Gram-Scale Synthesis of Graphitic Carbon Nitride Quantum Dots with Ultraviolet Photoluminescence for Fe3+ Ion Detection
Abstract
:1. Introduction
2. Materials and Methods
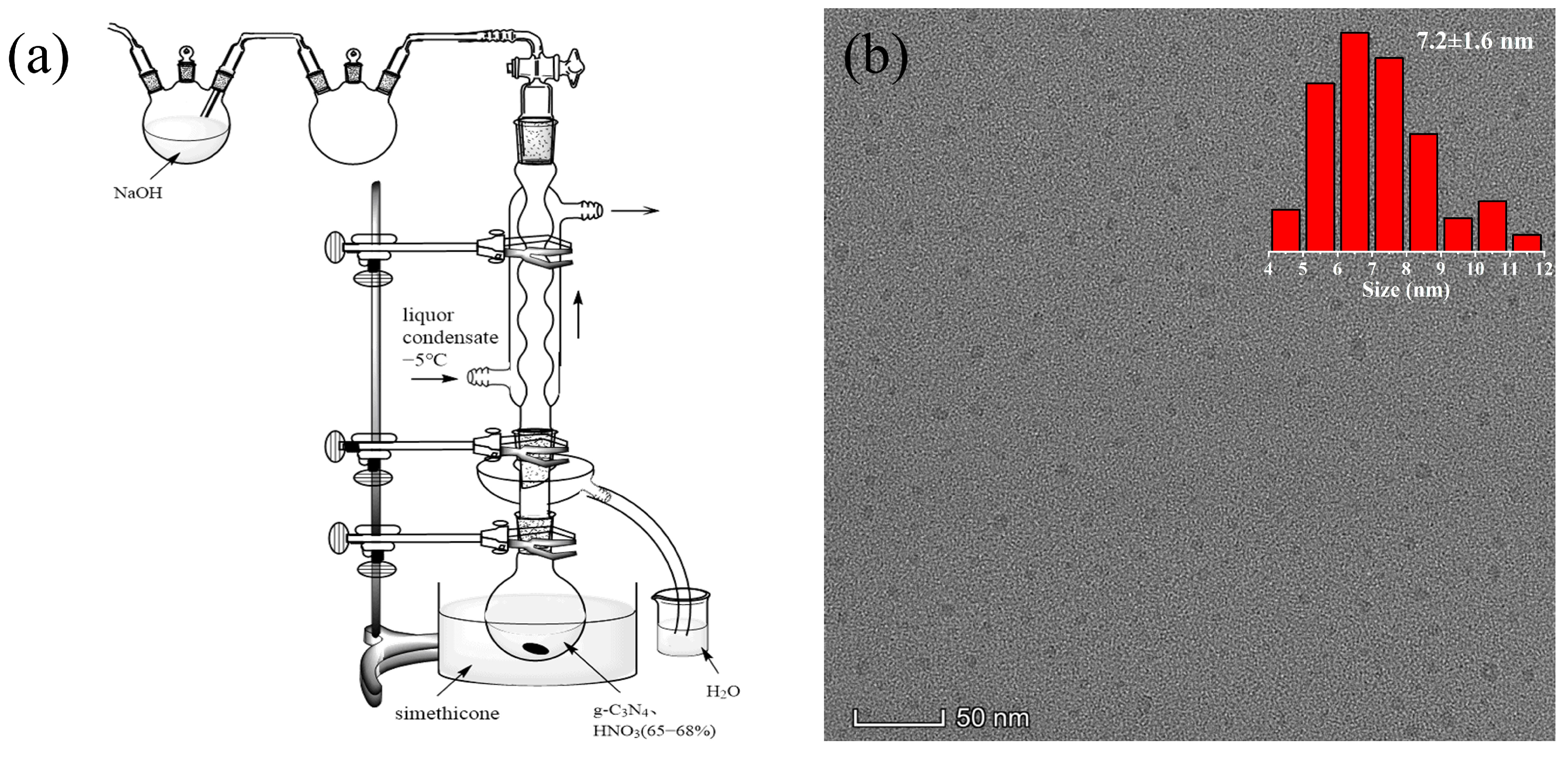
2.1. The Preparation of g-C3N4QDs
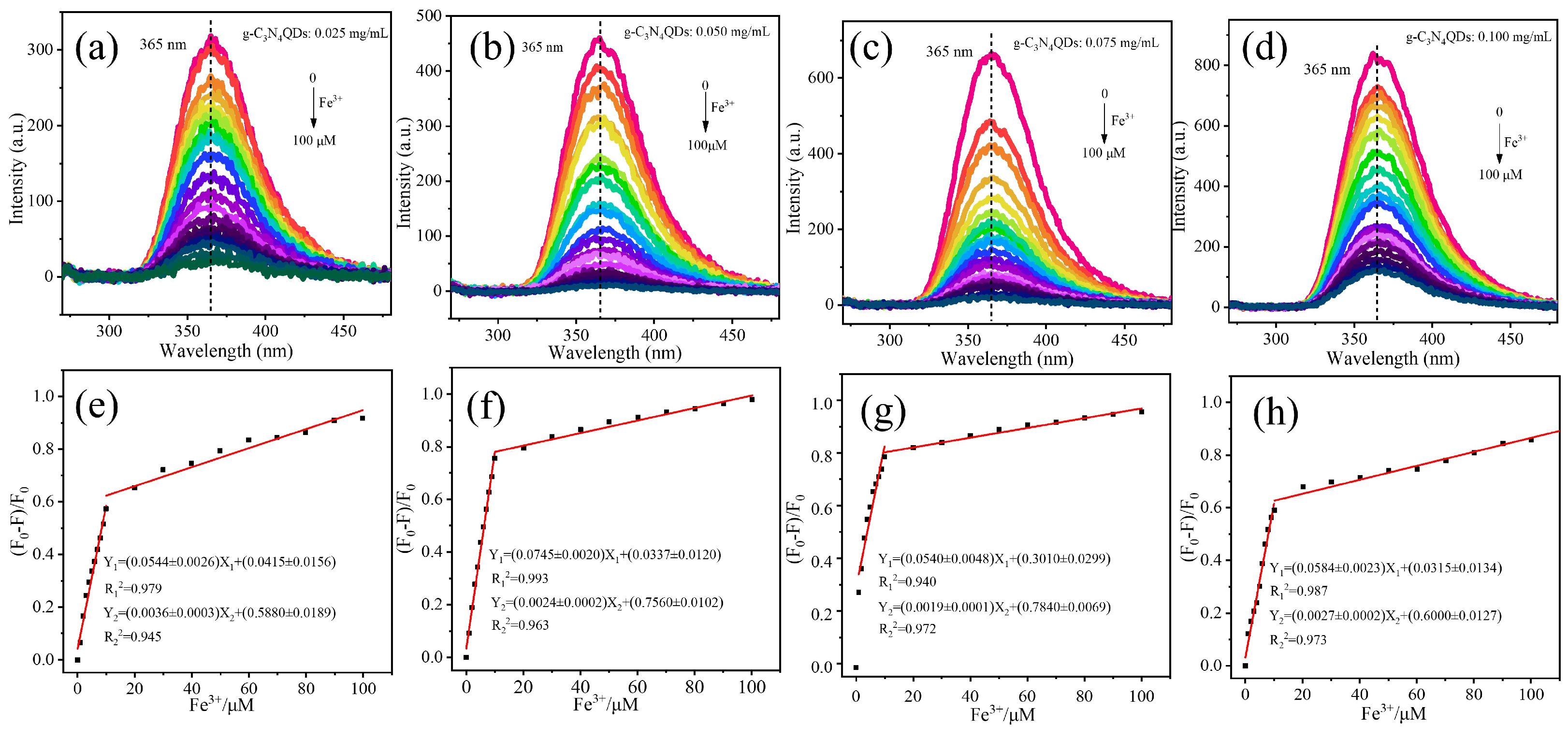
2.2. Fluorescence Detection of Fe3+
2.3. Characterization
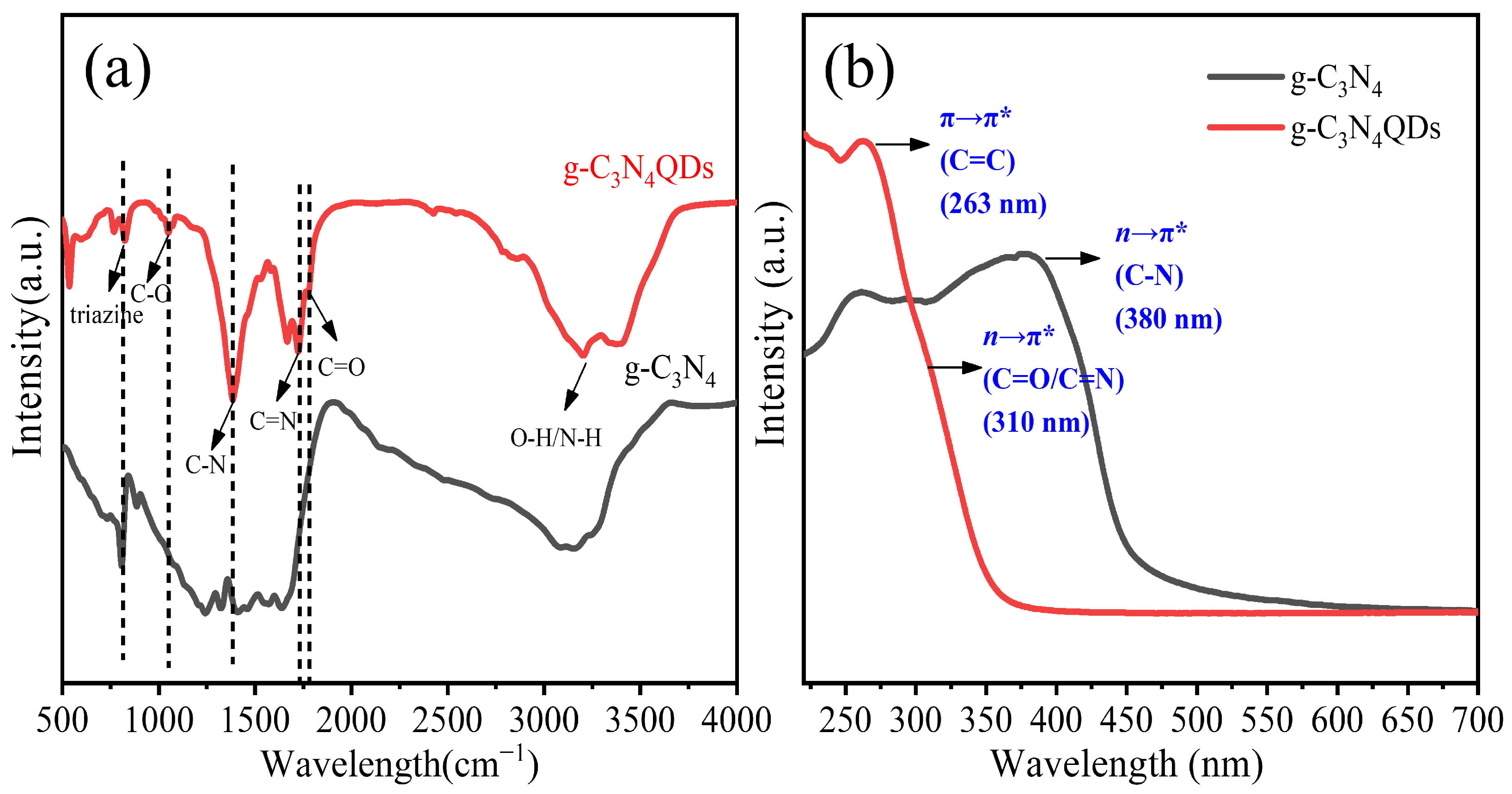
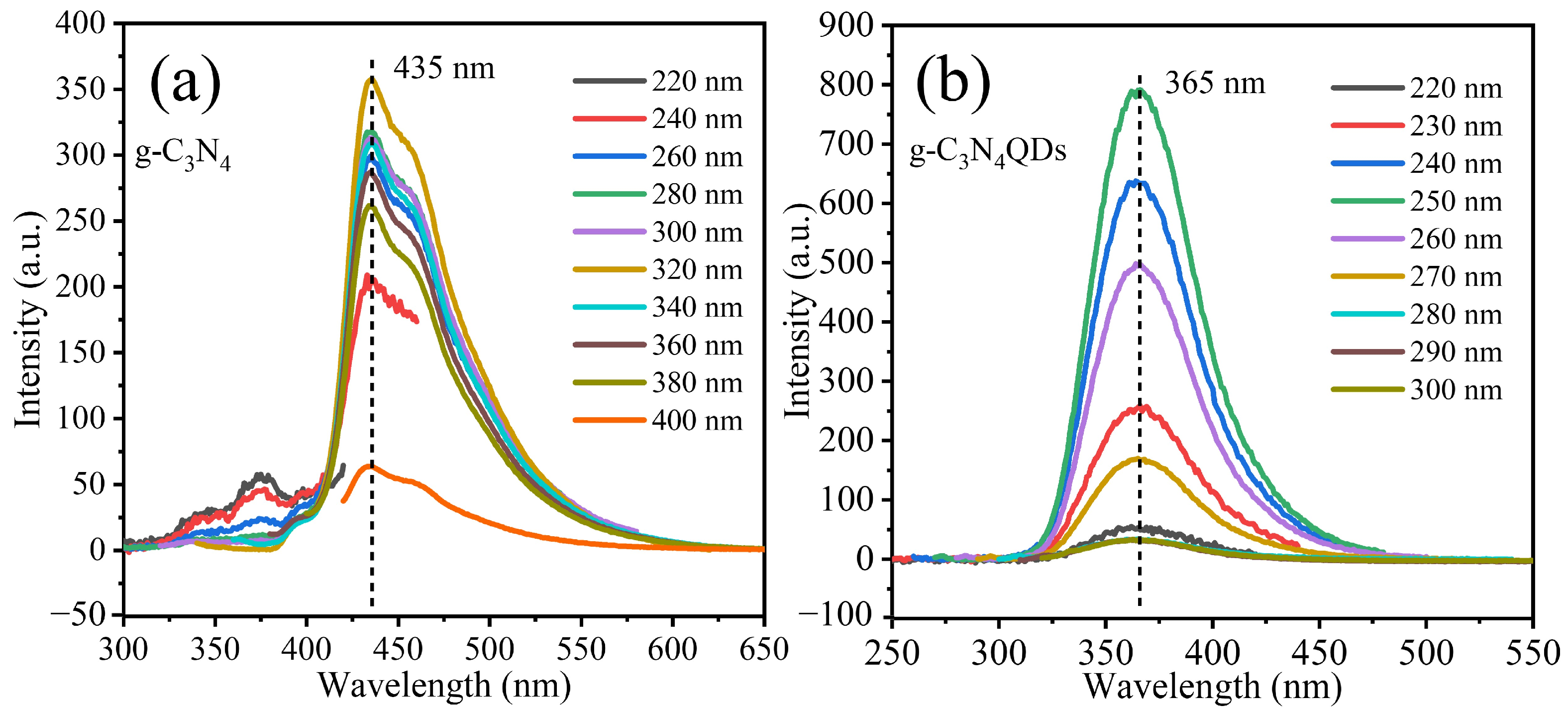
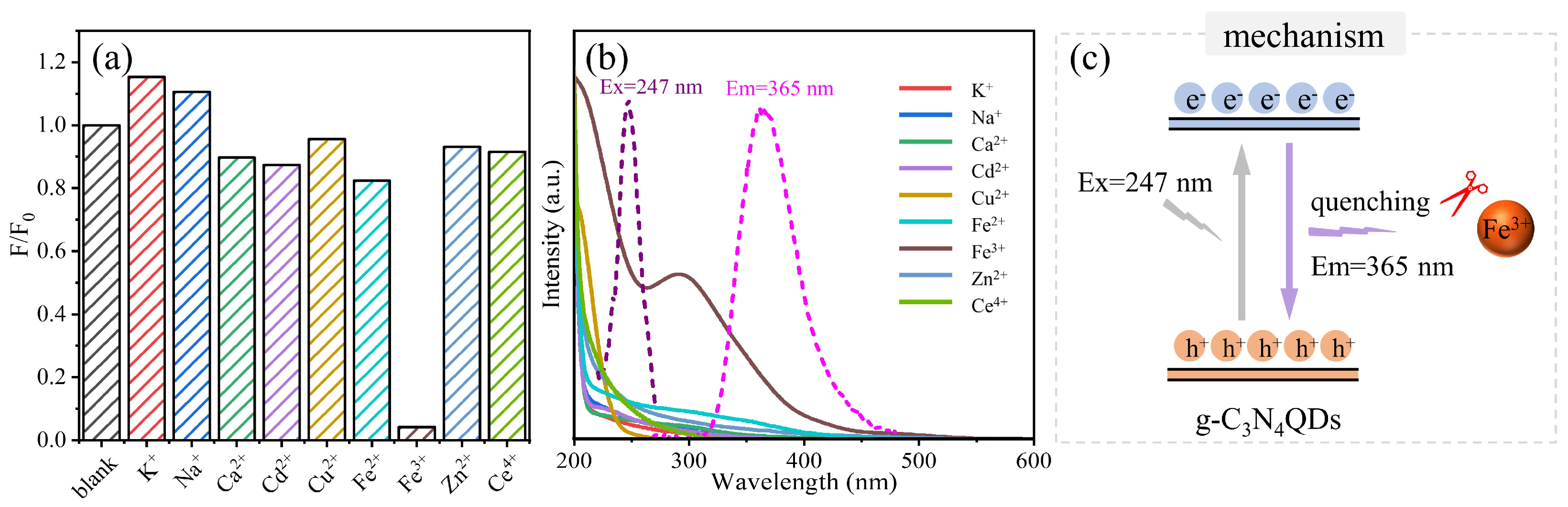
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, L.; Fei, M.; Chen, J.; Tian, Y.; Jiang, Y.; Huang, Y.; Xu, K.; Hu, J.; Zhao, Z.; Zhang, Q. Graphitic C3N4 quantum dots for next-generation QLED displays. Mater. Today 2019, 22, 76–84. [Google Scholar] [CrossRef]
- Devi, M.; Das, P.; Boruah, P.K.; Deka, M.J.; Duarah, R.; Gogoi, A.; Neog, D.; Dutta, H.S.; Das, M.R. Fluorescent graphitic carbon nitride and graphene oxide quantum dots as efficient nanozymes: Colorimetric detection of fluoride ion in water by graphitic carbon nitride quantum dots. J. Environ. Chem. Eng. 2021, 9, 104803. [Google Scholar] [CrossRef]
- Yin, M.; Chen, X.; Wan, Y.; Zhang, W.; Feng, L.; Zhang, L.; Wang, H. Doping carbon nitride quantum dots into melamine-silver matrix: An efficient photocatalyst with tunable morphology and photocatalysis for H2O2 evolution under visible light. ChemCatChem 2020, 12, 1512–1518. [Google Scholar] [CrossRef]
- Xu, J.; Shalom, M. Conjugated carbon nitride as an emerging luminescent material: Quantum dots, thin films and their applications in imaging, sensing, optoelectronic devices and photoelectrochemistry. ChemPhotoChem 2019, 3, 170–179. [Google Scholar] [CrossRef]
- Zhou, Z.; Niu, X.; Ma, L.; Wang, J. Revealing the pH-dependent photoluminescence mechanism of graphitic C3N4 quantum dots. Adv. Theory Simul. 2019, 2, 1900074. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.; Nie, R. Synthesis and biomedical applications of graphitic carbon nitride quantum dots. J. Mater. Chem. B 2019, 7, 5432–5448. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, H.; Zhang, Q.; Xie, J.; Tian, Y.; Wang, J.; Xie, Y. Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus. Adv. Mater. 2014, 26, 4438–4443. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, Z.; Liu, Q.; Huang, D.; Wei, Y.; Hu, Y.; Lian, X.; Hu, C. A facile and one-pot synthesis of fluorescent graphitic carbon nitride quantum dots for bio-imaging applications. New J. Chem. 2017, 41, 3930–3938. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Ge, X.; Hu, L.; Hu, Y. Electrochemiluminescence sensing platform for ultrasensitive DNA analysis based on resonance energy transfer between graphitic carbon nitride quantum dots and gold nanoparticles. Sens. Actuators B 2019, 297, 126790. [Google Scholar] [CrossRef]
- Fronczak, M.; Demby, K.; Strachowski, P.; Strawski, M.; Bystrzejewski, M. Graphitic carbon nitride doped with the s-block metals: Adsorbent for the removal of methyl blue and copper (II) ions. Langmuir 2018, 34, 7272–7283. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, Y.; Fang, X.; Cui, X.; Wang, J.; Yue, C.; Fang, W.; Zhao, H.; Li, Z. In situ sulfur-doped graphitic carbon nitride nanosheets with enhanced electrogenerated chemiluminescence used for sensitive and selective sensing of l-cysteine. J. Mater. Chem. B 2019, 7, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; Ang, H.; Tade, M.O.; Wang, S. Integrated oxygen-doping and dye sensitization of graphitic carbon nitride for enhanced visible light photodegradation. J. Colloid Interface Sci. 2016, 476, 193–199. [Google Scholar] [CrossRef]
- Zhuang, Q.; Guo, P.; Zheng, S.; Lin, Q.; Lin, Y.; Wang, Y.; Ni, Y. Green synthesis of luminescent graphitic carbon nitride quantum dots from human urine and its bioimaging application. Talanta 2018, 188, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.-L.T.; Choi, W.M.; Chung, J.S.; Hur, S.H. Highly biocompatible phenylboronic acid-functionalized graphitic carbon nitride quantum dots for the selective glucose sensor. Sens. Actuators B 2019, 282, 36–44. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, J.; Li, H.; Li, Y.; Liu, X.; Shi, D.; Wu, Q.; Jiao, Q. Graphitic carbon nitride quantum dot decorated three-dimensional graphene as an efficient metal-free electrocatalyst for triiodide reduction. J. Mater. Chem. A 2018, 6, 5603–5607. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Chung, J.S.; Hur, S.H. Aminoboronic acid-functionalized graphitic carbon nitride quantum dots for the photoluminescence multi-chemical sensing probe. Dye. Pigment. 2019, 168, 180–188. [Google Scholar] [CrossRef]
- Yousaf, M.U.; Pervaiz, E.; Minallah, S.; Afzal, M.J.; Honghong, L.; Yang, M. Tin oxide quantum dots decorated graphitic carbon nitride for enhanced removal of organic components from water: Green process. Results Phys. 2019, 14, 102455. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Wang, J.; Lu, Y.; Liu, Y.; Yu, J.; Chen, S.; Zhou, Y. Construction of plasmonic Ag and nitrogen-doped graphene quantum dots codecorated ultrathin graphitic carbon nitride nanosheet composites with enhanced photocatalytic activity: Full-spectrum response ability and mechanism insight. ACS Appl. Mater. Interfaces 2017, 9, 42816–42828. [Google Scholar] [CrossRef]
- Barman, S.; Sadhukhan, M. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 2012, 22, 21832–21837. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, X.; Qin, H.; Wang, F.; Li, Y.; Li, X.; Kang, S.; Zuo, Y.; Cui, L. Facile one-step synthesis of hybrid graphitic carbon nitride and carbon composites as high-performance catalysts for CO2 photocatalytic conversion. ACS Appl. Mater. Interfaces 2016, 8, 17212–17219. [Google Scholar] [CrossRef]
- Cai, A.; Wang, Q.; Chang, Y.; Wang, X. Graphitic carbon nitride decorated with S, N co-doped graphene quantum dots for enhanced visible-light-driven photocatalysis. J. Alloys Compd. 2017, 692, 183–189. [Google Scholar] [CrossRef]
- Shi, J.; Feng, S.; Chen, T.; Li, Y. Effect of porous modification on the synthesis and photocatalytic activity of graphitic carbon nitride/carbon quantum dot nanocomposite. J. Mater. Sci. Mater. Electron. 2018, 29, 17454–17462. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Chen, H.; Liu, L.; Zhang, W.; Sun, B.; Yang, M.; Wang, Y.; Dong, L. Fabrication of PEGylated graphitic carbon nitride quantum dots as traceable, pH-sensitive drug delivery systems. New J. Chem. 2018, 42, 14263–14270. [Google Scholar] [CrossRef]
- Lin, X.; Liu, C.; Wang, J.; Yang, S.; Shi, J.; Hong, Y. Graphitic carbon nitride quantum dots and nitrogen-doped carbon quantum dots co-decorated with BiVO4 microspheres: A ternary heterostructure photocatalyst for water purification. Sep. Purif. Technol. 2019, 226, 117–127. [Google Scholar] [CrossRef]
- Zhou, Z.; Shen, Y.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. Chemical cleavage of layered carbon nitride with enhanced photoluminescent performances and photoconduction. ACS Nano 2015, 9, 12480–12487. [Google Scholar] [CrossRef]
- Wang, T.; Nie, C.; Ao, Z.; Wang, S.; An, T. Recent progress in gC3N4 quantum dots: Synthesis, properties and applications in photocatalytic degradation of organic pollutants. J. Mater. Chem. A 2020, 8, 485–502. [Google Scholar] [CrossRef]
- Li, X.; Chai, C.; Zhang, Y.; Wang, Y.; Lv, J.; Bian, W.; Choi, M.M. Microwave synthesis of nitrogen and sulfur co-doped carbon dots for the selective detection of Hg2+ and glutathione. Opt. Mater. 2020, 99, 109559. [Google Scholar] [CrossRef]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron (III) ions and dopamine. Chem. Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef]
- Lu, W.; Gong, X.; Nan, M.; Liu, Y.; Shuang, S.; Dong, C. Comparative study for N and S doped carbon dots: Synthesis, characterization and applications for Fe3+ probe and cellular imaging. Anal. Chim. Acta 2015, 898, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Aslandaş, A.M.; Balcı, N.; Arık, M.; Şakiroğlu, H.; Onganer, Y.; Meral, K. Liquid nitrogen-assisted synthesis of fluorescent carbon dots from Blueberry and their performance in Fe3+ detection. Appl. Surf. Sci. 2015, 356, 747–752. [Google Scholar] [CrossRef]

| Samples (ppm) | Ca | Co | Cu | Fe | Mn | Na | Ni |
|---|---|---|---|---|---|---|---|
| g-C3N4QDs | 195 | <5 | <5 | 45 | <5 | 135 | <5 |
| Materials | Linear Range (μmol·L−1) | Detection Limit (μmol·L−1) | Reaction Time (min) | Ref |
|---|---|---|---|---|
| Carbon dots | 0–20 | 0.32 | 10 | [28] |
| g-CNQDs | 2–200 | 1 | 2 | [25] |
| N-CQDs | 3.32–32.26 | 0.7462 | 2 | [29] |
| S-GQDs | 0–0.7 | 0.0042 | 10 | [30] |
| N and S doped Carbon dots | 6.0–200 | 0.8 | 2 | [31] |
| C-dots | 12.5–100 | 9.97 | - | [32] |
| g-C3N4QDs | 0–100 | 0.259 | 1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Qin, H.; Cai, J.; Cui, Y.; Liao, L.; Lv, F.; Zhu, C.; Wang, L.; Liu, J.; Long, L.; et al. Gram-Scale Synthesis of Graphitic Carbon Nitride Quantum Dots with Ultraviolet Photoluminescence for Fe3+ Ion Detection. Nanomaterials 2022, 12, 2804. https://doi.org/10.3390/nano12162804
Lu X, Qin H, Cai J, Cui Y, Liao L, Lv F, Zhu C, Wang L, Liu J, Long L, et al. Gram-Scale Synthesis of Graphitic Carbon Nitride Quantum Dots with Ultraviolet Photoluminescence for Fe3+ Ion Detection. Nanomaterials. 2022; 12(16):2804. https://doi.org/10.3390/nano12162804
Chicago/Turabian StyleLu, Xuemei, Haijun Qin, Jiuzhang Cai, Yuhang Cui, Lixin Liao, Fengzhen Lv, Changming Zhu, Liguang Wang, Jun Liu, Lizhen Long, and et al. 2022. "Gram-Scale Synthesis of Graphitic Carbon Nitride Quantum Dots with Ultraviolet Photoluminescence for Fe3+ Ion Detection" Nanomaterials 12, no. 16: 2804. https://doi.org/10.3390/nano12162804






