI = −9.3–67.5), (ER = 0.6, 95% CI = −3.9–5.4), (ER = 2.3, 95% CI = 0.1–5.2). | |
| Long-term PM2.5 exposure studies | Badaloni et al. 2017 [116] | Rome Longitudinal Study (RoLS) | October 2001–December 2010 | 1,249,108 individuals | 30–44 years, 45–54 years, 55–64 years, 65–74 years, and >75 years | Italy, Rome | 5 | Mortality: IHD, CVD (hazard ratio (HR) = 1.05; 95% CI: 1.02–1.08), (HR = 1.06; 95% CI: 1.01–1.11). |
| Jerrett et al. 2017 [117] | American Cancer Society Cancer Prevention Study II (CPS-II) | 1982–2004 | 668,629 participants | ≥30 years | U.S., Washington, DC, and Puerto Rico | 10 | Mortality: IHD. | |
| Kim et al. 2017 [118] | National Health Insurance Service–National Sample Cohort (NHIS-NSC) | 2007–2013 | 1,025,340 individuals | ≥18 years | Korea-Seoul | 1 | Mortality: cardiovascular event (CE) (1.36 (95% confidence interval, 1.29–1.43)) and Incidence: Stroke. | |
| Pinault et al. 2017 [119] | Canadian Census Health and Environment Cohort (CanCHEC) | 2000–2008 | 2,448,500 participants | 25–89 | Canada | 10 | Mortality: IHD (HR = 1.16; 95% CI: 1.13–1.20). | |
| Pun et al. 2017 [120] | Medicare Beneficiaries | 2000–2008 | 52.9 million participants | 65–120 | U.S. | 10 | Mortality: CVD (RR = 1.56, 95% CI: 1.55, 1.57) | |
| Qiu et al. 2017 [121] | Elderly Health Service of the Department of Health in Hong Kong | 1998–2001 | 66,820 individuals | ≥65 years | Hong Kong | 10 | Incident: Stroke (1.14 (95% CI 1.02–1.27). | |
| Stockfelt et al. 2017 [122] | PPS cohort and the GOT-MONICA cohort | 1990–2011 | 10,350 participants | 25–64 years and 64–75 years | Sweden-Gothenburg | 5 | IHD (HR: 1.24 95% CI: 0.98–1.59) and Incident: Stroke (HR: 1.48; 95% CI: 0.88–2.49). | |
| Turner et al. 2017 [123] | American Cancer Society Cancer Prevention Study-II | 1999–2008 | 429,406 participants | <40 years and 40–80 years | U.S., Columbia, Puerto Rico etc. | 11–15 | Mortality: CV (relative excess risk due to interaction (RERI) = 0.10, attributal proportion (AP) = 0.05, synergy index (S) = 1.11). | |
| Yin et al. 2017 [124] | Disease Surveillance Points (DSPs), China | 1990–1991 | 189,793 participants | ≥40 years | China | 10 | Mortality: CVD (1.12 (1.10, 1.13)). | |
| Cakmak et al. 2018 [125] | Canadian Census Health and Environment Cohort (CanCHEC) | 1991–2011 | 3.6 million participants | ≥25 years | Canada | 10 | Mortality: IHD (1.13; 95% CI 1.08, 1.19). | |
| Gandini et al. 2018 [126] | Italian Longitudinal Study (ILS) | 1999–2000 | 140,011 individuals | >35 years | Italy | 10 | AMI (1.15 (1.12–1.18)) and Incidence: Stroke. | |
| Loop et al. 2018 [127] | REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort | 2003–2007 | 17,126 participants | ≥45 years | U.S. -Stroke Belt and Stroke Buckle | 2.7 | Mortality: CHD (0.94 (0.83–1.06)) and Non-fatal: AMI (0.85 (0.73–0.99)). | |
| Parker et al. 2018 [128] | National Health Interview Survey (NHIS) | 1997 to 2009 | 657,238 participants | ≥25 years | U. S. | 10 | Mortality: Heart disease ((HR, 1.16; 95% CI, 1.08–1.25)). | |
| Yitshak-Sade et al. 2018 [129] | Harvard School of Public Health Institutional Review Board | 2001−2011 | 2,015,660 participants | ≥65 years | U.S., New England | 2.3 | CVD (6.58% (5.90%; 7.26%)), and IS (0.82% (−0.68%; 2.35%)). | |
| Bai et al. 2019 [130] | Ontario Population Health and Environment Cohort (ONPHEC) | 2001 to 2015 | 6,248,299 participants | 35–85 years | Canada-Ontario | 9.6 | Mortality: CHF (1.05 (95% CI: 1.04–1.05)) and Incident: AMI (3%; (95% CI: 2–3%)). | |
| Danesh Yazdi et al. 2019 [131] | Medicare and Medicaid Services denominator file | 2000–2012 | 11,084,660 individuals | ≥65 years | Southeastern United States-Florida, Alabama, Mississippi, Georgia, North Carolina, South Carolina, and Tennessee | 1 | AMI and Stroke. | |
| Dirgawati et al. 2019 [132] | The Health in Men Study (HIMS) | Apr 1996–Jan 1999 | 12,203 participants | ≥65years | Perth | 5 | Fatal: Stroke. | |
| Heritier et al. 2019 [133] | Swiss National Cohort (SNC) | Dec 2000–Dec 2008 | 7.28 million observations | >30 years | Switzerland | 10 | Mortality: AMI (1.034, 95% CI: 1.014–1.055). | |
| Huang et al. 2019 [134] | China-PAR | 2000–2015 | 117,575 participants | <50 years >50 years | China | 10 | Incident: Stroke (13% (1.133, 1.09 to 1.17). | |
| Lim et al. 2019 [135] | National Institutes of Health–American Association for Retired Persons (NIH-AARP) | 1995–2011 | 548,845 participants | 50–71years | U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and metropolitan areas (Atlanta, Georgia; Detroit, Michigan) | 10 | CVD (1.13; 95% CI, 1.08–1.18)), IHD ((HR, 1.16; 95% CI, 1.10–1.23)). | |
| Ljungman et al. 2019 [136] | Swedish cohorts (includes the Primary Prevention Study (PPS) and the Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases (GOT-MONICA) | Jan 1990–Dec 2011 | 114,758 individuals | 25–64 years | Sweden-Gothenburg, Stockholm, and Umea | 1.94 | Incident: IHD (6.5% (95% CI: −0.5−0.5, 14)). | |
| Pope et al. 2019 [137] | National Health Interview Surveys (NHIS) | 1986–2014 | 1,599,329 participants | 18–84 years | U.S. | 10 | Mortality: CP (1.24 (95% CI: 1.20, 1.29)) and (1.23 (95% CI: 1.17, 1.29)). | |
| Shin et al. 2019 [138] | Ontario Population Health and Environment Cohort (ONPHEC) | Apr 2001–Mar 2015 | 5,071,956 participants | 35–85 years | Canada-Ontario | 10 | AF: HR (95% CI): 1.03 (1.01, 1.04) and Incidence: Stroke (HR (95% CI): 1.05 (1.03, 1.07)). | |
| Hayes et al. 2020 [139] | National Institutes of Health NIH-AARP | 2000–2005 | 565,477 participants | 50–71 years | U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and urban areas (Atlanta, GA, and Detroit, MI,) | 10 | Mortality: IHD (HR 1.16; 95% CI 1.09–1.22) and Stroke (HR 1.14; CI 1.02–1.27). |
6. PM2.5-Induced Risk of Cardiovascular Diseases
6.1. Acute Coronary Syndrome and Myocardial Infarction
6.2. Arrhythmia
6.3. Cardiovascular Mortality
6.4. Heart Failure and Ischemic Heart Disease
6.5. Blood Pressure and Hypertension
6.6. Vascular Dysfunction, Peripheral Arterial Disease, and Atherosclerosis
6.7. Thrombosis and Coagulation
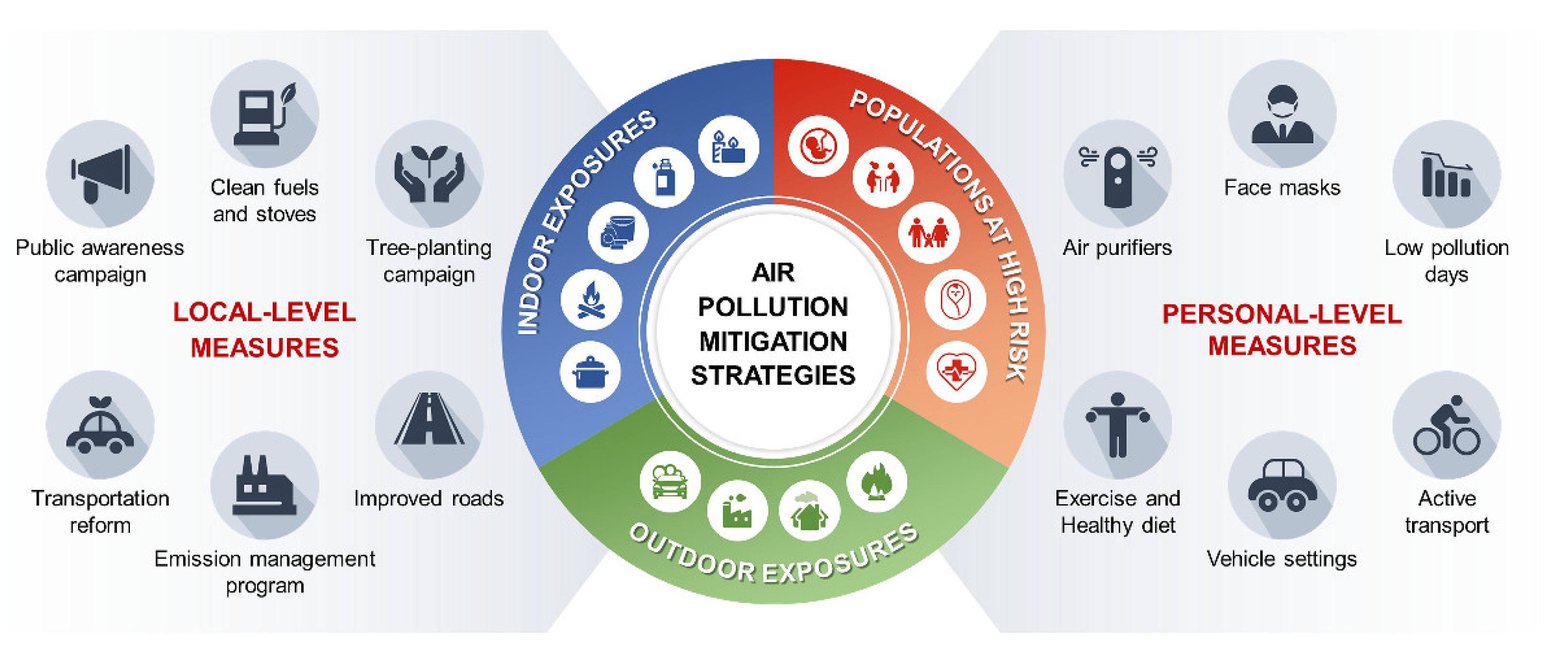
7. Strategies to Mitigate the Effects of PM2.5 on Cardiovascular Disease
7.1. Societal and Governmental Mitigation Strategies
7.2. Personal Mitigation Strategies
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brauer, M.; Casadei, B.; Harrington, R.A.; Kovacs, R.; Sliwa, K.; WHF Air Pollution Expert Group. Taking a Stand Against Air Pollution—The Impact on Cardiovascular Disease: A Joint Opinion from the World Heart Federation, American College of Cardiology, American Heart Association, and the European Society of Cardiology. Glob. Heart 2021, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., 3rd; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, P.M.; Harari, S.; Franchini, M. Novel evidence for a greater burden of ambient air pollution on cardiovascular disease. Haematologica 2019, 104, 2349–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: Car sick. Cardiovasc. Res. 2019, 116, 279–294. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Boogaard, H.; Walker, K.; Cohen, A.J. Air pollution: The emergence of a major global health risk factor. Int. Health 2019, 11, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Apte, J.S.; Brauer, M.; Cohen, A.J.; Ezzati, M.; Pope, C.A., III. Ambient PM2.5 Reduces Global and Regional Life Expectancy. Environ. Sci. Technol. Lett. 2018, 5, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, S.; Landrigan, P.J. Pollution and the Heart. N. Engl. J. Med. 2021, 385, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2014, 36, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R. Oxidative stress and the cardiovascular effects of air pollution. Free Radic. Biol. Med. 2020, 151, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and Temporal Variation in PM 2.5 Chemical Composition in the United States for Health Effects Studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boovarahan, S.R.; Kurian, G.A. Mitochondrial dysfunction: A key player in the pathogenesis of cardiovascular diseases linked to air pollution. Rev. Environ. Health 2018, 33, 111–122. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Olivieri, O.; Girelli, D. Air particulate matter and cardiovascular disease: A narrative review. Eur. J. Intern. Med. 2013, 24, 295–302. [Google Scholar] [CrossRef]
- Bourdrel, T.; Bind, M.-A.; Béjot, Y.; Morel, O.; Argacha, J.-F. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017, 110, 634–642. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryou, H.G.; Heo, J.; Kim, S.-Y. Source apportionment of PM10 and PM2.5 air pollution, and possible impacts of study characteristics in South Korea. Environ. Pollut. 2018, 240, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Weagle, C.L.; Snider, G.; Li, C.; van Donkelaar, A.; Philip, S.; Bissonnette, P.; Burke, J.; Jackson, J.; Latimer, R.; Stone, E.; et al. Global Sources of Fine Particulate Matter: Interpretation of PM2.5 Chemical Composition Observed by SPARTAN using a Global Chemical Transport Model. Environ. Sci. Technol. 2018, 52, 11670–11681. [Google Scholar] [CrossRef]
- Masri, S.; Kang, C.-M.; Koutrakis, P. Composition and sources of fine and coarse particles collected during 2002-2010 in Boston, MA. J. Air Waste Manag. Assoc. 2015, 65, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexeeff, S.E.; Liao, N.S.; Liu, X.; Eeden, S.K.V.D.; Sidney, S. Long-Term PM2.5 Exposure and Risks of Ischemic Heart Disease and Stroke Events: Review and Meta-Analysis. J. Am. Hear. Assoc. 2021, 10, e016890. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Chen, L.-C.; Gordon, T.; Ito, K.; Thurston, G.D. National Particle Component Toxicity (NPACT) Initiative: Integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res. Rep. Health Eff. Inst. 2013, 5–13. [Google Scholar]
- Kundu, S.; Stone, E.A. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ. Sci. Process. Impacts 2014, 16, 1360–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlwein, S.; Kappeler, R.; Joss, M.K.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines Involved in the Systemic Inflammatory Response Induced by Exposure to Particulate Matter Air Pollutants (PM10). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Törnqvist, H.; Mills, N.; Gonzalez, M.G.M.; Miller, M.R.; Robinson, S.D.; Megson, I.; MacNee, W.; Donaldson, K.; Söderberg, S.; Newby, D.E.; et al. Persistent Endothelial Dysfunction in Humans after Diesel Exhaust Inhalation. Am. J. Respir. Crit. Care Med. 2007, 176, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Shoenfelt, J.; Mitkus, R.J.; Zeisler, R.; Spatz, R.O.; Powell, J.; Fenton, M.J.; Squibb, K.A.; Medvedev, A.E. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 2009, 86, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.; Fenton, M.J.; Soukup, J.M. Involvement of Microbial Components and Toll-like Receptors 2 And 4 in Cytokine Responses to Air Pollution Particles. Am. J. Respir. Cell Mol. Biol. 2002, 27, 611–618. [Google Scholar] [CrossRef]
- Milici, A.; Talavera, K. TRP Channels as Cellular Targets of Particulate Matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef]
- Rückerl, R.; Ibald-Mulli, A.; Koenig, W.; Schneider, A.; Woelke, G.; Cyrys, J.; Heinrich, J.; Marder, V.; Frampton, M.; Wichmann, H.E.; et al. Air Pollution and Markers of Inflammation and Coagulation in Patients with Coronary Heart Disease. Am. J. Respir. Crit. Care Med. 2006, 173, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Zanobetti, A.; Martinelli, I.; Grillo, P.; Hou, L.; Giacomini, S.; Bonzini, M.; Lanzani, G.; Mannucci, P.M.; Bertazzi, P.A.; et al. Effects of exposure to air pollution on blood coagulation. J. Thromb. Haemost. 2007, 5, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Bonzini, M.; Tripodi, A.; Artoni, A.; Tarantini, L.; Marinelli, B.; Bertazzi, P.A.; Apostoli, P.; Baccarelli, A. Effects of inhalable particulate matter on blood coagulation. J. Thromb. Haemost. 2010, 8, 662–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgueira, S.A.; Lawrence, J.; Coull, B.; Murthy, G.G.K.; González-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002, 110, 749–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakey, P.; Berkemeier, T.; Tong, H.-J.; Arangio, A.; Lucas, K.; Pöschl, U.; Shiraiwa, M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016, 6, 32916. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Hoet, P.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of Inhaled Particles Into the Blood Circulation in Humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.M.; Verbruggen, A.; Nemery, B. Passage of Intratracheally Instilled Ultrafine Particles from the Lung into the Systemic Circulation in Hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; MacNee, W.; Donaldson, K.; Godden, D. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Q.-Y.; Cheng, Z.-P.; Hu, B.; Liu, J.-D.; Hu, Y. Air pollution and venous thrombosis: A meta-analysis. Sci. Rep. 2016, 6, 32794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.J.; Zanobetti, A.; Koutrakis, P.; Mittleman, M.A.; Sparrow, D.; Vokonas, P.; Schwartz, J. Associations Between Short-term Changes in Air Pollution and Correlates of Arterial Stiffness: The Veterans Affairs Normative Aging Study, 2007–2011. Am. J. Epidemiol. 2013, 179, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, P.; Mikkelsen, L.; Vesterdal, L.K.; Folkmann, J.K.; Forchhammer, L.; Roursgaard, M.; Danielsen, P.H.; Loft, S. Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis. Crit. Rev. Toxicol. 2011, 41, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D. Cardiovascular effects of air pollution. Clin. Sci. 2008, 115, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Tu, Y.; Yu, Z.; Lu, R. PM2.5 and Cardiovascular Diseases in the Elderly: An Overview. Int. J. Environ. Res. Public Health 2015, 12, 8187–8197. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.; Duan, Y.; Whitsel, E.A.; Zheng, Z.-J.; Heiss, G.; Chinchilli, V.M.; Lin, H.-M. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: A population-based study. Am. J. Epidemiol. 2004, 159, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.R.; Wellenius, G.; Coull, B.A.; Akiyama, I.; Diaz, E.A.; Lawrence, J.; Okabe, K.; Verrier, R.L.; Godleski, J.J. Concentrated Ambient Particles Alter Myocardial Blood Flow during Acute Ischemia in Conscious Canines. Environ. Health Perspect. 2009, 117, 333–337. [Google Scholar] [CrossRef]
- Ying, Z.; Xu, X.; Bai, Y.; Zhong, J.; Chen, M.; Liang, Y.; Zhao, J.; Liu, D.; Morishita, M.; Sun, Q.; et al. Long-Term Exposure to Concentrated Ambient PM 2.5 Increases Mouse Blood Pressure through Abnormal Activation of the Sympathetic Nervous System: A Role for Hypothalamic Inflammation. Environ. Health Perspect. 2014, 122, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Kurhanewicz, N.; McIntosh-Kastrinsky, R.; Tong, H.; Ledbetter, A.; Walsh, L.; Farraj, A.; Hazari, M. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol. Appl. Pharmacol. 2016, 324, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.; Thomson, A.L.; Carter, R.; Stott, H.R.; Shaw, C.A.; Hadoke, P.W.F.; Newby, D.E.; Miller, M.R.; Gray, G.A. Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV1 and β1 adrenoreceptors. Part. Fibre Toxicol. 2014, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazari, M.S.; Haykal-Coates, N.; Winsett, D.W.; Krantz, Q.T.; King, C.; Costa, D.L.; Farraj, A.K. TRPA1 and Sympathetic Activation Contribute to Increased Risk of Triggered Cardiac Arrhythmias in Hypertensive Rats Exposed to Diesel Exhaust. Environ. Health Perspect. 2011, 119, 951–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajat, A.; Roux, A.V.D.; Castro-Diehl, C.; Cosselman, K.; Golden, S.H.; Hazlehurst, M.F.; Szpiro, A.; Vedal, S.; Kaufman, J. The Association between Long-Term Air Pollution and Urinary Catecholamines: Evidence from the Multi-Ethnic Study of Atherosclerosis. Environ. Health Perspect. 2019, 127, 057007. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2014, 4, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, H.-M.; Panni, T.; Motta, V.; Hou, L.; Nordio, F.; Apostoli, P.; Bertazzi, P.A.; Baccarelli, A.A. Effects of airborne pollutants on mitochondrial DNA Methylation. Part. Fibre Toxicol. 2013, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojkovič, G.; Makarova, A.V.; Wanrooij, P.; Forslund, J.; Burgers, P.M.; Wanrooij, S. Oxidative DNA damage stalls the human mitochondrial replisome. Sci. Rep. 2016, 6, 28942. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leung, M.C.K.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a Target of Environmental Toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Xu, X.; Zhong, M.; Hotchkiss, I.P.; Lewandowski, R.P.; Wagner, J.G.; Bramble, L.A.; Yang, Y.; Wang, A.; Harkema, J.R.; et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol. 2011, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [Green Version]
- Di Gregorio, I.; Busiello, R.A.; Burgos-Aceves, M.A.; Lepretti, M.; Paolella, G.; Lionetti, L. Environmental Pollutants Effect on Brown Adipose Tissue. Front. Physiol. 2019, 9, 1891. [Google Scholar] [CrossRef]
- Marchini, T.; Magnani, N.; D’Annunzio, V.; Tasat, D.; Gelpi, R.; Alvarez, S.; Evelson, P. Impaired cardiac mitochondrial function and contractile reserve following an acute exposure to environmental particulate matter. Biochim. Biophys. Acta 2012, 1830, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Hiura, T.S.; Li, N.; Kaplan, R.; Horwitz, M.; Seagrave, J.-C.; Nel, A.E. The Role of a Mitochondrial Pathway in the Induction of Apoptosis by Chemicals Extracted from Diesel Exhaust Particles. J. Immunol. 2000, 165, 2703–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Volpi, S.; Sims, K.B.; Walter, J.E.; Traggiai, E. Powering the Immune System: Mitochondria in Immune Function and Deficiency. J. Immunol. Res. 2014, 2014, 164309. [Google Scholar] [CrossRef] [PubMed]
- Kapnick, S.M.; Pacheco, S.E.; McGuire, P.J. The emerging role of immune dysfunction in mitochondrial diseases as a paradigm for understanding immunometabolism. Metabolism 2018, 81, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Breda, C.N.D.S.; Davanzo, G.G.; Basso, P.J.; Câmara, N.O.S.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, M.; Khemka, V.K.; Chatterjee, G.; Ganguly, A.; Mukhopadhyay, S.; Chakrabarti, S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol. Cell. Biochem. 2014, 399, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cochemé, H.M.; Murphy, M.P. Complex I Is the Major Site of Mitochondrial Superoxide Production by Paraquat. J. Biol. Chem. 2008, 283, 1786–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurian, G.A.; Philip, S.; Varghese, T. Effect of aqueous extract of the Desmodium gangeticum DC root in the severity of myocardial infarction. J. Ethnopharmacol. 2005, 97, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Mügge, A. The role of reactive oxygen species in atherosclerosis. Z Kardiol. 1998, 87, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L.A. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, H.H.; Lame, M.W.; Gohil, K.; He, G.; Denison, M.S.; Rutledge, J.C.; Wilson, D.W. Comparative gene responses to collected ambient particles in vitro: Endothelial responses. Physiol. Genom. 2011, 43, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Li, Q.; Asweto, C.; Feng, L.; Yang, X.; Duan, F.; Duan, J.; Sun, Z. Fine particulate matter induces vascular endothelial activation via IL-6 dependent JAK1/STAT3 signaling pathway. Toxicol. Res. 2016, 5, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Montiel-Dávalos, A.; Ibarra-Sánchez, M.D.J.; Ventura-Gallegos, J.L.; Alfaro-Moreno, E.; López-Marure, R. Oxidative stress and apoptosis are induced in human endothelial cells exposed to urban particulate matter. Toxicol. Vitr. 2010, 24, 135–141. [Google Scholar] [CrossRef]
- Sivakumar, B.; Kurian, G.A. PM2.5 from diesel exhaust attenuated fisetin mediated cytoprotection in H9c2 cardiomyocytes subjected to ischemia reoxygenation by inducing mitotoxicity. Drug Chem. Toxicol. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, B.; Kurian, G.A. PM2.5 Exposure Lowers Mitochondrial Endurance During Cardiac Recovery in a Rat Model of Myocardial Infarction. Cardiovasc. Toxicol. 2022, 22, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yue, P.; Ying, Z.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Hwang, J.-S.; Zweier, J.L.; Chen, L.C.; Rajagopalan, S. Air Pollution Exposure Potentiates Hypertension Through Reactive Oxygen Species-Mediated Activation of Rho/ROCK. Arter. Thromb. Vasc. Biol. 2008, 28, 1760–1766. [Google Scholar] [CrossRef] [Green Version]
- Wittkopp, S.; Staimer, N.; Tjoa, T.; Gillen, D.; Daher, N.; Shafer, M.; Schauer, J.J.; Sioutas, C.; Delfino, R.J. Mitochondrial Genetic Background Modifies the Relationship between Traffic-Related Air Pollution Exposure and Systemic Biomarkers of Inflammation. PLoS ONE 2013, 8, e64444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippmann, M. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: Coherence and public health implications. Crit. Rev. Toxicol. 2014, 44, 299–347. [Google Scholar] [CrossRef]
- Nakano, T.; Otsuki, T. Environmental air pollutants and the risk of cancer. Gan kagaku ryoho. Cancer Chemother. 2013, 40, 1441–1445. [Google Scholar]
- Watkins, A.; Danilewitz, M.; Kusha, M.; Massé, S.; Urch, B.; Quadros, K.; Spears, D.; Farid, T.; Nanthakumar, K. Air Pollution and Arrhythmic Risk: The Smog Is Yet to Clear. Can. J. Cardiol. 2013, 29, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; De Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2013, 348, f7412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafoggia, M.; Cesaroni, G.; Peters, A.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; Cyrys, J.; De Faire, U.; De Hoogh, K.; et al. Long-Term Exposure to Ambient Air Pollution and Incidence of Cerebrovascular Events: Results from 11 European Cohorts within the ESCAPE Project. Environ. Health Perspect. 2014, 122, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.S.V.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, B.; Moebus, S.; Kröger, K.; Stang, A.; Möhlenkamp, S.; Dragano, N.; Schmermund, A.; Memmesheimer, M.; Erbel, R.; Jöckel, K.-H. Residential Exposure to Urban Air Pollution, Ankle–Brachial Index, and Peripheral Arterial Disease. Epidemiology 2009, 20, 280–288. [Google Scholar] [CrossRef]
- Baccarelli, A.; Martinelli, I.; Pegoraro, V.; Melly, S.; Grillo, P.; Zanobetti, A.; Hou, L.; Bertazzi, P.A.; Mannucci, P.M.; Schwartz, J. Living Near Major Traffic Roads and Risk of Deep Vein Thrombosis. Circulation 2009, 119, 3118–3124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.L.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air pollution and cardiovascular disease—A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Yan, M.; Shan, A.; Wang, C.; Yang, X.; Tang, N. Sex Differences in Cardiovascular Risk Associated With Long-Term PM2.5 Exposure: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Public Health 2022, 10, 802167. [Google Scholar] [CrossRef]
- Wu, T.; Yang, X.; Chu, A.; Xie, X.; Bai, M.; Peng, Y.; Zhang, Z. Acute effects of fine particulate matter (PM2.5) on hospital admissions for cardiovascular diseases in Lanzhou, China: A time-series study. Environ. Sci. Eur. 2022, 34, 55. [Google Scholar] [CrossRef]
- Xi, Y.; Richardson, D.B.; Kshirsagar, A.V.; Wade, T.J.; Flythe, J.E.; Whitsel, E.A.; Rappold, A.G. Association Between Long-term Ambient PM2.5 Exposure and Cardiovascular Outcomes Among US Hemodialysis Patients. Am. J. Kidney Dis. 2022. In Press. [Google Scholar] [CrossRef] [PubMed]
- Magari, S.R.; Hauser, R.; Schwartz, J.; Williams, P.L.; Smith, T.J.; Christiani, D.C. Association of Heart Rate Variability With Occupational and Environmental Exposure to Particulate Air Pollution. Circulation 2001, 104, 986–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarnat, S.E.; Suh, H.H.; Coull, B.A.; Schwartz, J.; Stone, P.H.; Gold, D.R. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occup. Environ. Med. 2006, 63, 700–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, N.L.; Törnqvist, H.; Gonzalez, M.C.; Vink, E.; Robinson, S.D.; Söderberg, S.; Boon, N.A.; Donaldson, K.; Sandström, T.; Blomberg, A.; et al. Ischemic and Thrombotic Effects of Dilute Diesel-Exhaust Inhalation in Men with Coronary Heart Disease. N. Engl. J. Med. 2007, 357, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Folino, A.F.; Scapellato, M.L.; Canova, C.; Maestrelli, P.; Bertorelli, G.; Simonato, L.; Iliceto, S.; Lotti, M. Individual exposure to particulate matter and the short-term arrhythmic and autonomic profiles in patients with myocardial infarction. Eur. Hear. J. 2009, 30, 1614–1620. [Google Scholar] [CrossRef]
- Chan, C.-C.; Chuang, K.-J.; Shiao, G.-M.; Lin, L.-Y. Personal Exposure to Submicrometer Particles and Heart Rate Variability in Human Subjects. Environ. Health Perspect. 2004, 112, 1063–1067. [Google Scholar] [CrossRef]
- Wu, S.; Deng, F.; Niu, J.; Huang, Q.; Liu, Y.; Guo, X. Association of Heart Rate Variability in Taxi Drivers with Marked Changes in Particulate Air Pollution in Beijing in 2008. Environ. Health Perspect. 2010, 118, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, H.H.; Zanobetti, A. Exposure Error Masks the Relationship Between Traffic-Related Air Pollution and Heart Rate Variability. J. Occup. Environ. Med. 2010, 52, 685–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekkanen, J.; Peters, A.; Hoek, G.; Tiittanen, P.; Brunekreef, B.; de Hartog, J.; Heinrich, J.; Ibald-Mulli, A.; Kreyling, W.; Lanki, T.; et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: The Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation 2002, 106, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Achilleos, S.; Kioumourtzoglou, M.-A.; Wu, C.-D.; Schwartz, J.D.; Koutrakis, P.; Papatheodorou, S.I. Acute effects of fine particulate matter constituents on mortality: A systematic review and meta-regression analysis. Environ. Int. 2017, 109, 89–100. [Google Scholar] [CrossRef]
- Newell, K.; Kartsonaki, C.; Lam, K.B.H.; Kurmi, O.P. Cardiorespiratory health effects of particulate ambient air pollution exposure in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e368–e380. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Yin, P.; Meng, X.; Liu, C.; Wang, L.; Xu, X.; Ross, J.A.; Tse, L.A.; Zhao, Z.; Kan, H.; et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am. J. Respir. Crit. Care Med. 2017, 196, 73–81. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, H.-R.; Chen, F.-Y.; Chen, Z.; Guan, W.-J.; Li, J.-H. Association between air pollution and cardiovascular mortality in China: A systematic review and meta-analysis. Oncotarget 2017, 8, 66438–66448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsalu, E.; Wang, T.; Li, H.; Liu, Y.; Wang, A.; Liu, X.; Tao, L.; Luo, Y.; Zhang, F.; Yang, X.; et al. Acute effects of fine particulate matter (PM2.5) on hospital admissions for cardiovascular disease in Beijing, China: A time-series study. Environ. Health 2019, 18, 70. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Liu, H.; Wu, Y.; Si, Y.; Song, J.; Cao, Y.; Li, M.; Wu, Y.; Wang, X.; Chen, L.; et al. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: Time series study in 184 major Chinese cities. BMJ 2019, 367, l6572. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, L.H.; Xi, Y.; Kshirsagar, A.; Di, Q.; Ward-Caviness, C.; Wade, T.J.; Cascio, W.E.; Rappold, A.G. Association of short-term exposure to ambient PM2.5 with hospital admissions and 30-day readmissions in end-stage renal disease patients: Population-based retrospective cohort study. BMJ Open 2020, 10, e041177. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wei, Y.; Wang, Y.; Di, Q.; Sofer, T.; Abu Awad, Y.; Schwartz, J. Inverse probability weighted distributed lag effects of short-term exposure to PM2.5 and ozone on CVD hospitalizations in New England Medicare participants—Exploring the causal effects. Environ. Res. 2019, 182, 109095. [Google Scholar] [CrossRef]
- Dahlquist, M.; Frykman, V.; Kemp-Gudmunsdottir, K.; Svennberg, E.; Wellenius, G.A.; Ljungman, P.L.S. Short-term associations between ambient air pollution and acute atrial fibrillation episodes. Environ. Int. 2020, 141, 105765. [Google Scholar] [CrossRef]
- Farhadi, Z.; Gorgi, H.A.; Shabaninejad, H.; Delavar, M.A.; Torani, S. Association between PM2.5 and risk of hospitalization for myocardial infarction: A systematic review and a meta-analysis. BMC Public Health 2020, 20, 314. [Google Scholar] [CrossRef]
- Ren, Q.; Li, S.; Xiao, C.; Zhang, J.; Lin, H.; Wang, S. The Impact of Air Pollution on Hospitalization for Cardiovascular and Cerebrovascular Disease in Shenyang, China. Iran. J. Public Health 2020, 49, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Geng, H.; Dong, C.; Bai, T. The short-term harvesting effects of ambient particulate matter on mortality in Taiyuan elderly residents: A time-series analysis with a generalized additive distributed lag model. Ecotoxicol. Environ. Saf. 2020, 207, 111235. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, F.; Li, F.; Chen, Y. Association between air pollutants and atrial fibrillation in general population: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 208, 111508. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, Ł.; Wańha, W.; Kralisz, P.; Kazmierski, M.; Bachórzewska-Gajewska, H.; Wojakowski, W.; Dobrzycki, S. Impact of short-term air pollution exposure on acute coronary syndrome in two cohorts of industrial and non-industrial areas: A time series regression with 6,000,000 person-years of follow-up (ACS—Air Pollution Study). Environ. Res. 2021, 197, 111154. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.; Zhuo, C.; Zheng, L. The Association Between Ambient Air Pollution and Atrial Fibrillation. Int. Hear. J. 2021, 62, 290–297. [Google Scholar] [CrossRef]
- Badaloni, C.; Cesaroni, G.; Cerza, F.; Davoli, M.; Brunekreef, B.; Forastiere, F. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ. Int. 2017, 109, 146–154. [Google Scholar] [CrossRef]
- Jerrett, M.; Turner, M.C.; Beckerman, B.S.; Pope, C.A.; van Donkelaar, A.; Martin, R.V.; Serre, M.; Crouse, D.; Gapstur, S.M.; Krewski, D.; et al. Comparing the Health Effects of Ambient Particulate Matter Estimated Using Ground-Based versus Remote Sensing Exposure Estimates. Environ. Health Perspect. 2017, 125, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.; Kim, S.; Kang, S.-H.; Kim, H.-J.; Kim, H.; Heo, J.; Yi, S.-M.; Kim, K.; Youn, T.-J.; et al. Cardiovascular Effects of Long-Term Exposure to Air Pollution: A Population-Based Study With 900 845 Person-Years of Follow-up. J. Am. Hear. Assoc. 2017, 6, e007170. [Google Scholar] [CrossRef] [Green Version]
- Pinault, L.L.; Weichenthal, S.; Crouse, D.L.; Brauer, M.; Erickson, A.; van Donkelaar, A.; Martin, R.V.; Hystad, P.; Chen, H.; Finès, P.; et al. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environ. Res. 2017, 159, 406–415. [Google Scholar] [CrossRef]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef]
- Qiu, H.; Sun, S.; Tsang, H.; Wong, C.-M.; Lee, R.S.-Y.; Schooling, C.M.; Tian, L. Fine particulate matter exposure and incidence of stroke. Neurology 2017, 88, 1709–1717. [Google Scholar] [CrossRef] [Green Version]
- Stockfelt, L.; Andersson, E.M.; Molnár, P.; Gidhagen, L.; Segersson, D.; Rosengren, A.; Barregard, L.; Sallsten, G. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ. Res. 2017, 158, 61–71. [Google Scholar] [CrossRef]
- Turner, M.C.; Cohen, A.; Burnett, R.T.; Jerrett, M.; Diver, W.R.; Gapstur, S.M.; Krewski, D.; Samet, J.M.; Pope, C.A. Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environ. Res. 2017, 154, 304–310. [Google Scholar] [CrossRef]
- Yin, P.; Brauer, M.; Cohen, A.; Burnett, R.T.; Liu, J.; Liu, Y.; Liang, R.; Wang, W.; Qi, J.; Wang, L.; et al. Long-term Fine Particulate Matter Exposure and Nonaccidental and Cause-specific Mortality in a Large National Cohort of Chinese Men. Environ. Health Perspect. 2017, 125, 117002. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, S.; Hebbern, C.; Pinault, L.; Lavigne, E.; Vanos, J.; Crouse, D.L.; Tjepkema, M. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ. Int. 2017, 111, 200–211. [Google Scholar] [CrossRef]
- Gandini, M.; Scarinzi, C.; Bande, S.; Berti, G.; Carnà, P.; Ciancarella, L.; Costa, G.; Demaria, M.; Ghigo, S.; Piersanti, A.; et al. Long term effect of air pollution on incident hospital admissions: Results from the Italian Longitudinal Study within LIFE MED HISS project. Environ. Int. 2018, 121, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Loop, M.S.; McClure, L.A.; Levitan, E.B.; Al-Hamdan, M.Z.; Crosson, W.L.; Safford, M.M. Fine particulate matter and incident coronary heart disease in the REGARDS cohort. Am. Hear. J. 2018, 197, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.D.; Kravets, N.; Vaidyanathan, A. Particulate Matter Air Pollution Exposure and Heart Disease Mortality Risks by Race and Ethnicity in the United States: 1997 to 2009 National Health Interview Survey With Mortality Follow-Up Through 2011. Circulation 2018, 137, 1688–1697. [Google Scholar] [CrossRef]
- Yitshak-Sade, M.; Bobb, J.F.; Schwartz, J.D.; Kloog, I.; Zanobetti, A. The association between short and long-term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short-term exposures. Sci. Total Environ. 2018, 639, 868–875. [Google Scholar] [CrossRef]
- Bai, L.; Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Goldberg, M.S.; Lavigne, E.; Copes, R.; Martin, R.V.; et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: A population-based study of 5.1 million Canadian adults living in Ontario. Environ. Int. 2019, 132, 105004. [Google Scholar] [CrossRef]
- Yazdi, M.D.; Wang, Y.; Di, Q.; Zanobetti, A.; Schwartz, J. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ. Int. 2019, 130, 104879. [Google Scholar] [CrossRef]
- Dirgawati, M.; Hinwood, A.; Nedkoff, L.; Hankey, G.; Yeap, B.B.; Flicker, L.; Nieuwenhuijsen, M.; Brunekreef, B.; Heyworth, J. Long-term Exposure to Low Air Pollutant Concentrations and the Relationship with All-Cause Mortality and Stroke in Older Men. Epidemiology 2019, 30, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Héritier, H.; Vienneau, D.; Foraster, M.; Eze, I.C.; Schaffner, E.; De Hoogh, K.; Thiesse, L.; Rudzik, F.; Habermacher, M.; Köpfli, M.; et al. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: A nationwide cohort study in Switzerland. Eur. Hear. J. 2018, 40, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Liang, F.; Yang, X.; Liu, F.; Li, J.; Xiao, Q.; Chen, J.; Liu, X.; Cao, J.; Shen, C.; et al. Long term exposure to ambient fine particulate matter and incidence of stroke: Prospective cohort study from the China-PAR project. BMJ 2019, 367, l6720. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.C.; Hayes, R.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Thurston, G.D. Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 2019, 139, 1766–1775. [Google Scholar] [CrossRef]
- Ljungman, P.L.S.; Andersson, N.; Stockfelt, L.; Andersson, E.M.; Sommar, J.N.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Lager, A.; Leander, K.; et al. Long-Term Exposure to Particulate Air Pollution, Black Carbon, and Their Source Components in Relation to Ischemic Heart Disease and Stroke. Environ. Health Perspect. 2019, 127, 107012. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A., 3rd; Lefler, J.S.; Ezzati, M.; Higbee, J.D.; Marshall, J.D.; Kim, S.-Y.; Bechle, M.; Gilliat, K.S.; Vernon, S.E.; Robinson, A.; et al. Mortality Risk and Fine Particulate Air Pollution in a Large, Representative Cohort of U.S. Adults. Environ. Health Perspect. 2019, 127, 077007. [Google Scholar] [CrossRef]
- Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; Van Donkelaar, A.; Brook, J.R.; Goldberg, M.S.; Tu, K.; Copes, R.; Martin, R.V.; et al. Ambient Air Pollution and the Risk of Atrial Fibrillation and Stroke: A Population-Based Cohort Study. Environ. Health Perspect. 2019, 127, 087009. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 2020, 49, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Yang, K.-Q.; Yang, Y.-K.; Zhou, X.-L. Potential Harmful Effects of PM2.5 on Occurrence and Progression of Acute Coronary Syndrome: Epidemiology, Mechanisms, and Prevention Measures. Int. J. Environ. Res. Public Health 2016, 13, 748. [Google Scholar] [CrossRef]
- Gardner, B.; Ling, F.; Hopke, P.K.; Frampton, M.W.; Utell, M.J.; Zareba, W.; Cameron, S.J.; Chalupa, D.; Kane, C.; Kulandhaisamy, S.; et al. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: A case-crossover study. Part. Fibre Toxicol. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Qi, W.; Yao, W.; Wang, M.; Chen, Y.; Zhou, Y. Ambient Particulate Matter (PM2.5/PM10) Exposure and Emergency Department Visits for Acute Myocardial Infarction in Chaoyang District, Beijing, China During 2014: A Case-Crossover Study. J. Epidemiol. 2016, 26, 538–545. [Google Scholar] [CrossRef] [Green Version]
- PopeIII, C.A.; Muhlestein, J.B.; Anderson, J.L.; Cannon, J.B.; Hales, N.M.; Meredith, K.G.; Le, V.; Horne, B.D. Short-Term Exposure to Fine Particulate Matter Air Pollution Is Preferentially Associated With the Risk of ST-Segment Elevation Acute Coronary Events. J. Am. Hear. Assoc. 2015, 4, e002506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of air pollution on the incidence of myocardial infarction. Heart 2009, 95, 1746–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafić, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Périer, M.-C.; Marijon, E.; Vernerey, D.; Empana, J.-P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA J. Am. Med Assoc. 2012, 307, 713–721. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, R.T.; Copes, R.; Kwong, J.C.; Villeneuve, P.; Goldberg, M.S.; Brook, R.D.; Van Donkelaar, A.; Jerrett, M.; Martin, R.V.; et al. Ambient Fine Particulate Matter and Mortality among Survivors of Myocardial Infarction: Population-Based Cohort Study. Environ. Health Perspect. 2016, 124, 1421–1428. [Google Scholar] [CrossRef]
- Tonne, C.; Wilkinson, P. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur. Hear. J. 2013, 34, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Dresen, W.F.; Ferguson, J.D. Ventricular Arrhythmias. Cardiol. Clin. 2018, 36, 129–139. [Google Scholar] [CrossRef]
- Song, X.; Liu, Y.; Hu, Y.; Zhao, X.; Tian, J.; Ding, G.; Wang, S. Short-Term Exposure to Air Pollution and Cardiac Arrhythmia: A Meta-Analysis and Systematic Review. Int. J. Environ. Res. Public Health 2016, 13, 642. [Google Scholar] [CrossRef] [Green Version]
- Folino, F.; Buja, G.; Zanotto, G.; Marras, E.; Allocca, G.; Vaccari, D.; Gasparini, G.; Bertaglia, E.; Zoppo, F.; Calzolari, V.; et al. Association between air pollution and ventricular arrhythmias in high-risk patients (ARIA study): A multicentre longitudinal study. Lancet Planet. Health 2017, 1, e58–e64. [Google Scholar] [CrossRef]
- Kim, I.-S.; Yang, P.-S.; Lee, J.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. Long-term exposure of fine particulate matter air pollution and incident atrial fibrillation in the general population: A nationwide cohort study. Int. J. Cardiol. 2019, 283, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, J.; Hong, Y.S.; Chang, Y.; Ryu, S.; Park, J.; Cho, J.; Guallar, E.; Shin, H.C.; Zhao, D. Long-Term Particulate Matter Exposure and Incidence of Arrhythmias: A Cohort Study. J. Am. Hear. Assoc. 2020, 9, e016885. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, T.; Korantzopoulos, P.; Zhang, Z.; Zhao, J.; Li, G. Association between air pollution and development of atrial fibrillation: A meta-analysis of observational studies. Heart Lung. 2016, 45, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020, 17, 656–672. [Google Scholar] [CrossRef]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Mills, I.C.; Atkinson, R.W.; Kang, S.; Walton, H.; Anderson, H.R. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 2015, 5, e006946. [Google Scholar] [CrossRef]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio- respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Krewski, D.; Jerrett, M.; Burnett, R.T.; Ma, R.; Hughes, E.; Shi, Y.; Turner, M.C.; Pope, C.A., 3rd; Thurston, G.; Calle, E.E.; et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009, 5–114, discussion 115–136. [Google Scholar]
- Crouse, D.L.; Peters, P.A.; Van Donkelaar, A.; Goldberg, M.S.; Villeneuve, P.J.; Brion, O.; Khan, S.; Atari, D.O.; Jerrett, M.; Pope, C.A.; et al. Risk of Nonaccidental and Cardiovascular Mortality in Relation to Long-term Exposure to Low Concentrations of Fine Particulate Matter: A Canadian National-Level Cohort Study. Environ. Health Perspect. 2012, 120, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, G.; Zhao, D.; Xie, X.; Wei, Z.; Wang, W.; Wang, M.; Li, G.; Liu, W.; Sun, J.; et al. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart 2015, 101, 257–263. [Google Scholar] [CrossRef]
- Hart, J.E.; Chiuve, S.E.; Laden, F.; Albert, C.M. Roadway proximity and risk of sudden cardiac death in women. Circulation 2014, 130, 1474–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tian, Y.; Song, J.; Cao, Y.; Xiang, X.; Huang, C.; Li, M.; Hu, Y. Effect of Ambient Air Pollution on Hospitalization for Heart Failure in 26 of China’s Largest Cities. Am. J. Cardiol. 2018, 121, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, B.; Ke, W.; Feng, B.; Lin, H.; Xiao, J.; Zeng, W.; Li, X.; Tao, J.; Yang, Z.; et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension. Hypertension 2016, 68, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.-Y.; Qian, Z.; Howard, S.W.; Vaughn, M.G.; Fan, S.-J.; Liu, K.-K.; Dong, G.-H. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut. 2018, 235, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhang, B.; Zhao, X.; Ruan, Y.; Lian, H.; Fan, Z. Effect of exposure to PM2.5 on blood pressure. J. Hypertens. 2014, 32, 2130–2140, discussion 2141. [Google Scholar] [CrossRef]
- Thillaiappan, N.B.; Chakraborty, P.; Hasan, G.; Taylor, C.W. IP3 receptors and Ca2+ entry. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1866, 1092–1100. [Google Scholar] [CrossRef]
- Lin, H.; Guo, Y.; Zheng, Y.; Di, Q.; Liu, T.; Xiao, J.; Li, X.; Zeng, W.; Cummings-Vaughn, L.A.; Howard, S.W.; et al. Long-Term Effects of Ambient PM 2.5 on Hypertension and Blood Pressure and Attributable Risk Among Older Chinese Adults. Hypertension 2017, 69, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Guo, C.; Lau, A.K.; Chan, T.-C.; Chuang, Y.C.; Lin, C.; Jiang, W.K.; Yeoh, E.-K.; Tam, T.; Woo, K.S.; et al. Long-Term Exposure to Fine Particulate Matter, Blood Pressure, and Incident Hypertension in Taiwanese Adults. Environ. Health Perspect. 2018, 126, 017008. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, Z.; Fang, Y. Spatial analysis of the effects of PM2.5 on hypertension among the middle-aged and elderly people in China. Int. J. Environ. Health Res. 2019, 31, 729–740. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, M.; Liang, Q.; Wang, F.; Lin, L.; Li, T.; Duan, J.; Sun, Z. The relationship between long-term exposure to PM2.5 and hypertension in women: A meta-analysis. Ecotoxicol. Environ. Saf. 2020, 208, 111492. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, J.; Ning, R.; Du, Z.; Liu, J.; Batibawa, J.W.; Duan, J.; Sun, Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part. Fibre Toxicol. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Camilli, M.; Russo, M.; Termite, C.; La Vecchia, G.; Iannaccone, G.; Rinaldi, R.; Gurgoglione, F.; Del Buono, M.G.; Sanna, T.; et al. Air Pollution and Coronary Plaque Vulnerability and Instability. JACC: Cardiovasc. Imaging 2021, 15, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, H.; Ge, P.; Hu, T.; Zhang, Y.; Zhang, X.; Xu, B.; Wang, B.; Xie, J. PM2.5 promotes plaque vulnerability at different stages of atherosclerosis and the formation of foam cells via TLR4/MyD88/NFκB pathway. Ecotoxicol. Environ. Saf. 2019, 176, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhu, P.; Liu, Y.; Zhu, H.; Geng, J.; Wang, B.; Yuan, G.; Peng, Y.; Xu, B. PM2.5 induces endothelial dysfunction via activating NLRP3 inflammasome. Environ. Toxicol. 2021, 36, 1886–1893. [Google Scholar] [CrossRef]
- Krishnan, R.M.; Adar, S.D.; Szpiro, A.A.; Jorgensen, N.W.; Van Hee, V.C.; Barr, R.G.; O’Neill, M.S.; Herrington, D.M.; Polak, J.F.; Kaufman, J.D. Vascular Responses to Long- and Short-Term Exposure to Fine Particulate Matter. J. Am. Coll. Cardiol. 2012, 60, 2158–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloog, I. Fine particulate matter (PM2.5) association with peripheral artery disease admissions in northeastern United States. Int. J. Environ. Health Res. 2016, 26, 572–577. [Google Scholar] [CrossRef]
- Zhang, S.; Wolf, K.; Breitner, S.; Kronenberg, F.; Stafoggia, M.; Peters, A.; Schneider, A. Long-term effects of air pollution on ankle-brachial index. Environ. Int. 2018, 118, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Roux, A.V.D.; Gassett, A.J.; Jacobs, D.R.; et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016, 388, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Provost, E.B.; Madhloum, N.; Panis, L.I.; De Boever, P.; Nawrot, T.S. Carotid Intima-Media Thickness, a Marker of Subclinical Atherosclerosis, and Particulate Air Pollution Exposure: The Meta-Analytical Evidence. PLoS ONE 2015, 10, e0127014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chan, T.-C.; Guo, C.; Chang, L.-Y.; Lin, C.; Chuang, Y.C.; Jiang, W.K.; Ho, K.F.; Tam, T.; Woo, K.S.; et al. Long-term exposure to ambient particulate matter (PM2.5) is associated with platelet counts in adults. Environ. Pollut. 2018, 240, 432–439. [Google Scholar] [CrossRef]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part. Fibre Toxicol. 2018, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mengoli, C.; Cruciani, M.; Bonfanti, C.; Mannucci, P.M. Association between particulate air pollution and venous thromboembolism: A systematic literature review. Eur. J. Intern. Med. 2015, 27, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Fongsodsri, K.; Chamnanchanunt, S.; Desakorn, V.; Thanachartwet, V.; Sahassananda, D.; Rojnuckarin, P.; Umemura, T. Particulate Matter 2.5 and Hematological Disorders From Dust to Diseases: A Systematic Review of Available Evidence. Front. Med. 2021, 8, 692008. [Google Scholar] [CrossRef]
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous thromboembolism. Lancet 2021, 398, 64–77. [Google Scholar] [CrossRef]
- Kloog, I.; Zanobetti, A.; Nordio, F.; Coull, B.A.; Baccarelli, A.A.; Schwartz, J. Effects of airborne fine particles (PM2.5 ) on deep vein thrombosis admissions in the northeastern United States. J. Thromb. Haemost. 2015, 13, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet Health. 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Hadley, M.B.; Baumgartner, J.; Vedanthan, R. Developing a Clinical Approach to Air Pollution and Cardiovascular Health. Circulation 2018, 137, 725–742. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Mitigation of air pollution by greenness: A narrative review. Eur. J. Intern. Med. 2018, 55, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Balakrishnan, K.; Guttikunda, S.; Roychowdhury, A.; Smith, K.R. India Leads the Way: A Health-Centered Strategy for Air Pollution. Environ. Health Perspect. 2016, 124, A116–A117. [Google Scholar] [CrossRef] [Green Version]
- Ezzati, M.; Baumgartner, J.C. Household energy and health: Where next for research and practice? Lancet 2016, 389, 130–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Kaufman, J. Cardiovascular Disease and Air Pollutants: Evaluating and Improving Epidemiological Data Implicating Traffic Exposure. Inhal. Toxicol. 2007, 19, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, A.; Chen, H.; Zhao, Z.; Cai, J.; Wang, C.; Yang, C.; Li, H.; Xu, X.; Ha, S.; et al. Cardiopulmonary Benefits of Reducing Indoor Particles of Outdoor Origin. J. Am. Coll. Cardiol. 2015, 65, 2279–2287. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Dominici, F. Adverse Health Effects of Particulate Air Pollution. Epidemiology 2009, 20, 682–686. [Google Scholar] [CrossRef] [Green Version]
- Langrish, J.P.; Mills, N.L.; Chan, J.K.; Leseman, D.L.; Aitken, R.J.; Fokkens, P.H.; Cassee, F.R.; Li, J.; Donaldson, K.; Newby, D.E.; et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part. Fibre Toxicol. 2009, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langrish, J.P.; Li, X.; Wang, S.; Lee, M.M.Y.; Barnes, G.D.; Miller, M.R.; Cassee, F.R.; Boon, N.A.; Donaldson, K.; Li, J.; et al. Reducing Personal Exposure to Particulate Air Pollution Improves Cardiovascular Health in Patients with Coronary Heart Disease. Environ. Health Perspect. 2012, 120, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Lin, Z.; Chen, R.; Wang, C.; Yang, C.; Cai, J.; Lin, J.; Xu, X.; Ross, J.A.; Zhao, Z.; et al. Cardiovascular Benefits of Wearing Particulate-Filtering Respirators: A Randomized Crossover Trial. Environ. Health Perspect. 2017, 125, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Shin, T.H.; Manavalan, B.; Lee, D.Y.; Basith, S.; Seo, C.; Paik, M.J.; Kim, S.W.; Seo, H.; Lee, J.Y.; Kim, J.Y.; et al. Silica-coated magnetic-nanoparticle-induced cytotoxicity is reduced in microglia by glutathione and citrate identified using integrated omics. Part. Fibre Toxicol. 2021, 18, 42. [Google Scholar] [CrossRef]
- Romieu, I.; Castro-Giner, F.; Künzli, N.; Sunyer, J. Air pollution, oxidative stress and dietary supplementation: A review. Eur. Respir. J. 2008, 31, 179–197. [Google Scholar] [CrossRef]
- Tong, H.; Rappold, A.; Diaz-Sanchez, D.; Steck, S.E.; Berntsen, J.; Cascio, W.E.; Devlin, R.B.; Samet, J.M. Omega-3 Fatty Acid Supplementation Appears to Attenuate Particulate Air Pollution–Induced Cardiac Effects and Lipid Changes in Healthy Middle-Aged Adults. Environ. Health Perspect. 2012, 120, 952–957. [Google Scholar] [CrossRef] [Green Version]
- McCracken, J.P.; Smith, K.R.; Diaz, A.; Mittleman, M.; Schwartz, J. Chimney Stove Intervention to Reduce Long-term Wood Smoke Exposure Lowers Blood Pressure among Guatemalan Women. Environ. Health Perspect. 2007, 115, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.; Smith, K.R.; Stone, P.; Díaz, A.; Arana, B.; Schwartz, J. Intervention to Lower Household Wood Smoke Exposure in Guatemala Reduces ST-Segment Depression on Electrocardiograms. Environ. Health Perspect. 2011, 119, 1562–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bräuner, E.V.; Forchhammer, L.; Møller, P.; Barregard, L.; Gunnarsen, L.; Afshari, A.; Wåhlin, P.; Glasius, M.; Dragsted, L.O.; Basu, S.; et al. Indoor Particles Affect Vascular Function in the Aged. Am. J. Respir. Crit. Care Med. 2008, 177, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, C.; Salvi, S.; Wong, G.; Chung, K.F. Personal strategies to minimise effects of air pollution on respiratory health: Advice for providers, patients and the public. Eur. Respir. J. 2020, 55, 1902056. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs; Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- These Five Cities are Taking Aim at Air Pollution. Available online: https://www.unep.org/news-and-stories/story/these-five-cities-are-taking-aim-air-pollution (accessed on 20 September 2021).
- Niu, J.; Liberda, E.N.; Qu, S.; Guo, X.; Li, X.; Zhang, J.; Meng, J.; Yan, B.; Li, N.; Zhong, M.; et al. The Role of Metal Components in the Cardiovascular Effects of PM2.5. PLoS ONE 2013, 8, e83782. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Stafoggia, M.; Cesaroni, G.; Andersen, Z.J.; Beelen, R.; Galassi, C.; Hennig, F.; Migliore, E.; Penell, J.; Ricceri, F.; et al. Long-term Exposure to Particulate Matter Constituents and the Incidence of Coronary Events in 11 European Cohorts. Epidemiology 2015, 26, 565–574. [Google Scholar] [CrossRef] [Green Version]
| Study | Study Model, Pollutant and Year Published | Main Findings |
|---|---|---|
| Aung et al. [73] | In vitro model (human aortic endothelial cells (HAEC) exposed to both fine (PM1.8) and ultrafine particles (UFPs–PM0.1)), 2011 | Gene responses involved in xenobiotic and oxidoreductase activities, inflammatory pathways, and transcription factors were affected. |
| Hu et al. [74] | In vitro model (HUVECs exposed to PM2.5), 2016 | Decreased cell viability, increased LDH activity, increased ROS and MDA productions, inhibition of SOD activity, and increased levels of proinflammatory cytokines, cell adhesion molecules, and tissue factor. Upregulation of IL-6 dependent JAK1/STAT3 pathway. |
| Montiel-Dávalos et al. [75] | In vitro model (human umbilical vein endothelial cells (HUVEC) exposed to PM2.5), 2010 | Increased production of reactive oxygen species (ROS) and nitric oxide (NO), and increased translocation of nuclear factor-kappa B (NF-κB) leading to apoptosis. |
| Sivakumar et al. [76] | In vitro model (H9c2 cardiomyocytes exposed to 100 µg/mL PM2.5), 2021 | Augmented mitochondrial dysfunction and inactivation of PI3K/Akt signaling pathway (mitotoxicity). |
| Sivakumar et al. [77] | In vivo model (rat model of myocardial infarction (MI) exposed to PM2.5), 2022 | Lowers mitochondrial endurance during cardiac recovery. |
| Sun et al. [78] | In vivo model (rats exposed to PM2.5 or filtered air for 10 weeks), 2008 | Increase in mitochondrial superoxide production mediated by activation of Rho/ROCK pathway. |
| Wittkopp et al. [79] | Cohort study model (Elderly adults > 65 years with coronary artery disease—Measured hourly PM2.5), 2013 | Toxic effects of air pollutants depend on the mitochondrial haplotype. Haplogroup H are more sensitive to air pollutants than haplogroup U. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basith, S.; Manavalan, B.; Shin, T.H.; Park, C.B.; Lee, W.-S.; Kim, J.; Lee, G. The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials 2022, 12, 2656. https://doi.org/10.3390/nano12152656
Basith S, Manavalan B, Shin TH, Park CB, Lee W-S, Kim J, Lee G. The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials. 2022; 12(15):2656. https://doi.org/10.3390/nano12152656
Chicago/Turabian StyleBasith, Shaherin, Balachandran Manavalan, Tae Hwan Shin, Chan Bae Park, Wang-Soo Lee, Jaetaek Kim, and Gwang Lee. 2022. "The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer" Nanomaterials 12, no. 15: 2656. https://doi.org/10.3390/nano12152656
APA StyleBasith, S., Manavalan, B., Shin, T. H., Park, C. B., Lee, W.-S., Kim, J., & Lee, G. (2022). The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials, 12(15), 2656. https://doi.org/10.3390/nano12152656







