One-Pot Preparation of HTPB/nNi and Its Catalyst for AP
Abstract
:1. Introduction
2. Experiment
2.1. Reagents and Instruments
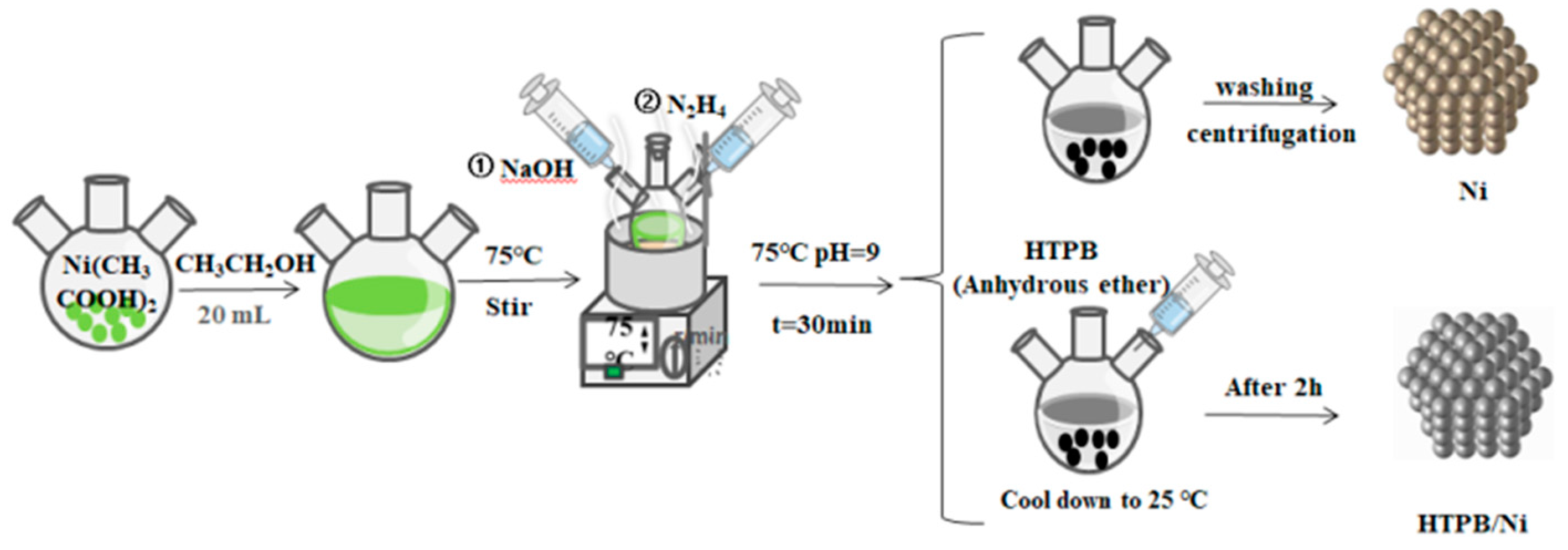
2.2. Preparation of nNi and HTPB/nNi
2.3. The Catalytic Effect of HTPB/nNi on Thermal Decomposition of AP
3. Results and Discussion
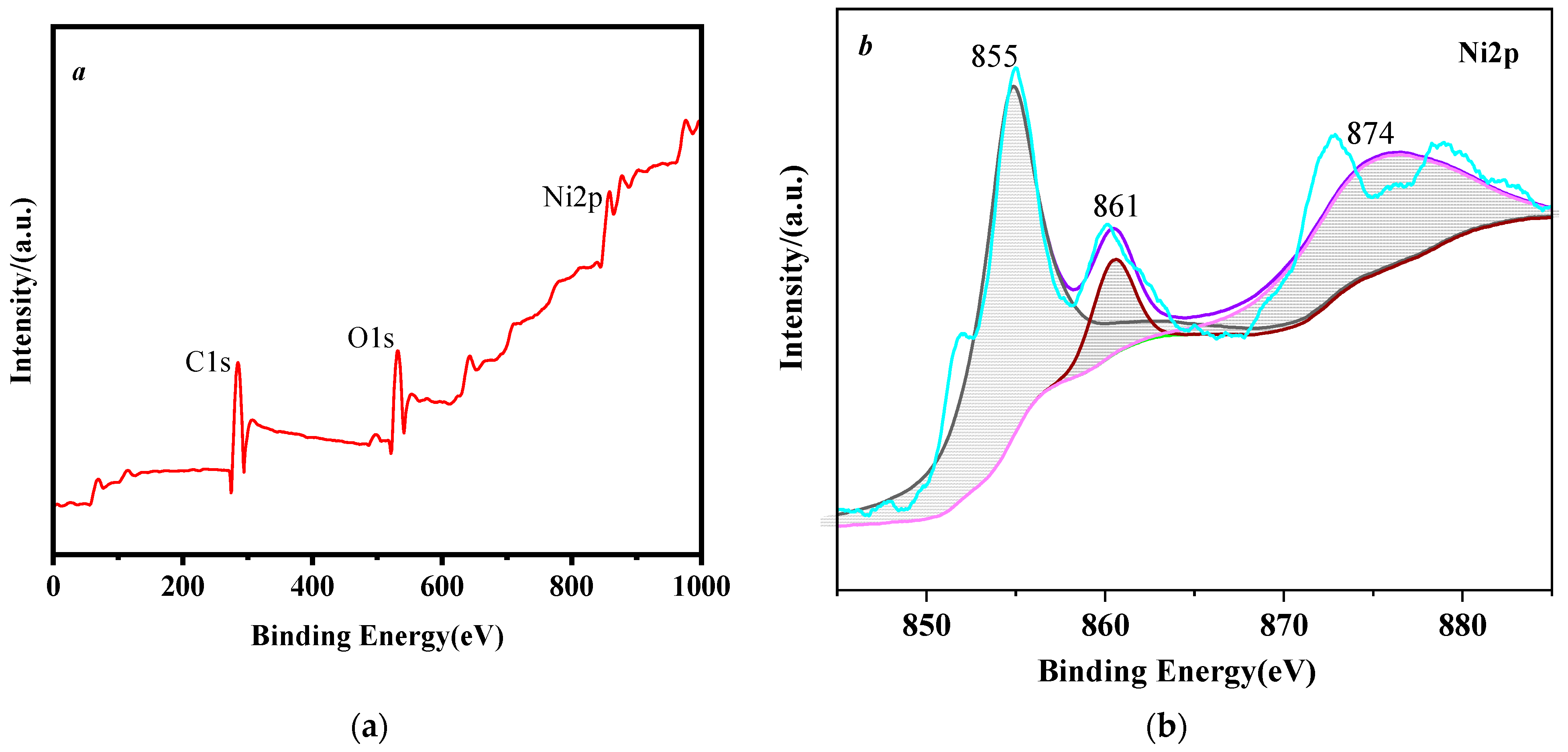
3.1. Identification of Nickel Powder
3.2. Characterization of X-ray Powder Diffraction (XRD)
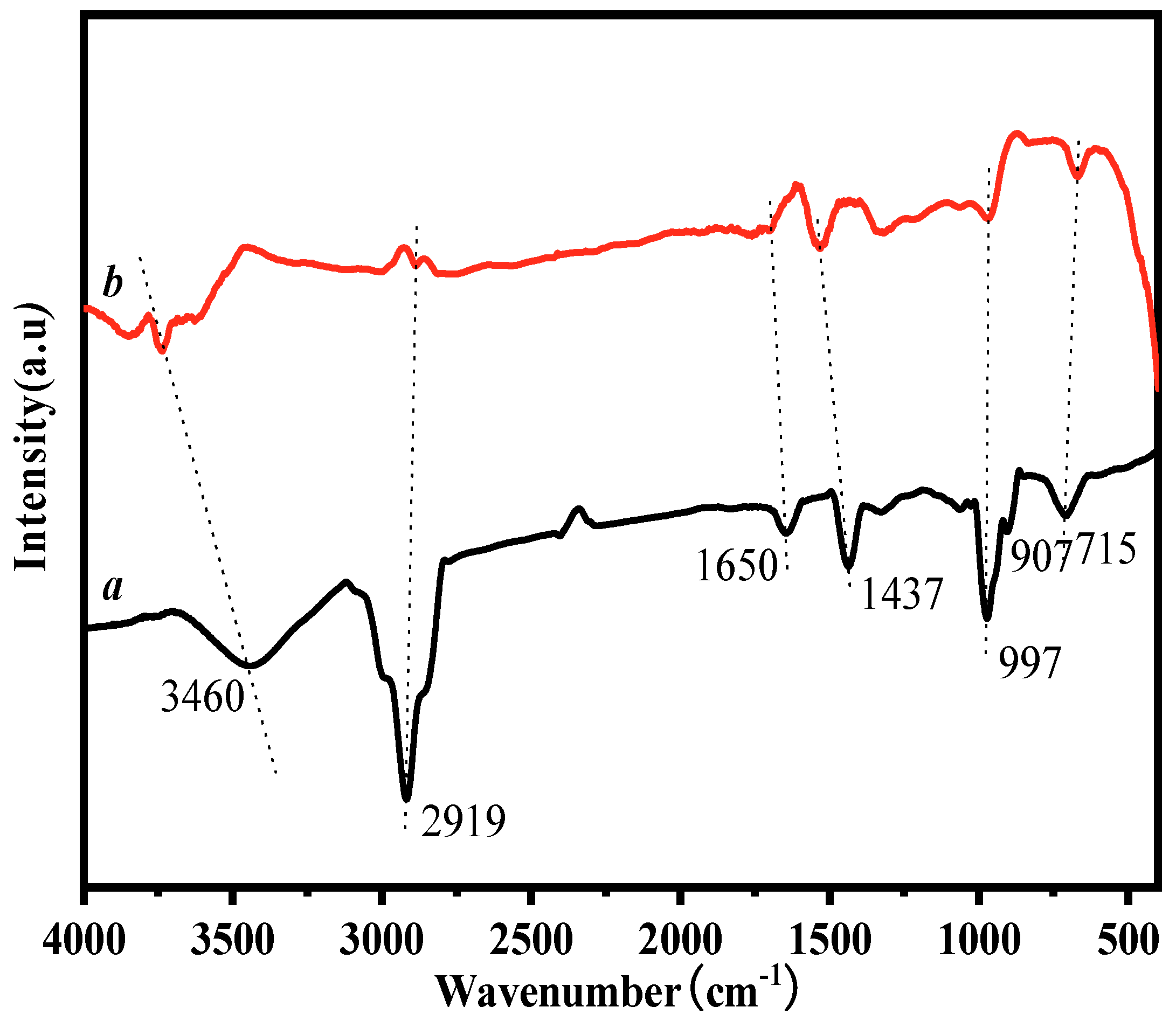
3.3. FT-IR Characterization
3.4. X-ray Diffraction Characterization
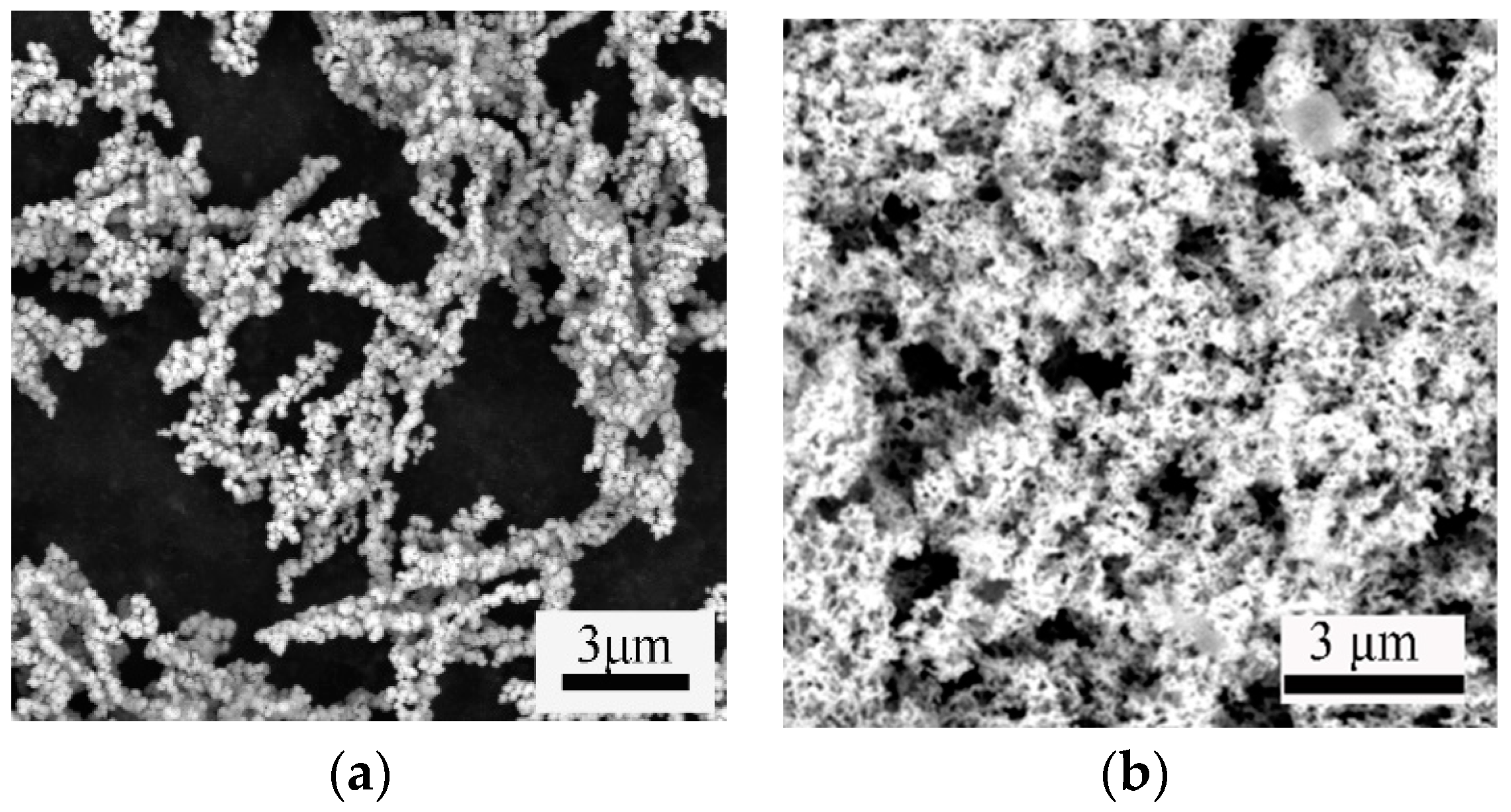
3.5. Morphology Characterization
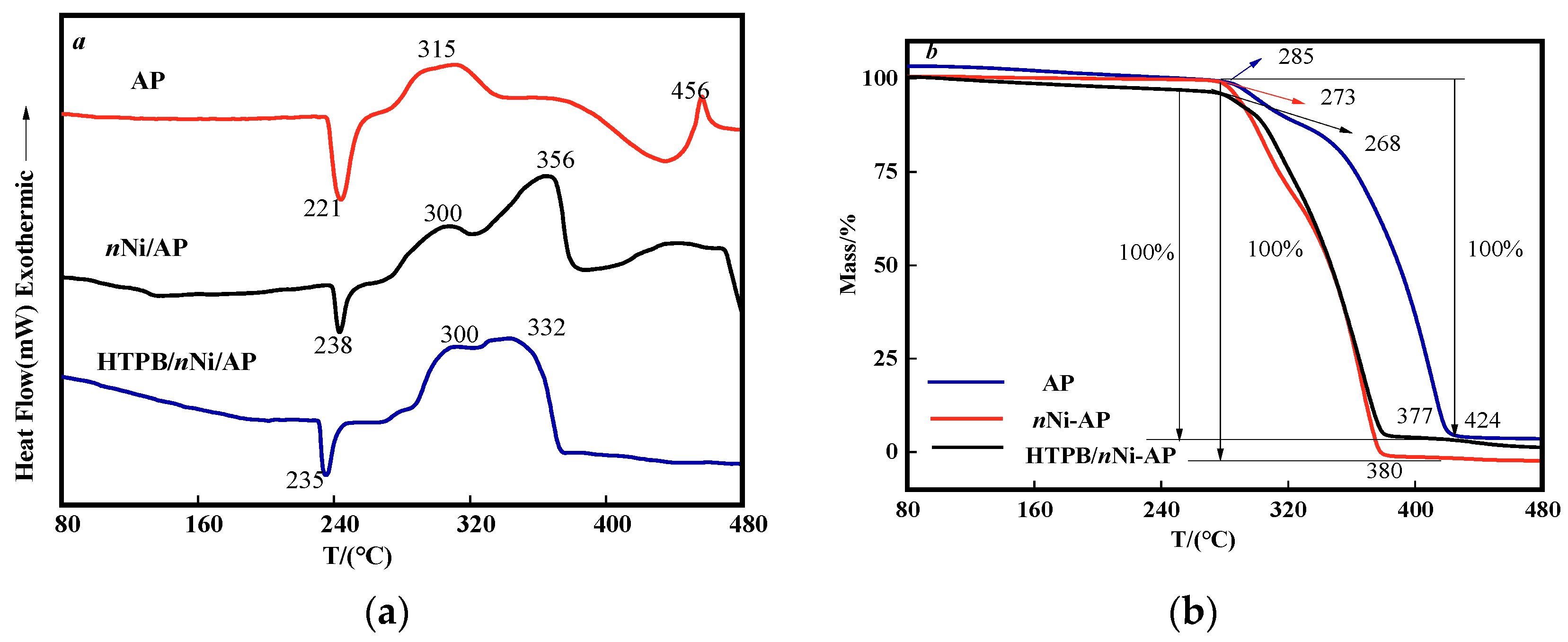
3.6. Thermal Performance Analysis of nNi and HTPB/nNi
3.7. Analysis of HTPB/nNi on the Thermal Decomposition Performance of AP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.Y.; Xu, C.; Chen, C.; Zhao, G.Z.; Liu, Y.Q. Flower-like hierarchical nickel microstructures: Facile synthesis, growth mechanism, and their magnetic properties. Mater. Res. Bull. 2012, 47, 1839–1844. [Google Scholar] [CrossRef]
- Agrawal, M.; Rana, B.; Barman, A. Magnetization Reversal in Chains and Clusters of Exchange-Coupled Nickel Nanoparticles. J. Phys. Chem. C 2010, 114, 11115–11118. [Google Scholar] [CrossRef]
- Yang, W.P.; Li, J.R.; Liu, S.Z.; Shi, Z.X.; Zhao, J.Q.; Wang, X.G. Orientation dependence of transverse tensile properties of nickel-based third generation single crystal superalloy DD9 from 760 to 1100 degrees C. Trans. Nonferrous Met. Soc. China 2019, 29, 558–568. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.; Chen, G.; Qin, H.Y.; Wang, C.J. Grain size based low cycle fatigue life prediction model for nickel-based superalloy. Trans. Nonferrous Met. Soc. China 2018, 28, 2102–2106. [Google Scholar] [CrossRef]
- Voisin, C.; Del Fatti, N.; Christofilos, D.; Vallee, F. Ultrafast electron dynamics and optical nonlinearities in metal nanoparticles. J. Phys. Chem. Part B 2001, 105, 2264–2280. [Google Scholar] [CrossRef]
- Qiu, X.P. Synthesis and characterization of magnetic nanoparticles. Chin. Chem. Engl. Version 2000, 18, 834–837. [Google Scholar]
- Flauraud, V.M.; Mastrangeli, M.; Bernasconi, G.D.; Butet, J.; Alexander, D.T.L.; Shahrabi, E.; Martin, O.J.F.; Brugger, J. Nanoscale topographical control of capillary assembly of nanoparticles. Nat. Nanotechnol. 2017, 12, 73–80. [Google Scholar] [CrossRef]
- Ma, L.; Chen, J.B.; Zheng, W.; Zhang, C.R. Effects of nickel powder on the combustion performance of RDX-CMDB propellant. Initiat. Pyrotech. 2015, 4, 47–49. [Google Scholar]
- Jiang, Z.; Li, F.S.; Li, K.; Zhang, G.C.; Wang, H.; Ma, X.M. Research on the ignition and combustion properties of composite propellant containing nano metal powders. J. Solid Rocket. Technol. 2004, 27, 117–120. [Google Scholar]
- Jiang, Z.; Li, F.S.; Zhao, F.Q.; Liu, Z.R.; Yin, C.M.; Luo, Y.; Li, S.W. Research on the combustion properties of propellants with low content of nano metal powders. Propellants Explos. Pyrotech. 2006, 31, 139–147. [Google Scholar]
- Sezer, I. Effect of nano materials additives on fuel properties and combustion characteristics. J. Fac. Eng. Archit. Gazi Univ. 2019, 34, 115–135. [Google Scholar]
- Yang, Z.X.; Zhong, W.A.; Du, J.Y. An environment-benign solvothermal method for the synthesis of flower-like hierarchical nickel and zinc compounds and their transformation to nanoporous NiO and ZnO. Crystengcomm 2011, 13, 1831–1837. [Google Scholar] [CrossRef]
- Bai, L.Y.; Peng, Y.; Fang, L.L.; Tang, J.L. RF plasma synthesis of nickel nanopowders via hydrogen reduction of nickel hydroxide/carbonate. J. Alloys Compd. 2009, 481, 563–567. [Google Scholar] [CrossRef]
- Salvetr, P.; Kubatik, T.F.; Novak, P. Preparation of Ni-Ti shape memory alloy by spark plasma sintering method. Manuf. Technol. 2016, 16, 45–46. [Google Scholar] [CrossRef]
- Li, L.Y.; Zhang, Z.Z.; Zhao, F.X.; Shou, F.L. Preparation of ultrafine nickel powder by high-energy ball milling. Surf. Technol. 2012, 41, 74–76. [Google Scholar]
- Zhang, T.S.; Hong, B.Z.; Ya, B.L.; Qing, F.; Wei, Y.W. Hollow flower-like nickel particles as the promoter of ammonium perchlorate-based solid propellant. Appl. Surf. Sci. 2021, 552, 457–460. [Google Scholar] [CrossRef]
- Wu, X.G.; Yan, Q.L.; Guo, X.Q.; Xiao, F.L.; Xiao, J.W. Combustion efficiency and pyrochemical properties of micron-sized metal particles as the components of modified double-base propellant. Acta Astronaut. 2011, 68, 1098–1112. [Google Scholar] [CrossRef]
- Chang, C.P.; Liu, C.D.; Huang, S.W.; Chao, D.Y. Synthesis and characterization of high dielectric constant polyaniline/polyurethane blends. Synth. Met. 2004, 142, 275–281. [Google Scholar] [CrossRef]
- Berta, M.; Lindsay, C.P.; Camino, G.G. Effect of chemical structure on combustion and thermal behaviour of polyurethane elastomer layered silicate nanocomposites. Polym. Degrad. Stab. 2006, 91, 1179–1191. [Google Scholar] [CrossRef]
- Cao, X.L.; Li, W.D.; Macosko, C.T. Polyurethane/clay nanocomposites foams: Processing, structure and properties. Polymer 2005, 46, 775–783. [Google Scholar] [CrossRef]
- Feng, M.Y.; Luo, H.P.; Kai, H. A molecular dynamics study on oxidation of aluminum hydride/hydroxyl-terminated polybutadiene (HTPB) solid fuel. Proc. Combust. Inst. 2021, 38, 4469–4476. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; De, L.C.; Reina, L.T.; Lerner, M.I.; Rodkevich, N.G. Effects of HTPB-coating on nano-sized aluminum in solid rocket propellant performance. Sci. Technol. Energ. Mater. 2015, 76, 105–109. [Google Scholar]
- Ju, Z.Y.; An, J.L.; Guo, C.Y.; Li, T.R.; Jia, Z.Y.; Wu, R.F. The oxidation reaction and sensitivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene. J. Energ. Mater. 2021, 39, 299–312. [Google Scholar] [CrossRef]
- Hao, J.; Ding, A.; Huang, J.H.; Zhang, Y.S.; Qian, K.M.; Ji, S. Cladding assembly and characterization of HTPB-TDI coated high activity nano-aluminum powder. Chin. J. Rare Met. 2018, 42, 168–174. [Google Scholar]
- Zha, M.X.; Ma, Z.Y.; Xu, J.; Qin, H.; Xu, S.Y.; Zhao, F.Q. Preparation of aluminum nanoparticles by liquid chemical method and their characterizati. Acta Armamentarii 2014, 35, 1575–1580. [Google Scholar]
- Liu, X.D.; Yang, Y.; Li, F.S. Fabricating nanometer Cu/Al composite powder by electroless copper plating. J. Funct. Mater. 2006, 37, 1335–1337. [Google Scholar]
- Li, T.R.; Guo, C.Y.; Bao, S.X.; Zhao, Y.Y.; Du, Z.G.; Wu, R.F. Preparation of HTPB/Cu/μAl and Its effect on the thermal decomposition properties of AP. Chin. J. Energ. Mater. 2021, 29, 897–903. [Google Scholar]
- Xue, F.; Qiang, C.M.; Yu, M.; Li, S.M.; Liu, J.H. Preparation and characterization of nano-NiO/hollow glass sphere composite particles. Acta Mater. Compos. Sin. 2008, 25, 100–104. [Google Scholar]
- Zhang, S.W.; Yao, Y.X.; Gao, H.F.; Ren, L.L. Application of X-ray Photoelectron Spectroscopy in Material Surface Analysis. Metrol. Sci. Technol. 2021, 6, 40–44. [Google Scholar]
- Wei, Z.Q.; Wu, Y.F.; Liu, L.G.; Feng, W.J.; Yang, H.; Zhang, C.R. Preparation and characterization of SiO2 encapsulated Ni nanoparticles. Trans. Mater. Heat Treat. 2013, 34, 28–31. [Google Scholar]
- Wang, Y.H.; Liu, L.L.; Wang, L.Y.; Ze, X. Thermal decomposition of HTPB/AP and HTPB/HMX mixtures with low content of oxidizer. J. Therm. Anal. Calorim. 2015, 119, 1673–1678. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, F.S.; Tan, L.H.; Yang, Y.; Yin, Q. Preparation of nanometer Ni and amorphous Ni-B alloys and their effects on the thermal decomposition characteristics of ammonium perchlorate. Acta Armamentarii 2004, 25, 428–430. [Google Scholar]
- Huo, J.D.; Yan, Z.Z.; Lu, Y.W.; Han, J.M.; Yang, L. Preparation of porous polyacrylate and its catalysis on thermal decomposition of ammonium perchlorate. Acta Armamentarii 2021, 42, 1215–1222. [Google Scholar]
- Dennis, C.B.; Brian, N.A. On the combustion of heterogeneous AP/HTPB composite propellants: A review. Fuel 2019, 254, 115646. [Google Scholar] [CrossRef]
- Han, Z.W.; Yu, C.X. Preparation of nano-cerium dioxide and its effect on the thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2014, 116, 273–278. [Google Scholar] [CrossRef]
- Xu, B.Q.; Wei, J.M.; Yu, Y.T.; Li, Y.; Li, J.L.; Zhu, Q.M. Size limit of support particles in an oxide-supported metal catalyst: Nanocomposite Ni/ZrO2 for utilization of natural gas. J. Phys. Chem. Part B 2003, 107, 5203–5207. [Google Scholar] [CrossRef]
- Feng, H.T.; Mintz, K.J.; Augsten, R.A.; Jones, D.E.G. Thermal analysis of branched GAP. Thermochim. Acta 1998, 311, 105–111. [Google Scholar] [CrossRef]
- Zhou, T.T.; Cai, F.L.; Wu, B.D. Preparation of butterfly wing-shaped TiO2 and its catalytic effects on the thermal decomposition of ammonium perchlorate. Chin. J. Energ. Mater. 2020, 28, 1054–1060. [Google Scholar]
- Chinnam, A.K.; Petrutik, N.; Wang, K.C.; Shlomovich, A.; Shamis, O.; Tov, D.S. Effects of closo-icosahedral periodoborane salts on hypergolic reactions of 70% H2O2 with energetic ionic liquids. J. Mater. Chem. A 2018, 6, 19989–19997. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.X.; Wang, X.J.; Huang, Y.C. Preparation of nanoparticle nickel and its catalytic performance for thermal decomposition of ammonium perchlorate. Ind. Catal. 2008, 16, 71–73. [Google Scholar]



| Sample | Ni | O | C | Total Amount |
|---|---|---|---|---|
| nNi | 93.31 | 6.69 | — | 100.00 |
| HTPB/nNi | 93.48 | 5.93 | 0.59 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, S.; Li, T.; Guo, C.; Zhao, Y.; Zhang, H.; Wu, R.; Shi, H. One-Pot Preparation of HTPB/nNi and Its Catalyst for AP. Nanomaterials 2022, 12, 2669. https://doi.org/10.3390/nano12152669
Bao S, Li T, Guo C, Zhao Y, Zhang H, Wu R, Shi H. One-Pot Preparation of HTPB/nNi and Its Catalyst for AP. Nanomaterials. 2022; 12(15):2669. https://doi.org/10.3390/nano12152669
Chicago/Turabian StyleBao, Shuxia, Tingrun Li, Chunyu Guo, Yangyang Zhao, Huijuan Zhang, Ruifeng Wu, and Heping Shi. 2022. "One-Pot Preparation of HTPB/nNi and Its Catalyst for AP" Nanomaterials 12, no. 15: 2669. https://doi.org/10.3390/nano12152669






