Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy
Abstract
:1. Introduction
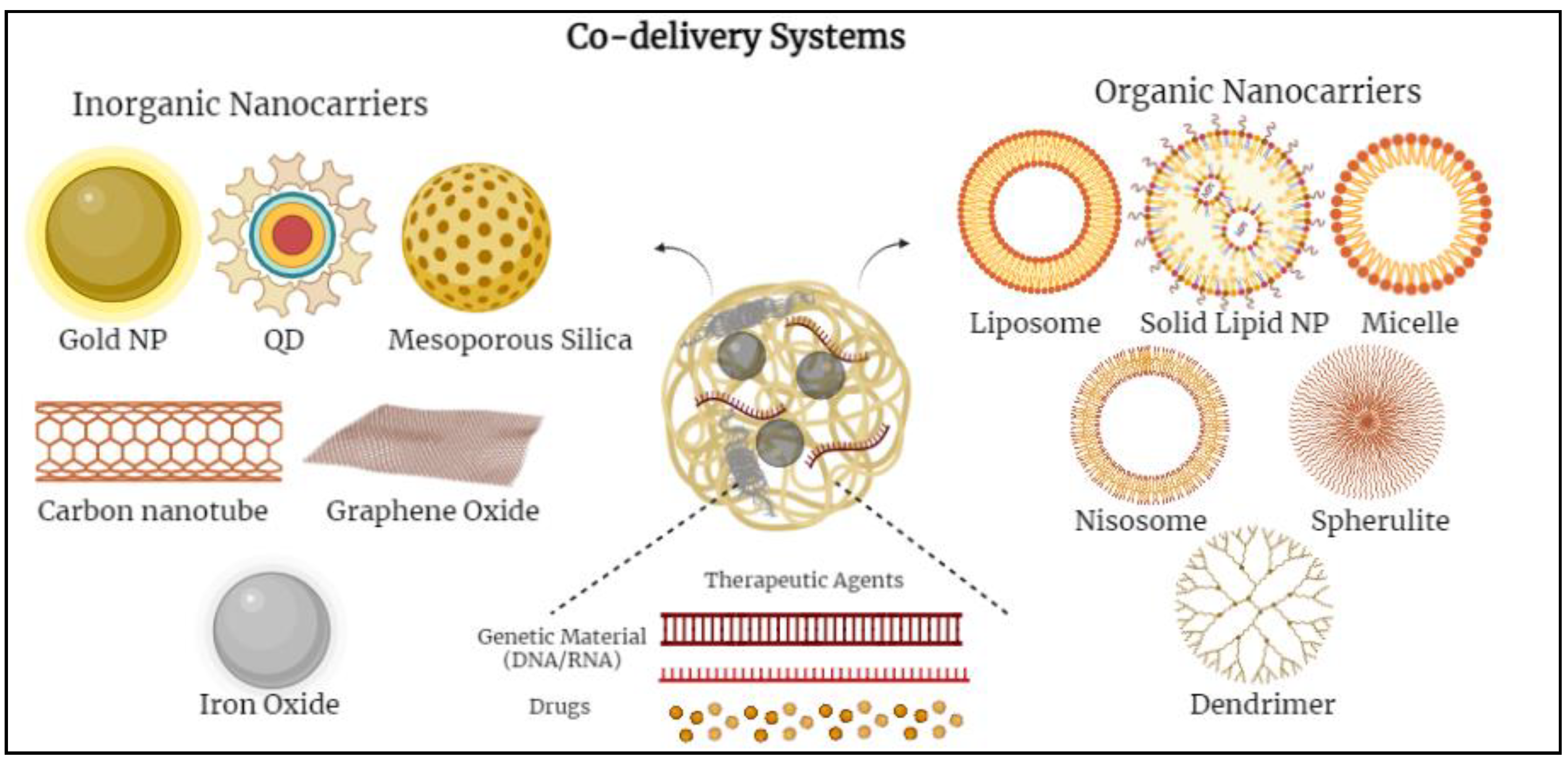
2. Nanocarriers Used in Co-Delivery Systems
2.1. Inorganic-Based Nanoparticles
2.1.1. Gold Nanoparticles (Au NPs)
2.1.2. Mesoporous Silica Nanoparticles (MSNs)
2.1.3. Copper Nanoparticles (Cu NPs)
2.2. Organic-Based Nanoparticles
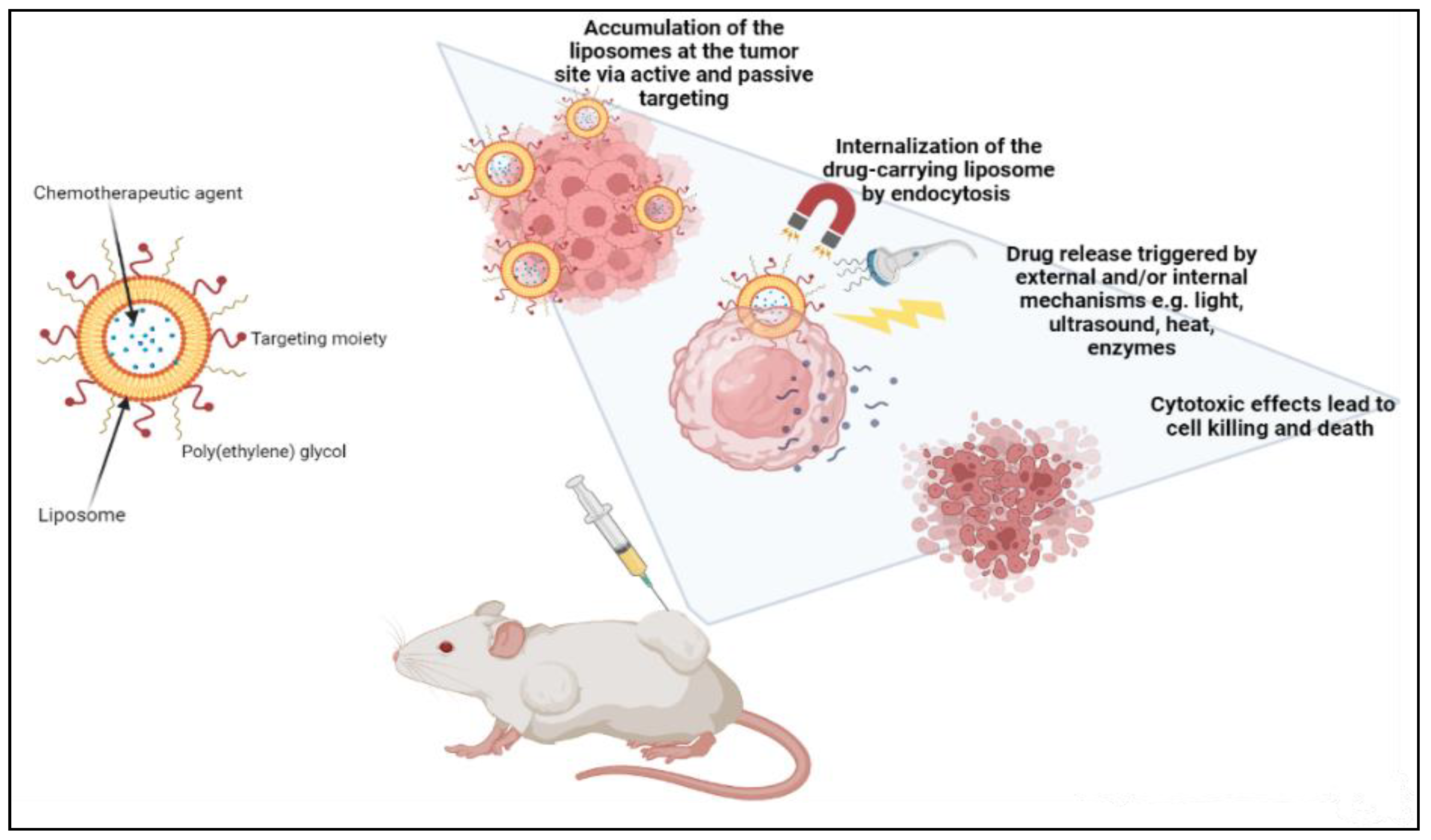
2.2.1. Liposomes
2.2.2. Lipid Nano-Emulsion (NE)
| Commercialized Formulation (Active Ingredient) | Nanocarrier Type | Indications | Company | Clinical Trial Phase | Reference |
|---|---|---|---|---|---|
| Onco-TCS (Vincristine) | Liposomes | Non-Hodgkin Lymphoma | INEX Pharmaceuticals | Clinical phase 1/2 | [78] |
| OSI-211 (Lurtotecan) | Liposomes | Lung cancer Recurrent ovarian cancer | OSI | Clinical phase 2 | [79] |
| LEP-ETU (Paclitaxel) | Liposomes | Ovarian, breast, and lung cancers | Neopharma | Clinical phase 1/2 | [78] |
| Auroshell | Gold-silica nanoshells | AuroLase therapy for cancer | Nanospectra Biosciences | Clinical phase 1 | [80] |
| Thermodox (Doxorubicin) | Liposomes | Hepatocellular carcinoma | Celsion | Clinical phase 3 | [78] |
| Aroplatin (Cisplatin analog) | Liposomes | Colorectal cancer | Antigenics, Inc. | Clinical phase 1/2 | [79] |
| Nektar-102 (PEGylated irinotecan) | Liposomes | Breast, colorectal cancer | Nektar therapeutics | Clinical phase 3 | [78] |
| NKTR-105 (PEG-Docetaxel) | Polymer-drug conjugate | Solid tumors | Nektar therapeutics | Clinical phase 1 | [80] |
| CYT-6091 Aurimmune (TNF-α) | TNF-α bound to colloidal gold nanoparticles | Head and neck cancer | Cytimmune Sciences | Clinical phase 2 | [78] |
| Paclical (Paclitaxel) | Polymeric micelles | Ovarian cancer | Oasmia Pharmaceutical AB | Clinical phase 3 | [79] |
| Lipoplatin (Cisplatin) | Liposomes | Pancreatic, head and neck, breast cancer | Regulon | Clinical phase 3 | [78] |
2.2.3. Polymeric Micelles
2.2.4. Cubosome Nanoparticles
2.2.5. Polymersomes
| Nanomaterial Type | Common Trade Name | Composition | Delivery | Indication | Approval Date | Company | Reference |
|---|---|---|---|---|---|---|---|
| Lipid-based nanoparticles | Doxil®/Caelyx® | PEGylated liposomal doxorubicin | Non-targeted delivery Immunoevasion | Metastatic ovarian, breast cancer, multiple myeloma, HIV-associated Kaposi’s sarcoma (KS) | FDA—1995 EMA—1996 | Orthobiotech/Schering-Plough Canada Inc. Janssen-Cilag, Europe | [97,98,99] |
| Lipodox® | FDA—2013 | Sun Pharmaceutical Industries Ltd. (SPIL) | [97,100] | ||||
| DepoCyt® | Liposomal cytarabine | Non-targeted delivery | Lymphomatous meningitis | FDA—1999 | Skye Pharma, Enzon | [77] | |
| DaunoXome® | Liposomal daunorubicin | Non-targeted delivery | HIV-associated Kaposi’s sarcoma (KS) | FDA—1996 | Galen Ltd., USA/Gilead Science, Inc., Ireland | [78,101] | |
| Onivyde® | PEGylated liposomal irinotecan | Non-targeted delivery Immunoevasion | Metastatic pancreatic cancer | FDA—2015 | Merrimack Pharmaceuticals Inc., Massachusetts, USA | [99,102] | |
| Lipid-based nanoparticles | Myocet® | Non-PEGylated liposomal doxorubicin | Non-targeted delivery | Breast cancer | EMA—2000 (Approved in Europe and Canada) | Enzon Pharmaceuticals for Cephalon in Europe Elan Pharmaceuticals/Sopherion Therapeutics in Canada | [103,104,105] |
| Mepact® | Liposomal mifamurtide | Non-targeted delivery | Osteogenic sarcoma | EMA—2009 (Approved in Europe) | Takeda France SAS | [101,106] | |
| Marqibo® | Liposomal vincristine sulfate | Non-targeted delivery Sustained Release | Acute lymphoblastic leukemia | FDA—2012 | Talon Therapeutic, Inc., California, USA | [102] | |
| Lipid-based nanoparticles | Lipusu® | Liposomal paclitaxel | Non-targeted delivery Sustained Release | Breast cancer, NSCLC, ovarian cancer | Approved in China—2006 | Luye Pharma Group | [107,108,109] |
| Vyxeos® | Liposomal daunorubicin and cytarabine | Combinatorial delivery | Acute myeloid leukemia | FDA—2017 EMA—2018 | Jazz Pharmaceutics, Inc. | [106,110] | |
| Polymer-based nanoparticle | Genexol-PM® | Paclitaxel micellar | Sustained Release | Breast, ovarian, gastric cancer, and NSCLC | Approved in South Korea—2007 | Samyang, Seongnam, South Korea | [111,112] |
| Eligard® | Leuprolide acetate and polymer | Non-targeted delivery | Prostate cancer | FDA—2002 | Tolmar Pharmaceuticals Inc. | [113,114] | |
| Protein- drug conjugate | Pazenir® | Paclitaxel | Non-targeted delivery | Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, NSCLC | EMA—2019 | Ratiopharm GmbH San Francisco, CA, USA | [107] |
| Oncaspar® | PEGylated L-asparaginase conjugate | Non-targeted delivery | Acute lymphocytic leukemia | FDA—1994 | Enzon Pharmaceuticals Inc. | [114,115,116] | |
| Protein nanoparticle | Abraxane® | Albumin-bound paclitaxel | Non-targeted delivery Sustained Release | Metastatic breast cancer Lung cancer, and NSCLC | FDA—2005 EMA—2008 FDA—2012 | Abraxis Bioscience, AstraZeneca | [117,118,119] |
| Nab-paclitaxel in combination with gemcitabine | Metastatic pancreatic adenocarcinoma | FDA—2013 | Celgene Pharmaceutical Co. Ltd. | ||||
| Inorganic nanoparticle | Hensify® | Hafnium oxide nanoparticles | Non-targeted delivery Radiation-activated | Locally advanced squamous cell carcinoma | EMA—2019 | Nanobiotix | [110] |
| Nano-therm | Iron oxide (Fe2O3) | Hyperthermia | Glioblastoma, prostate, and pancreatic cancer. | FDA—2010 EMA—2013 | Magforce | [102] |
3. Combination Treatment Strategies
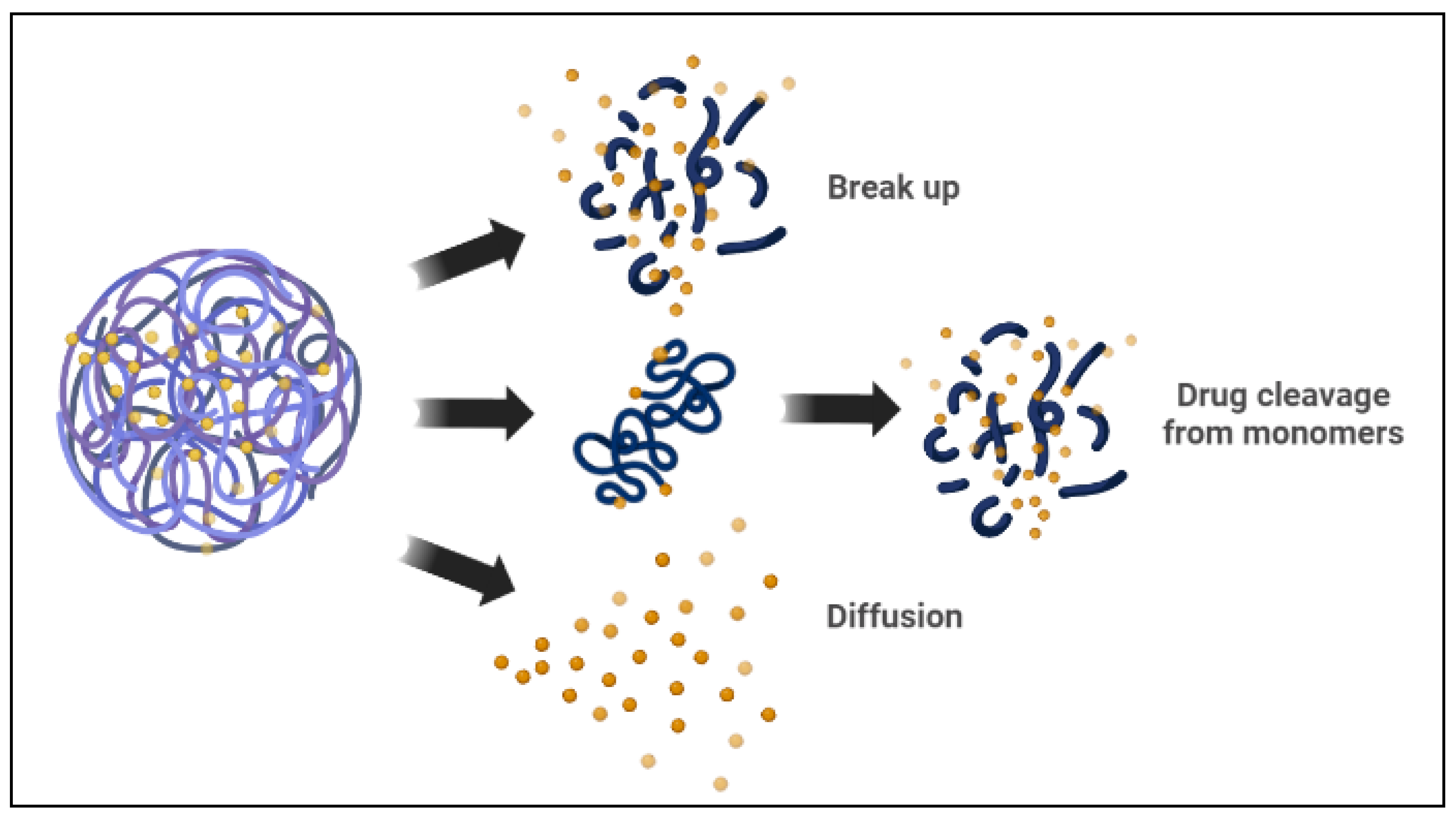
3.1. Co-Delivery of Chemotherapeutic Drugs
3.2. Co-Delivery of Chemotherapeutic Drugs and Genes
3.3. Co-Delivery of Multiple Genes
4. Limitations and Challenges
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Global Cancer Observatory—United Arab Emirates. March 2021. Available online: https://gco.iarc.fr/today/data/factsheets/populations/784-united-arab-emirates-fact-sheets.pdf (accessed on 5 March 2022).
- UAE Department of Health. Cancer—Chronic Diseases and Natural Disorders. 24 October 2018. Available online: https://u.ae/en/information-and-services/health-and-fitness/chronic-diseases-and-natural-disorders/cancer-#:~:text=In%20the%20UAE%2C%20approximately%204%2C500,18%20per%20cent%20by%202021 (accessed on 12 April 2022).
- Murphy, S.L.; Kochanek, K.D.; Xu, J.; Arias, E. Mortality in the United States, 2020; National Center for Health Statistics (U.S.): Hyattsville, MD, USA, 2021. [Google Scholar] [CrossRef]
- Chung, S.L.; Yee, M.S.-L.; Hii, L.-W.; Lim, W.-M.; Ho, M.Y.; Khiew, P.S.; Leong, C.-O. Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment. Nanomaterials 2021, 11, 2467. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Cong, H.L.; Hu, H.; Xu, F.-J. Rational design and latest advances of codelivery systems for cancer therapy. Mater. Today Bio 2020, 7, 100056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Kong, Y.Y.; Sun, J.H.; Huo, S.J.; Zhou, M.; Gui, Y.L.; Mu, X.; Chen, H.; Yu, S.Q.; Xu, Q. Co-delivery nanoparticles with characteristics of intracellular precision release drugs for overcoming multidrug resistance. Int. J. Nanomed. 2017, 12, 2081–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adolphe, M. (Ed.) Chemotherapy: Proceedings of the International Congress of Pharmacology, Paris, 1978—Advances in Pharmacology and Therapeutics, 1st ed.; Pergamon Press: Oxford, UK; New York, NY, USA, 1979; ISBN 978-0-08-023200-3. [Google Scholar]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Zhao, R.; Nie, G. Engineering the Assemblies of Biomaterial Nanocarriers for Delivery of Multiple Theranostic Agents with Enhanced Antitumor Efficacy. Adv. Mater. 2013, 25, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of Multidrug Resistance in Cancer by Multi-Functional Flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Sirisha, S. A Review on Delivery of Anti-Cancer Drugs by Smart Nanocarriers: Data Obtained from Past One Decade. Res. J. Pharm. Dos. Forms Technol. 2020, 12, 185–190. [Google Scholar] [CrossRef]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; de la Torre, L.G. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2021, 9, 1208–1237. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Dahmani, F.Z.; Qiao, J.; Ni, J.; Xiong, H.; Liu, T.; Zhou, J.; Yao, J. A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer. Acta Biomater. 2018, 75, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D.E.; Allinson, L.M.; Al Amri, W.S.; Poulter, J.A.; Pramanik, A.; Thorne, J.L.; Verghese, E.T.; Hughes, T.A. MiR-195 and Its Target SEMA6D Regulate Chemoresponse in Breast Cancer. Cancers 2021, 13, 5979. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, S.A.; Websdale, A.; Cioccoloni, G.; Røberg-Larsen, H.; Lianto, P.; Kim, B.; Rose, A.; Soteriou, C.; Pramanik, A.; Wastall, L.M.; et al. Liver x receptor alpha drives chemoresistance in response to side-chain hydroxycholesterols in triple negative breast cancer. Oncogene 2021, 40, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Gwili, N.; Jones, S.J.; Amri, W.A.; Carr, I.M.; Harris, S.; Hogan, B.V.; Hughes, W.E.; Kim, B.; Langlands, F.E.; Millican-Slater, R.A.; et al. Transcriptome profiles of stem-like cells from primary breast cancers allow identification of ITGA7 as a predictive marker of chemotherapy response. Br. J. Cancer 2021, 125, 983–993. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs’ Dosage Ratio Effect. Molecules 2019, 24, 1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsharzadeh, M.; Hashemi, M.; Mokhtarzadeh, A.; Abnous, K.; Ramezani, M. Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1095–1110. [Google Scholar] [CrossRef]
- Carrick, S.; Parker, S.; Thornton, C.; Ghersi, D.; Simes, J.; Wilcken, N. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst. Rev. 2009, 2021, CD003372. [Google Scholar] [CrossRef]
- Fumoto, S.; Nishida, K. Co-delivery Systems of Multiple Drugs Using Nanotechnology for Future Cancer Therapy. Chem. Pharm. Bull. 2020, 68, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lv, S.; Li, Y.; He, H.; Ji, Y.; Zheng, M.; Liu, Y.; Yin, L. Co-delivery of dual chemo-drugs with precisely controlled, high drug loading polymeric micelles for synergistic anti-cancer therapy. Biomater. Sci. 2020, 8, 949–959. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Zhang, Z.; Wang, B.; Chen, G.; Zhang, Y.; Deng, H.; Tang, Z.; Mao, J.; Wang, L. Combination Chemotherapy of Lung Cancer—Co-Delivery of Docetaxel Prodrug and Cisplatin Using Aptamer-Decorated Lipid–Polymer Hybrid Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Mogheri, F.; Jokar, E.; Afshin, R.; Akbari, A.A.; Dadashpour, M.; Firouzi-amandi, A.; Serati-Nouri, H.; Zarghami, N. Co-delivery of metformin and silibinin in dual-drug loaded nanoparticles synergistically improves chemotherapy in human non-small cell lung cancer A549 cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102752. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; Bao, Y.; Xia, J.; Guo, L.; Wang, J.; Li, Y. Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy. Mater. Sci. Eng. C 2017, 70, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol. Med. 2010, 16, 594–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakhuis, H.M.; Kloet, S.K.; Kezic, S.; Kuper, F.; Park, M.V.D.Z.; Bellmann, S.; van der Zande, M.; Le Gac, S.; Krystek, P.; Peters, R.J.B.; et al. Progress and future of in vitro models to study translocation of nanoparticles. Arch. Toxicol. 2015, 89, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, C.; Wei, T.; Gayet, O.; Loncle, C.; Borge, L.; Dusetti, N.; Ma, X.; Marson, D.; Laurini, E.; et al. Dendrimeric nanosystem consistently circumvents heterogeneous drug response and resistance in pancreatic cancer. Exploration 2021, 1, 21–34. [Google Scholar] [CrossRef]
- Mujokoro, B.; Adabi, M.; Sadroddiny, E.; Adabi, M.; Khosravani, M. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: A review. Mater. Sci. Eng. C 2016, 69, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Jaskula-Sztul, R.; Javadi, A.; Xu, W.; Eide, J.; Dammalapati, A.; Kunnimalaiyaan, M.; Chen, H.; Gong, S. Co-delivery of doxorubicin and siRNA using octreotide-conjugated gold nanorods for targeted neuroendocrine cancer therapy. Nanoscale 2012, 4, 7185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Du, X.; Zhang, S.; Wang, Q.; Yin, Y.; Qiu, X.; Da, P.; Yue, H.; Wu, H.; Xu, F. Achaete-scute complex homologue-1 promotes development of laryngocarcinoma via facilitating the epithelial–mesenchymal transformation. Tumour Biol. 2017, 39, 101042831770575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef]
- Ye, H.; Chu, X.; Cao, Z.; Hu, X.; Wang, Z.; Li, M.; Wan, L.; Li, Y.; Cao, Y.; Diao, Z.; et al. A Novel Targeted Therapy System for Cervical Cancer: Co-Delivery System of Antisense LncRNA of MDC1 and Oxaliplatin Magnetic Thermosensitive Cationic Liposome Drug Carrier. Int. J. Nanomed. 2021, 16, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, D.; Cao, Z.; Zhang, C.; Cheng, D.; Liu, J.; Shuai, X. Drug and gene co-delivery systems for cancer treatment. Biomater. Sci. 2015, 3, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Wang, S.; Gao, Y.; Song, Z.; Chen, L.; Chen, Z. Advances in metal graphitic nanocapsules for biomedicine. Exploration 2022, 2, 20210223. [Google Scholar] [CrossRef]
- Zhang, H.; Song, F.; Dong, C.; Yu, L.; Chang, C.; Chen, Y. Co-delivery of nanoparticle and molecular drug by hollow mesoporous organosilica for tumor-activated and photothermal-augmented chemotherapy of breast cancer. J. Nanobiotechnol. 2021, 19, 290. [Google Scholar] [CrossRef]
- Pontón, I.; Martí del Rio, A.; Gómez Gómez, M.; Sánchez-García, D. Preparation and Applications of Organo-Silica Hybrid Mesoporous Silica Nanoparticles for the Co-Delivery of Drugs and Nucleic Acids. Nanomaterials 2020, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Argyo, C.; Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 8693. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; McBain, S.C.; El Haj, A.J.; Dobson, J. A triple-layer design for polyethyleneimine-coated, nanostructured magnetic particles and their use in DNA binding and transfection. Nanotechnology 2007, 18, 435601. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Quan, G.; Yang, P.; Pan, X.; Wu, C. The Serum-Resistant Transfection Evaluation and Long-Term Stability of Gene Delivery Dry Powder Based on Mesoporous Silica Nanoparticles and Polyethyleneimine by Freezing-Drying. AAPS PharmSciTech 2017, 18, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Zhang, K.; Sun, B.; Wang, L.; Meng, L.; Liu, Q.; Zheng, C.; Yang, B.; Sun, H. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int. J. Nanomed. 2017, 13, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Kazemi Oskuee, R.; Hanafi-Bojd, M.Y.; Gholami, L.; Ansari, L.; Malaekeh-Nikouei, B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm. Dev. Technol. 2019, 24, 127–132. [Google Scholar] [CrossRef]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an Optimal Drug/siRNA Combination Using Mesoporous Silica Nanoparticles to Overcome Drug Resistance in Breast Cancer in Vitro and in Vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Shi, K.; Tan, L.; Qu, Y.; Shen, G.; Chu, B.; Zhang, S.; Su, X.; Li, X.; Wei, Y.; et al. The use of cationic MPEG-PCL-g-PEI micelles for co-delivery of Msurvivin T34A gene and doxorubicin. Biomaterials 2014, 35, 4536–4547. [Google Scholar] [CrossRef]
- Lin, D.; Cheng, Q.; Jiang, Q.; Huang, Y.; Yang, Z.; Han, S.; Zhao, Y.; Guo, S.; Liang, Z.; Dong, A. Intracellular cleavable poly(2-dimethylaminoethyl methacrylate) functionalized mesoporous silica nanoparticles for efficient siRNA delivery in vitro and in vivo. Nanoscale 2013, 5, 4291. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.P.; Santra, S.; Mandal, S.K.; Sengupta, T.K. Singlet oxygen mediated DNA degradation by copper nanoparticles: Potential towards cytotoxic effect on cancer cells. J. Nanobiotechnol. 2011, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A. Copper Nanomaterials as Drug Delivery System against Infectious Agents and Cancerous Cells. J. Appl. Life Sci. Int. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Mabrouk, M.; Kenawy, S.H.; El-Bassyouni, G.E.T.; Ibrahim Soliman, A.A.E.-F.; Aly Hamzawy, E.M. Cancer Cells Treated by Clusters of Copper Oxide Doped Calcium Silicate. Adv. Pharm. Bull. 2019, 9, 102–109. [Google Scholar] [CrossRef]
- Pramanik, A.; Laha, D.; Chattopadhyay, S.; Dash, S.K.; Roy, S.; Pramanik, P.; Karmakar, P. Targeted delivery of “copper carbonate” nanoparticles to cancer cells in vivo. Toxicol. Res. 2015, 4, 1604–1612. [Google Scholar] [CrossRef]
- Chen, W.; Yang, W.; Chen, P.; Huang, Y.; Li, F. Disulfiram Copper Nanoparticles Prepared with a Stabilized Metal Ion Ligand Complex Method for Treating Drug-Resistant Prostate Cancers. ACS Appl. Mater. Interfaces 2018, 10, 41118–41128. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-M.; Xu, X.-M.; Zhao, D.; Cai, X.-G.; Zhou, B. Biosynthesis, characterization of PLGA coated folate-mediated multiple drug loaded copper oxide (CuO) nanoparticles and it’s cytotoxicity on nasopharyngeal cancer cell lines. AMB Express 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Shan, X.; Hao, L.; Feng, Q.; Zhang, Z. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomater. 2017, 54, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, E.; Karmakar, B.; Awwad, N.S.; Ibrahium, H.A.; Osman, H.-E.H.; El-kott, A.F.; Abdel-Daim, M.M. Green preparation of copper nanoparticle-loaded chitosan/alginate bio-composite: Investigation of its cytotoxicity, antioxidant and anti-human breast cancer properties. Arab. J. Chem. 2022, 15, 103638. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Zhuang, X.; Kang, Y.; Zhao, L.; Guo, S. Design and synthesis of copper nanoparticles for the treatment of human esophageal cancer: Introducing a novel chemotherapeutic supplement. J. Exp. Nanosci. 2022, 17, 274–284. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Lema, M.I.; Otero-Espinar, F.J. Design, Optimization, and Characterization of Lactoferrin-Loaded Chitosan/TPP and Chitosan/Sulfobutylether-β-cyclodextrin Nanoparticles as a Pharmacological Alternative for Keratoconus Treatment. ACS Appl. Mater. Interfaces 2021, 13, 3559–3575. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; He, Y.; Xu, T.; Pi, C.; Jiang, Q.; Wei, Y.; Zhao, L. Co-delivery system of chemotherapy drugs and active ingredients from natural plants: A brief overview of preclinical research for cancer treatment. Expert Opin. Drug Deliv. 2020, 17, 665–675. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Paul, V.; Kawak, P.S.; Al-Sayah, M.H.; Husseini, G.A. Transferrin-modified liposomes triggered with ultrasound to treat HeLa cells. Sci. Rep. 2021, 11, 11589. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Liu, T.; Deng, L.; Zhang, L.; Li, X.; Xu, J.; Hao, L.; Li, P.; Ran, H.; Chen, H.; et al. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials 2018, 165, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levit, S.L.; Tang, C. Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer. Nanomaterials 2021, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissig, V.; Pettinger, T.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 2014, 4357–4373. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lu, Y.; Lee, A.; Pan, X.; Yang, X.; Zhao, X.; Lee, R.J. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J. Pharm. Pharm. Sci. 2007, 10, 350–357. [Google Scholar] [PubMed]
- Zou, L.; Wang, D.; Hu, Y.; Fu, C.; Li, W.; Dai, L.; Yang, L.; Zhang, J. Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget 2017, 8, 60453–60468. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Claro de Souza, M.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment—A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, A.; Zhang, A.; Sun, Y.; Liu, J. Polymer-Based Nanocarriers for Co-Delivery and Combination of Diverse Therapies against Cancers. Nanomaterials 2018, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Pillai, G. Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development. SOJ Pharm. Pharm. Sci. 2014, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Pillai, G.; Ceballos-Coronel, M.L. Science and technology of the emerging nanomedicines in cancer therapy: A primer for physicians and pharmacists. SAGE Open Med. 2013, 1, 205031211351375. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Tu, P. Synergistically Improved Anti-tumor Efficacy by Co-delivery Doxorubicin and Curcumin Polymeric Micelles: Co-Delivery Micelles For Anti-Tumor Therapy. Macromol. Biosci. 2015, 15, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Suo, A.; Qian, J.; Zhang, Y.; Liu, R.; Xu, W.; Wang, H. Comb-like amphiphilic polypeptide-based copolymer nanomicelles for co-delivery of doxorubicin and P-gp siRNA into MCF-7 cells. Mater. Sci. Eng. C 2016, 62, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Ning, Q.; Mo, Z.; Tang, S. Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wu, S.; Hu, C.; Chen, Z.; Wang, H.; Fan, F.; Qin, Y.; Wang, C.; Sun, H.; Leng, X.; et al. Folate-targeted polymersomes loaded with both paclitaxel and doxorubicin for the combination chemotherapy of hepatocellular carcinoma. Acta Biomater. 2017, 58, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Wang, C.-Q.; Zhuo, R.-X.; Cheng, S.-X. Multi-drug delivery system based on alginate/calcium carbonate hybrid nanoparticles for combination chemotherapy. Colloids Surf. B Biointerfaces 2014, 123, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.; Nan, K.; Nie, G.; Chen, H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-cell-targeted theranostic cubosomes. Langmuir 2014, 30, 6228–6236. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An Overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef] [Green Version]
- Murgia, S.; Bonacchi, S.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Talmon, Y.; et al. Drug-loaded fluorescent cubosomes: Versatile nanoparticles for potential theranostic applications. Langmuir 2013, 29, 6673–6679. [Google Scholar] [CrossRef]
- Meli, V.; Caltagirone, C.; Falchi, A.M.; Hyde, S.T.; Lippolis, V.; Monduzzi, M.; Obiols-Rabasa, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; et al. Docetaxel-Loaded Fluorescent Liquid-Crystalline Nanoparticles for Cancer Theranostics. Langmuir 2015, 31, 9566–9575. [Google Scholar] [CrossRef]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Du, Z.; Tang, S.; Yang, R.; Tang, X.; Ji, T.; Guo, W. Use of an Artificial Ligament Decreases Hip Dislocation and Improves Limb Function After Total Femoral Prosthetic Replacement Following Femoral Tumor Resection. J. Arthroplast. 2018, 33, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Berges, T.; Granai, C.; Gordinier, M.; Gajewski, W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev. Anticancer Ther. 2002, 2, 143–150. [Google Scholar] [CrossRef]
- Franco, Y.; Vaidya, T.; Ait-Oudhia, S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer Targets Ther. 2018, 10, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Mathew, L.; Burney, M.; Nyshadham, P.; Coleman, R.L. Equivalency challenge: Evaluation of Lipodox® as the generic equivalent for Doxil® in a human ovarian cancer orthotropic mouse model. Gynecol. Oncol. 2016, 141, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bednár, J. The present status of laboratory diagnosis of thyropathies. An analytical review. Ceska Slov. Farm. 1994, 43, 64–68. [Google Scholar]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hong, K.; Park, J.W.; Kirpotin, D.B. Development of a Highly Active Nanoliposomal Irinotecan Using a Novel Intraliposomal Stabilization Strategy. Cancer Res. 2006, 66, 3271–3277. [Google Scholar] [CrossRef] [Green Version]
- Batist, G.; Barton, J.; Chaikin, P.; Swenson, C.; Welles, L. Myocet (liposome-encapsulated doxorubicin citrate): A new approach in breast cancer therapy. Expert Opin. Pharmacother. 2002, 3, 1739–1751. [Google Scholar] [CrossRef]
- Balazsovits, J.A.E.; Mayer, L.D.; Bally, M.B.; Cullis, P.R.; McDonell, M.; Ginsberg, R.S.; Falk, R.E. Analysis of the effect of liposome encapsulation on the vesicant properties, acute and cardiac toxicities, and antitumor efficacy of doxorubicin. Cancer Chemother. Pharmacol. 1989, 23, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R. Regulating Nanomedicine—Can the FDA Handle It? Curr. Drug Deliv. 2011, 8, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yan, J.; Chen, W.; Yang, J.; Liu, M.; Zhang, Y.; Shen, X.; Ma, Y.; Hu, X.; Wang, Y.; et al. Population Pharmacokinetics and Exposure–Safety Relationship of Paclitaxel Liposome in Patients With Non-small Cell Lung Cancer. Front. Oncol. 2021, 10, 1731. [Google Scholar] [CrossRef]
- Koudelka, Š.; Turánek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Mayer-Nicolai, C.; Pfaff, O. Approval probabilities and regulatory review patterns for anticancer drugs in the European Union. Crit. Rev. Oncol./Hematol. 2013, 87, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef]
- Kim, J.; Shin, S. Cost-Effectiveness of Genexol-PM for Treating Metastatic Breast Cancer. J. Breast Cancer 2010, 13, 104. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Kim, D.-W.; Chung, J.-Y.; Shin, S.G.; Kim, S.-C.; Heo, D.S.; Kim, N.K.; Bang, Y.-J. Phase I and Pharmacokinetic Study of Genexol-PM, a Cremophor-Free, Polymeric Micelle-Formulated Paclitaxel, in Patients with Advanced Malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [Green Version]
- Berges, R. Eligard®: Pharmacokinetics, Effect on Testosterone and PSA Levels and Tolerability. Eur. Urol. Suppl. 2005, 4, 20–25. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 2009, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, D.; Lv, Y.; Yao, Y.; Miao, X.; Wang, Q.; Xiao, X.; Yin, J.; Shi, Y.; Shi, M.; Zhang, X.; et al. Efficacy and safety of Abraxane in treatment of progressive and recurrent non-small cell lung cancer patients: A retrospective clinical study: Abraxane in NSCLC. Thorac. Cancer 2012, 3, 341–347. [Google Scholar] [CrossRef]
- Choi, M.; Al-Hajeili, M.; Azmi, A. Nab-paclitaxel: Potential for the treatment of advanced pancreatic cancer. OncoTargets Ther. 2014, 2014, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef]
- Pan, L.; Liu, J.; He, Q.; Wang, L.; Shi, J. Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials 2013, 34, 2719–2730. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Li, M.; Tang, Z.; Song, W.; Sun, H.; Liu, H.; Chen, X. Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy. Acta Biomater. 2013, 9, 9330–9342. [Google Scholar] [CrossRef]
- Duong, H.H.P.; Yung, L.-Y.L. Synergistic co-delivery of doxorubicin and paclitaxel using multi-functional micelles for cancer treatment. Int. J. Pharm. 2013, 454, 486–495. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ren, T.; Zhao, J.; Wong, C.-H.; Chan, H.Y.E.; Zuo, Z. Exclusion of unsuitable CNS drug candidates based on their physicochemical properties and unbound fractions in biomatrices for brain microdialysis investigations. J. Pharm. Biomed. Anal. 2020, 178, 112946. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Mo, R.; Lu, Y.; Jiang, T.; Gu, Z. Folding graft copolymer with pendant drug segments for co-delivery of anticancer drugs. Biomaterials 2014, 35, 7194–7203. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Thambi, T.; Lee, D.S. Co-Delivery of Drugs and Genes Using Polymeric Nanoparticles for Synergistic Cancer Therapeutic Effects. Adv. Healthc. Mater. 2018, 7, 1700886. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, P.; Huang, C.; Song, Y.; Garg, S.; Luan, Y. Co-delivery of doxorubicin hydrochloride and verapamil hydrochloride by pH-sensitive polymersomes for the reversal of multidrug resistance. RSC Adv. 2015, 5, 77986–77995. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Xiong, X.; Guo, X.; Zhang, L.; Zhang, X.; Zhou, S. Codelivery of a π–π Stacked Dual Anticancer Drug Combination with Nanocarriers for Overcoming Multidrug Resistance and Tumor Metastasis. Adv. Funct. Mater. 2016, 26, 8266–8280. [Google Scholar] [CrossRef]
- Yaghoubi, F.; Motlagh, N.S.H.; Naghib, S.M.; Haghiralsadat, F.; Jaliani, H.Z.; Moradi, A. A functionalized graphene oxide with improved cytocompatibility for stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment. Sci. Rep. 2022, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, L.; Chen, X.; Xie, Z.; Ding, J.; He, C.; Zhang, J.; Chen, X. pH-responsive metallo-supramolecular nanogel for synergistic chemo-photodynamic therapy. Acta Biomater. 2015, 25, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Jin, H.; Tang, Y.; Wu, A.; Xu, Q.; Huang, Y. Prodrug-Like, PEGylated Protein Toxin Trichosanthin for Reversal of Chemoresistance. Mol. Pharm. 2017, 14, 1429–1438. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv. Sci. 2018, 5, 1800811. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y.; Xu, S.; Liu, Y.; Yin, J.; Lovejoy, D.B.; Zheng, M.; Liang, X.; Park, J.B.; Efremov, Y.M.; et al. Brain Co-Delivery of Temozolomide and Cisplatin for Combinatorial Glioblastoma Chemotherapy. Adv. Mater. 2022, 2203958. [Google Scholar] [CrossRef]
- Ismail, M.; Yang, W.; Li, Y.; Chai, T.; Zhang, D.; Du, Q.; Muhammad, P.; Hanif, S.; Zheng, M.; Shi, B. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials 2022, 287, 121608. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zhang, D.; Sun, Y.; Li, F.; Zheng, M.; Lovejoy, D.B.; Zou, Y.; Shi, B. Brain co-delivery of first-line chemotherapy drug and epigenetic bromodomain inhibitor for multidimensional enhanced synergistic glioblastoma therapy. Exploration 2022, 20210274. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Guo, Y.; Wang, P.; Su, Y.; Jin, X.; Zhu, X.; Zhang, C. Drug-grafted DNA as a novel chemogene for targeted combinatorial cancer therapy. Exploration 2022, 2, 20210172. [Google Scholar] [CrossRef]
- Creixell, M.; Peppas, N.A. Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today 2012, 7, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, C.; Lengauer, T. Managing drug resistance in cancer: Lessons from HIV therapy. Nat. Rev. Cancer 2012, 12, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Khelghati, N.; Soleimanpour Mokhtarvand, J.; Mir, M.; Alemi, F.; Asemi, Z.; Sadeghpour, A.; Maleki, M.; Samadi Kafil, H.; Jadidi-niaragh, F.; Majidinia, M.; et al. The importance of co-delivery of nanoparticle-siRNA and anticancer agents in cancer therapy. Chem. Biol. Drug Des. 2021, 97, 997–1015. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-M.; Jie, J.; Zhang, Y.; Liu, H.; Peng, L.-P. A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Jin, G.; Kang, L.; Chen, L.; Gao, Z.; Huang, W. Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int. J. Nanomed. 2018, 13, 2405–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Liu, W.; Hu, Y.; Li, W.; Di, W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 2020, 10, 3325–3339. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, B.; Zheng, C.; Ji, R.; Ren, X.; Guo, F.; Sun, S.; Shi, J.; Zhang, H.; Zhang, Z.; et al. The tumor-targeting core-shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J. Control. Release 2015, 220, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Muthuswamy, E.; Wani, A.; Brichacek, M.; Castañeda, A.L.; Brock, S.L.; Oupicky, D. Enhanced Gene and siRNA Delivery by Polycation-Modified Mesoporous Silica Nanoparticles Loaded with Chloroquine. Pharm. Res. 2010, 27, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.B.; Gao, X.C.; Yu, S.Y.; Ma, Y.; Ji, C.H. Fabrication and potential application of a di-functional magnetic system: Magnetic hyperthermia therapy and drug delivery. CrystEngComm 2016, 18, 1133–1138. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, W.; Sun, C.; Wu, J.; Tang, J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf. B Biointerfaces 2016, 138, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, Y.; Cheng, D.; Chen, J.; Wang, L.; Shuai, X. Co-Delivery of Doxorubicin and siRNA with Reduction and pH Dually Sensitive Nanocarrier for Synergistic Cancer Therapy. Small 2014, 10, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.-T. Enhanced apoptosis of ovarian cancer cells via nanocarrier-mediated codelivery of siRNA and doxorubicin. Int. J. Nanomed. 2012, 2012, 3823–3835. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Meng, Y.; Ye, J.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Jin, D.; Liu, Y. Sequential delivery of VEGF siRNA and paclitaxel for PVN destruction, anti-angiogenesis, and tumor cell apoptosis procedurally via a multi-functional polymer micelle. J. Control. Release 2018, 287, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Joshi, U.; Filipczak, N.; Khan, M.M.; Attia, S.A.; Torchilin, V. Hypoxia-sensitive micellar nanoparticles for co-delivery of siRNA and chemotherapeutics to overcome multi-drug resistance in tumor cells. Int. J. Pharm. 2020, 590, 119915. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, S.; Hui, W.; He, J.; Liu, Z.; Cheng, J. Chitosan based pH-responsive polymeric prodrug vector for enhanced tumor targeted co-delivery of doxorubicin and siRNA. Carbohydr. Polym. 2020, 250, 116781. [Google Scholar] [CrossRef]
- Qu, M.-H.; Zeng, R.-F.; Fang, S.; Dai, Q.-S.; Li, H.-P.; Long, J.-T. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int. J. Pharm. 2014, 474, 112–122. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, C.; Fang, E.; Lu, X.; Wang, G.; Tong, Q. Co-Delivery of Doxorubicin and SATB1 shRNA by Thermosensitive Magnetic Cationic Liposomes for Gastric Cancer Therapy. PLoS ONE 2014, 9, e92924. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, R.; Li, J.; Liu, S.; Huang, S.; Jiang, C. Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials 2011, 32, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.; Gao, Z.; Huang, W.; Jin, M.; Wang, Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 2015, 5, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Zhang, H.; Huang, L. Smart Polymeric Nanoparticles for Cancer Gene Delivery. Mol. Pharm. 2015, 12, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.-Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes: GENE THERAPIES FOR CANCER. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, Y.; Kim, S. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Z.; Lin, L.; Hu, Y.; Tian, H.; Chen, M.; Chen, X. Combination therapy of pDNA and siRNA by versatile carriers composed of poly(l-serine) modified polyethylenimines. Mater. Chem. Front. 2017, 1, 937–946. [Google Scholar] [CrossRef]
- Risnayanti, C.; Jang, Y.-S.; Lee, J.; Ahn, H.J. PLGA nanoparticles co-delivering MDR1 and BCL2 siRNA for overcoming resistance of paclitaxel and cisplatin in recurrent or advanced ovarian cancer. Sci. Rep. 2018, 8, 7498. [Google Scholar] [CrossRef] [Green Version]
- Chang Kang, H.; Bae, Y.H. Co-delivery of small interfering RNA and plasmid DNA using a polymeric vector incorporating endosomolytic oligomeric sulfonamide. Biomaterials 2011, 32, 4914–4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles Modified with Tumor-targeting scFv Deliver siRNA and miRNA for Cancer Therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, E.H.; Li, Y.T.; Chua, M.J.; Kunnath, A.P. Reversing multidrug resistance in breast cancer cells by silencing ABC transporter genes with nanoparticle-facilitated delivery of target siRNAs. Int. J. Nanomed. 2012, 2012, 2473–2481. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.J.; Tzeng, S.Y.; Green, J.J. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015, 11, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nastiuk, K.L.; Krolewski, J.J. Opportunities and challenges in combination gene cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 35–40. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines—Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Kim, G.J.; Nie, S. Targeted cancer nanotherapy. Mater. Today 2005, 8, 28–33. [Google Scholar] [CrossRef]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Bresseleers, J.; Varela-Moreira, A.; Sandre, O.; Meeuwissen, S.; Schiffelers, R.; Metselaar, J.; van Nostrum, C.; van Hest, J.; Hennink, W. Effect of Formulation and Processing Parameters on the Size of mPEG-b-p(HPMA-Bz) Polymeric Micelles. Langmuir 2018, 34, 15495–15506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L. (Ed.) Biomedical Composites, 2nd ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK; ISBN 978-0-08-100759-4.
| Delivery System | Drug-Drug | Cell-Line | Indication | Reference |
|---|---|---|---|---|
| π–π stacked dual anti-cancer drug combination with an actively targeted, pH- and reduction-sensitive polymer micellar platform | DOX and HCPT (10-hydroxycamptothecin) | MCF-7/ADR | Breast cancer | [120,130] |
| Inorganic nanoparticle Graphene oxide functionalized with a carboxyl group (GO-COOH) | DOX and Curcumin (CUR) | AGS, PC3, A2780, and HFF | Gastric, prostate, and ovarian cancer | [121,131] |
| Transferrin conjugated Liposomes | DOX and Verapamil (VER) | K562 | Leukemia | [72] |
| Amphiphilic methoxy polyethylene glycol-polylactic-co-glycolic acid (mPEG-PLGA) nanoparticles | DOX and Paclitaxel (PTX) | A549, SK-MEL-3 | Non-small lung cancer and melanoma | [13,88] |
| pH-responsive Metallo-supramolecular nanogel (SNG) | DOX and Tetraphenylporphyrin zinc | HepG2, A431 | Liver cancer, and epidermoid carcinoma | [122,132] |
| Liposome | PTX and trichosanthin (TCS) | A549 | Lung cancer | [123,133] |
| Hyaluronic acid nanogel (HANG) | Cisplatin (CDDP) and DOX | K7 cells (mouse osteosarcoma) | Osteosarcoma | [124,134] |
| pH-sensitive biomimetic nanoparticles (MNPs) | Temozolomide (TMZ) and Cisplatin (CDDP) | U87MG | Glioblastoma (GBM) | [125,135] |
| ApoE-functionalized liposomes based on artesunate-phosphatidylcholine (ARTPC) encapsulated with temozolomide (ApoE-ARTPC@TMZ) | Artesunate (ART) and temozolomide (TMZ) | U251-TR | Glioblastoma (GBM) | [126,136] |
| pH-sensitive ApoE peptide decorated biomimetic nanomedicine (ABNM@TMZ/OTX) | Temozolomide (TMZ) and OTX015 (OTX) | GL261 GBM | Glioblastoma multiforme (GBM) | [127,137] |
| Nanocarrier | Delivery System | Drug-Gene | Cell-Line | Indication | Reference |
|---|---|---|---|---|---|
| Mesoporous silica nanoparticle (MSN) | Mesoporous silica nanoparticles modified with polyethyleneimine MSNP-PEI-PEG | DOX—ABCB1 (P-gp) | KB-V1 | Oral squamous carcinoma | [50] |
| Micelle | Triblock copolymer functionalized with folic acid: PEG-PCL-PEI | DOX—(P-gp) siRNA | MCF-7, ADR | Breast cancer | [149] |
| A reduction and pH dual sensitive ternary block copolymer PEG-PAsp(AED)-PDPA | DOX—BCL-2 siRNA | SKOV3 | Ovarian cancer | [150] | |
| Self-assembled cationic micelle | Folate conjugated ternary copolymer FA-PEG-PEI-PCL | DOX—BCL-2 siRNA | SKOV3 | Ovarian cancer | [151] |
| Polymeric micelle | Targeted multi-functional polymeric micelle (TMPM): triblock copolymer PCL-PEG-PHIS | Paclitaxel—VEGF siRNA (siVEGF) | HUVECs, MCF-7 | Breast Cancer | [152] |
| Polymer-based nanomaterials | Hypoxia-sensitive PEG-azobenzene-PEI-DOPE (PAPD) nanoparticles | DOX—ABCB1 siRNA | A2780 ADR, MCF7 ADR | Ovarian cancer and breast cancer | [153] |
| Chitosan-based pH-responsive polymeric prodrug vector GA-CS-PEI-HBA-DOX | DOX—BCL-2 siRNA | HUVEC, HepG2 | Liver cancer | [154] | |
| Cationic liposome | PEGylated liposomes | Docetaxel—BCL2 siRNA | A549, H226 | Lung cancer | [155] |
| Thermosensitive magnetic cationic liposomes (TSMCL) | DOX—SATB1-shRNA | MKN-28 | Gastric adenocarcinoma | [156] | |
| Dendrimer | PAMAM-PEG-T7 | Doxorubicin—plasmid pORF-hTRAIL | Bel-7402 | Liver cancer | [157] |
| Nanocarrier | Delivery System | Active Agents | Cell-Line | Indication | Reference |
|---|---|---|---|---|---|
| Polymer-based | Polyethylenimine–poly(L-serine) (PEI–PSer) | Bcl-2-siRNA and pKH-rev-casp-3 (siRNA and pDNA) | HeLa/293T-GFP | Cervical carcinoma, kidney cancer | [165] |
| Poly(DL-lactide-co-glycolide acid) (PLGA) nanoparticles and poly-L-lysine (PLL) as a complexing reagent (PLGA NPs) | MDR1-siRNA and Bcl-2-siRNA (siRNA and siRNA) | SKOV-3/A2780-CP20 | Ovarian cancer | [166] | |
| poly(l-lysine) (PLL)—oligomeric sulfonamides (OSA) | luc-siRNA and pLuc (siRNA and pDNA) | HEK293 (human embryonic kidney cell line) | Renal cancer | [167] | |
| Lipid-based | Lipid nanoparticle (LNP) | anti-VEGF-siRNA and KSP-siRNA | SCID/Hep3B | Hepatocellular carcinoma | [161] |
| LPH (liposome-polycation-hyaluronic acid (HA)) nanoparticle formulation modified with tumor-targeting single-chain antibody fragment (scFv) | siRNA and miRNA (c-myc/MDM2/VEGF-siRNA and miR-34a) | B16F10 | Lung cancer | [168] | |
| anti-EGFR aptamer coupled cationic lipid nanocarriers incorporated with hydrophobic quantum dots (QDs) QD-lipid nanocarriers (QLs) | Quantum dots (QDs) and siRNA-siRNA (Bcl-2-siRNA and PKCl-siRNA | MDA-MB-231/MDA-MD-453 | Breast cancer | [93] | |
| Inorganic-based | pH-sensitive carbonate apatite (CO3Ap) nanoparticles | siRNA-siRNA (ABCG2-siRNA and ABCB1-siRNA | MCF-7 | Breast cancer | [169] |
| Polymer-Coated Gold Nanoparticles (MAu-P-D-SS37-siRNA-447) | anti-eGFP siRNA and pEGFP-N1, pDsRed-Max-N1 (siRNA-pDNA) | GBM319 | Brain cancer | [170] |
| Advantages | Disadvantages |
|---|---|
| Reducing cytotoxicity | Higher risk of drug interactions |
| Enhancing synergistic effects | Difficulty in reducing particle size |
| Improving the quality of life for patients | Challenge in coordinated drug release |
| Synchronized biodistribution | Complicated preparation and high cost |
| Reducing the likelihood of MDR | Antagonistic effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Bostami, R.D.; Abuwatfa, W.H.; Husseini, G.A. Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomaterials 2022, 12, 2672. https://doi.org/10.3390/nano12152672
Al Bostami RD, Abuwatfa WH, Husseini GA. Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomaterials. 2022; 12(15):2672. https://doi.org/10.3390/nano12152672
Chicago/Turabian StyleAl Bostami, Rouba D., Waad H. Abuwatfa, and Ghaleb A. Husseini. 2022. "Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy" Nanomaterials 12, no. 15: 2672. https://doi.org/10.3390/nano12152672
APA StyleAl Bostami, R. D., Abuwatfa, W. H., & Husseini, G. A. (2022). Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomaterials, 12(15), 2672. https://doi.org/10.3390/nano12152672






