Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts
Abstract
:1. Introduction
- The porous structure of MOFs exposes more active sites and facilitates;
- Ordered crystal structure of MOFs can separate electron-hole pairs;
- MOFs structure can be tailored;
- MOFs are easy to separate and recycle from the reaction system;
- MOFs have the tendency for electron transfer between ligands and metals due to the large π-conjugated systems in the organic linkers;
- A long-wavelength absorbing organic bridging ligand can enhance the optical absorption of MOFs.
2. Different Synthesis Methods of MOFs and MOFs-Based Heterostructures
2.1. Synthesis of MOFs
2.1.1. Microwave-Assisted Synthesis
2.1.2. Solvothermal Method
2.1.3. Vapor Assisted Synthesis
2.1.4. Sonochemical Method
2.2. Synthesis Method of MOFs-Based Heterostructures
2.2.1. Addition of Linkers
2.2.2. Modification of its Metal Clusters
3. Applications of MOFs and MOFs-Based Heterostructures
3.1. Photocatalytic Evolution of H2
3.2. Photocatalytic Degradation of Organic Pollutants
3.3. Photocatalytic Reduction of CO2
4. Photocatalytic Mechanisms of MOFs and MOFs-Based Heterostructures
4.1. Photocatalysis of Pristine MOFs Structures
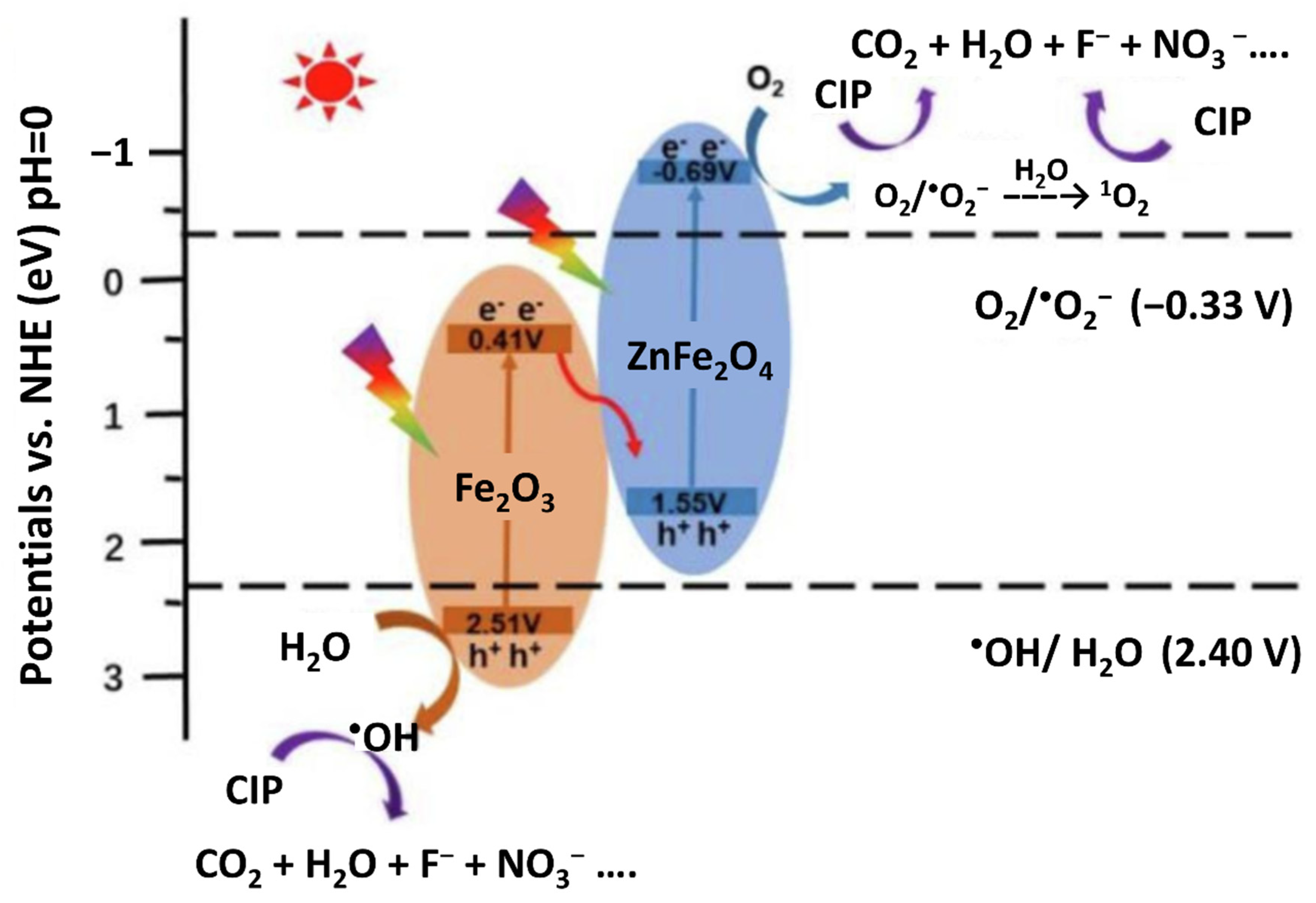
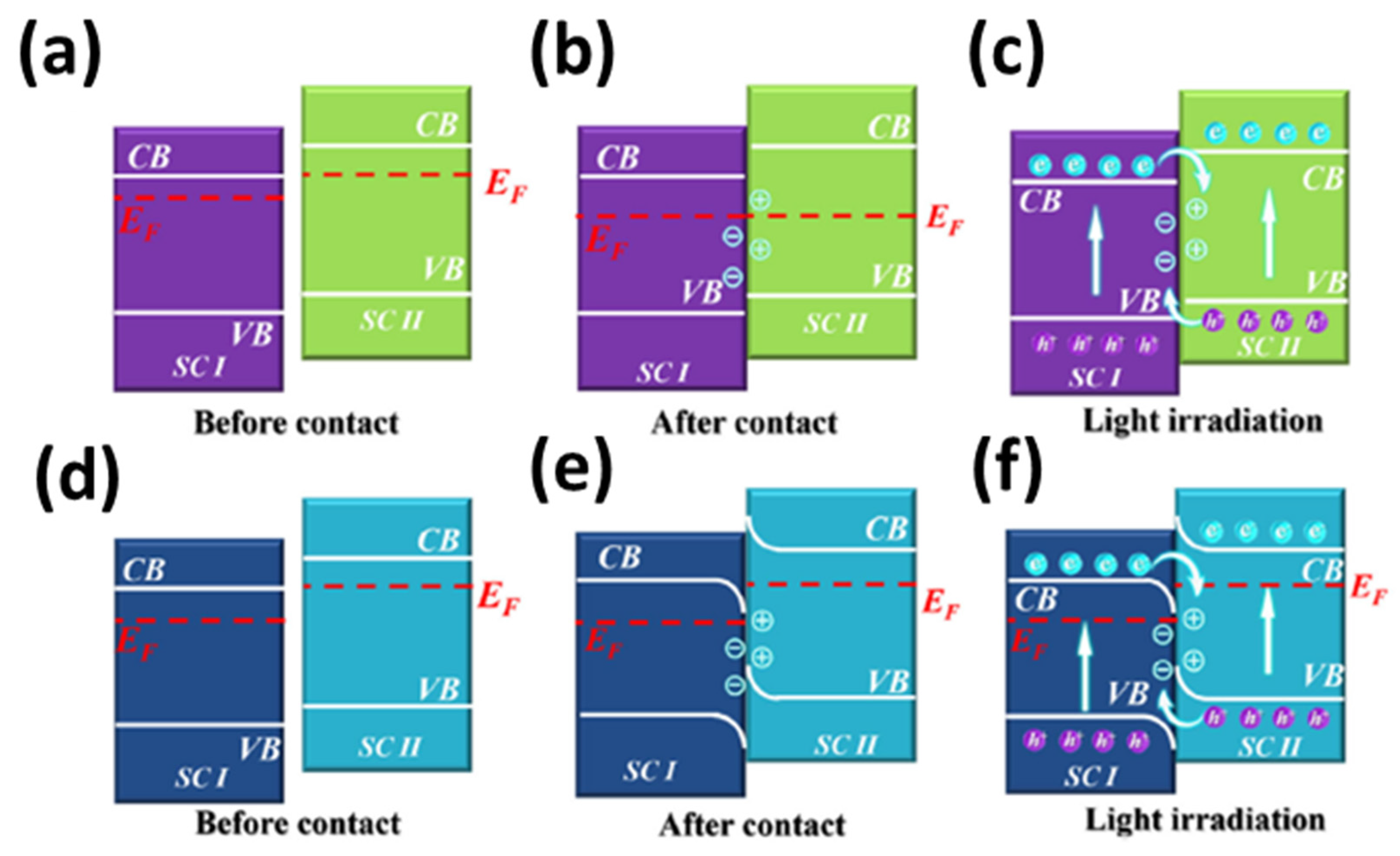
4.2. Photocatalysis of MOFs-Based Heterostructures
5. Future Outlook
- Firstly, most MOF synthesis methods require high production costs which may restrict their applications in various fields. Therefore, a simple and cost-effective synthesis method to prepare MOFs should be introduced;
- Recyclability, reusability, and stability of MOFs have not been discussed widely. These qualities are crucial for preparing MOFs with excellent properties;
- In-depth study of the physicochemical properties of these materials, such as light absorption in the visible region, electronic structure, crystallographic properties, porosity, and surface area, is highly required with the aid of advanced characterization techniques;
- More detailed studies to improve the stability of MOFs by in-situ growth of MOFs on wood should be carried out;
- Huge efforts should be exerted by carrying out more intensive research works on the determination of the MOFs photocatalytic mechanisms such as the Z-schemes and S-schemes since it is considered to be the key to developing and engineering photocatalysts with efficient charge separation and transport.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khan, M.M.; Pradhan, D.; Sohn, Y. Nanocomposites for Visible Light-Induced Photocatalysis. In Springer Series on Polymer and Composite Materials; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Min, B.K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1723. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Zinc Oxide and Zinc Oxide-Based Nanostructures. In Biogenic and Phytogenic Synthesis, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2021; Volume 44, pp. 1333–1372. [Google Scholar] [CrossRef]
- Matussin, S.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Plant-extract-mediated SnO2 nanoparticles: Synthesis and applications. ACS Sustain. Chem. Eng. 2020, 8, 3040–3054. [Google Scholar] [CrossRef]
- Naidi, S.N.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Green-Synthesized CeO2 Nanoparticles for photocatalytic, antimicrobial, antioxidant and cytotoxicity activities. J. Mater. Chem. B 2021, 9, 5599–5620. [Google Scholar] [CrossRef] [PubMed]
- Matussin, S.N.; Rahman, A.; Khan, M.M. Role of Anions in the Synthesis and Crystal Growth of Selected Semiconductors. Front. Chem. 2022, 10, 881518. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Green synthesis, photocatalytic and photoelectrochemical performance of an Au–Graphene nanocomposite. RSC Adv. 2015, 5, 26897–26904. [Google Scholar] [CrossRef]
- Mohammad, A.; Karim, M.R.; Khan, M.E.; Khan, M.M.; Cho, M.H. Biofilm-assisted fabrication of Ag@SnO2-g-C3N4 nanostructures for visible light-induced photocatalysis and photoelectrochemical performance. J. Phys. Chem. C 2019, 123, 20936–20948. [Google Scholar] [CrossRef]
- Rahman, A.; Khan, M.M. Chalcogenides as photocatalysts. New J. Chem. 2021, 45, 19622–19635. [Google Scholar] [CrossRef]
- Khan, M.M. Chalcogenide-Based Nanomaterials as Photocatalysts, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kolobov, N.; Goesten, M.G.; Gascon, J. Metal-organic frameworks: Molecules or semiconductors in photocatalysis? Angew. Chem. Int. Ed. 2021, 60, 26038–26052. [Google Scholar] [CrossRef]
- Hao, Y.-C.; Chen, L.-W.; Li, J.; Guo, Y.; Su, X.; Shu, M.; Zhang, Q.; Gao, W.-Y.; Li, S.; Yu, Z.-L.; et al. Metal-organic framework membranes with single-atomic centers for photocatalytic CO2 and O2 reduction. Nat. Commun. 2021, 12, 2682. [Google Scholar] [CrossRef]
- Chuhadiya, S.; Suthar, D.; Patel, S.L.; Dhaka, M.S. Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review. Coord. Chem. Rev. 2021, 446, 214115. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.-Q.; Kumar, A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Nemiwal, M.; Gosu, V.; Zhang, T.C.; Kumar, D. Metal organic frameworks as electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrog. Energy 2021, 46, 10216–10238. [Google Scholar] [CrossRef]
- Nordin, N.A.; Mohamed, M.A.; Salehmin, M.N.I.; Mohd Yusoff, S.F. Photocatalytic active metal-organic Framework and its derivatives for solar-driven environmental remediation and renewable energy. Coord. Chem. Rev. 2022, 468, 214639. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Hua, T.; Lan, X.; Han, S.; Cheng, J.; Du, K.-S.; Hu, Y.; Chen, Y. Micro/macrostructure and multicomponent design of catalysts by MOF-derived strategy: Opportunities for the application of nanomaterials-based advanced oxidation processes in wastewater treatment. Sci. Total Environ. 2022, 804, 150096. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing hydrogen storage in MOFs through engineering of crystal morphology and control of crystal size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef]
- Qiu, T.; Liang, Z.; Guo, W.; Tabassum, H.; Gao, S.; Zou, R. Metal-organic framework-based materials for energy conversion and storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef]
- Morshedy, A.S.; Abd El Salam, H.M.; El Naggar, A.M.A.; Zaki, T. Hydrogen production and in situ storage through process of water splitting using mono/binary metal-organic framework (MOF) structures as new chief photocatalysts. Energy Fuels 2020, 34, 11660–11669. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, S.; Huang, D.; Liu, Z.; Shao, B.; Liang, Q.; Wu, T.; Pan, Y.; Huang, J.; Liu, Y.; et al. Recent advances of Zr based metal organic frameworks photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2021, 448, 214177. [Google Scholar] [CrossRef]
- Liu, M.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in core—Shell metal organic frame-based photocatalysts for solar energy conversion. Coord. Chem. Rev. 2021, 446, 214123. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Liu, X.-Y.; Wang, X.-X.; Cao, M.-S. Metal-organic frameworks based photocatalysts: Architecture strategies for efficient solar energy conversion. Chem. Eng. J. 2021, 419, 129459. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Li, X.; Huo, P.; Shi, W. Design of Metal-organic frameworks (MOFs)-based photocatalyst for solar fuel production and photo-degradation of pollutants. Chin. J. Catal. 2021, 42, 872–903. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Hanikel, N.; Prévot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid cycling and exceptional yield in a metal-organic framework water harvester. ACS Cent. Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Huang, D.-D.; Wu, Y.-P.; Zhao, J.; Liu, X.; Dong, W.-W.; Li, S.; Li, D.-S.; Li, J.-R. In situ synthesis of nano CuS-embedded MOF hierarchical structures and application in dye adsorption and hydrogen evolution reaction. ACS Appl. Energy Mater. 2019, 2, 5698–5706. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, T.; Zhao, X.; Jiang, W.-J.; Pan, H.; Gao, D.; Xu, C. Expediting in-situ electrochemical activation of two-dimensional metal-organic frameworks for enhanced OER intrinsic activity by iron incorporation. ACS Catal. 2019, 9, 7356–7364. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A review for metal-organic frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Li, S.; Shan, S.; Chen, S.; Li, H.; Li, Z.; Liang, Y.; Fei, J.; Xie, L.; Li, J. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Adegboyega, S.A.; Giwa, A.-R.A. Remediation potentials of composite metal-organic frameworks (MOFs) for dyes as water contaminants: A comprehensive review of recent literatures. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100568. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.-M.; Yu, L. Construction of Z-scheme titanium-MOF/plasmonic silver nanoparticle/NiFe layered double hydroxide photocatalysts with enhanced dye and antibiotic degradation activity under visible light. Sep. Purif. Technol. 2022, 278, 119525. [Google Scholar] [CrossRef]
- Deng, Z.; Li, M.; Hu, Y.; He, Y.; Tao, B.; Yuan, Z.; Wang, R.; Chen, M.; Luo, Z.; Cai, K. Injectable biomimetic hydrogels encapsulating gold/metal-organic frameworks nanocomposites for enhanced antibacterial and wound healing activity under visible light actuation. Chem. Eng. J. 2021, 420, 129668. [Google Scholar] [CrossRef]
- Ghasempour, H.; ZareKarizi, F.; Morsali, A.; Yan, X.-W. Development of a highly porous Fe-based MOF using symmetrically incompatible building blocks: Selective oxidation of benzyl alcohols. Appl. Mater. Today 2021, 24, 101157. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Lu, G.; Chu, F.; Huang, X.; Li, Y.; Liang, K.; Wang, G. Recent advances in metal-organic frameworks-based materials for photocatalytic selective oxidation. Coord. Chem. Rev. 2022, 450, 214240. [Google Scholar] [CrossRef]
- Li, T.-T.; Dang, L.-L.; Zhao, C.-C.; Lv, Z.-Y.; Yang, X.-G.; Zhao, Y.; Zhang, S.-H. A self-sensitized Co (II)-MOF for efficient visible-light-driven hydrogen evolution without additional cocatalysts. J. Solid State Chem. 2021, 304, 122609. [Google Scholar] [CrossRef]
- Mahmoud Idris, A.; Jiang, X.; Tan, J.; Cai, Z.; Lou, X.; Wang, J.; Li, Z. Dye-sensitized Fe-MOF nanosheets as visible-light driven photocatalyst for high efficient photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 607, 1180–1188. [Google Scholar] [CrossRef]
- Tang, H.L.; Sun, X.J.; Zhang, F.M. Development of MOF-based heterostructures for photocatalytic hydrogen evolution. Dalton Trans. 2020, 49, 12136–12144. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, X.; Yang, N.; Zhang, F. Heterostructured MOFs photocatalysts for water splitting to produce hydrogen. J. Energy Chem. 2021, 58, 508–522. [Google Scholar] [CrossRef]
- Zhang, F.-M.; Dong, H.; Zhang, X.; Sun, X.-J.; Liu, M.; Yang, D.-D.; Liu, X.; Wei, J.-Z. Postsynthetic modification of ZIF-90 for potential targeted codelivery of two anticancer drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gualdron, D.A.; Gutov, O.V.; Krungleviciute, V.; Borah, B.; Mondloch, J.E.; Hupp, J.T.; Yildirim, T.; Farha, O.K.; Snurr, R.Q. Computational design of metal-organic frameworks based on stable zirconium building units for storage and delivery of methane. Chem. Mater. 2014, 26, 5632–5639. [Google Scholar] [CrossRef]
- Deria, P.; Gómez-Gualdrón, D.A.; Hod, I.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Framework-topology-dependent catalytic activity of zirconium-based (porphinato)zinc(II) MOFs. J. Am. Chem. Soc. 2016, 138, 14449–14457. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Chen, W.; Du, L.; Wu, C. Hydrothermal Synthesis of MOFs; Woodhead Publishing: Sawston, UK, 2020; pp. 141–157. [Google Scholar] [CrossRef]
- Denisov, G.L.; Primakov, P.V.; Korlyukov, A.A.; Novikov, V.V.; Nelyubina, Y.V. Solvothermal synthesis of the metal-organic framework MOF-5 in autoclaves prepared by 3D printing. Russ. J. Coord. Chem. Koord. Khimiya 2019, 45, 836–842. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, Y.; Li, J.; Kang, L.; Xie, Y.; Qiao, W.; Zhu, C.; Luo, H. Rapid room-temperature preparation of hierarchically porous metal-organic frameworks for efficient uranium removal from aqueous solutions. Nanomaterials 2020, 10, 1539. [Google Scholar] [CrossRef]
- Vakili, R.; Xu, S.; Al-Janabi, N.; Gorgojo, P.; Holmes, S.M.; Fan, X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): Optimization and gas adsorption. Microporous Mesoporous Mater. 2018, 260, 45–53. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Bellido, E.; Berthelot, T.; Simón-Yarza, T.; Hidalgo, T.; Simón-Vázquez, R.; González-Fernández, Á.; Avila, J.; Asensio, M.C.; Gref, R.; et al. GraftFast surface engineering to improve MOF nanoparticles furtiveness. Small 2018, 14, 1801900. [Google Scholar] [CrossRef]
- Xia, T.; Lin, Y.; Li, W.; Ju, M. Photocatalytic degradation of organic pollutants by MOFs based materials: A review. Chin. Chem. Lett. 2021, 32, 2975–2984. [Google Scholar] [CrossRef]
- Wang, X.R.; Huang, Z.; Du, J.; Wang, X.Z.; Gu, N.; Tian, X.; Li, Y.; Liu, Y.Y.; Huo, J.Z.; Ding, B. Hydrothermal preparation of five rare-earth (Re = Dy, Gd, Ho, Pr, and Sm) luminescent cluster-based coordination materials: The first MOFs-based ratiometric fluorescent sensor for lysine and bifunctional sensing platform for insulin and Al3+. Inorg. Chem. 2018, 57, 12885–12899. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Ghosh, D.; Chernyshev, V.V.; Dey, A.; Pradhan, D.; Biradha, K. 2D MOFs with Ni(II), Cu(II), and Co(II) as efficient oxygen evolution electrocatalysts: Rationalization of catalytic performance vs. structure of the MOFs and potential of the redox couples. ACS Appl. Mater. Interfaces 2020, 12, 33679–33689. [Google Scholar] [CrossRef] [PubMed]
- Remya, V.R.; Kurian, M. Synthesis and catalytic applications of metal-organic frameworks: A review on recent literature. Int. Nano Lett. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Saenz, N.; Reed, K.; Zavalij, P.; Mowery, J.; Bauchan, G. Facile and template-free solvothermal synthesis of mesoporous/macroporous metal-organic framework nanosheets. RSC Adv. 2018, 8, 33059–33064. [Google Scholar] [CrossRef] [PubMed]
- Virmani, E.; Rotter, J.M.; Mähringer, A.; von Zons, T.; Godt, A.; Bein, T.; Wuttke, S.; Medina, D.D. On-surface synthesis of highly oriented thin metal-organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 2018, 140, 4812–4819. [Google Scholar] [CrossRef]
- Stassin, T.; Rodríguez-Hermida, S.; Schrode, B.; Cruz, A.J.; Carraro, F.; Kravchenko, D.; Creemers, V.; Stassen, I.; Hauffman, T.; De Vos, D.; et al. Vapour-phase deposition of oriented copper dicarboxylate metal-organic framework thin films. Chem. Commun. 2019, 55, 10056–10059. [Google Scholar] [CrossRef]
- Kim, K.J.; Culp, J.T.; Ohodnicki, P.R.; Thallapally, P.K.; Tao, J. Synthesis of high-quality Mg-MOF-74 thin films via vapor-assisted crystallization. ACS Appl. Mater. Interfaces 2021, 13, 35223–35231. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Kim, J.; Kim, S.-N.; Ahn, W.-S. High yield 1-L scale synthesis of ZIF-8 via a sonochemical route. Microporous Mesoporous Mater. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-rela. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Reif, B.; Somboonvong, J.; Hartmann, M.; Kaspereit, M.; Schwieger, W. Synthesis of ZIF-11 membranes: The influence of preparation technique and support type. Membranes 2021, 11, 523. [Google Scholar] [CrossRef]
- Liu, J.; Wöll, C. Surface-supported metal-organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017, 46, 5730–5770. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.-T.; Choi, S.B.; Sim, J.; Kim, J.; Ahn, W.-S. Control of catenation in CuTATB-n metal-organic frameworks by sonochemical synthesis and its effect on CO2 adsorption. J. Mater. Chem. 2011, 21, 3070. [Google Scholar] [CrossRef]
- Mahendran, N.; Praveen, K. BiPO4/Fe-metal organic framework composite: A promising photocatalyst toward the abatement of tetracycline hydrochloride, indigo carmine and reduction of 4-nitrophenol. J. Ind. Eng. Chem. 2021, 100, 220–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhou, J.; Wang, L. Metal-organic framework-derived multifunctional photocatalysts. Chin. J. Catal. 2022, 43, 971–1000. [Google Scholar] [CrossRef]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, A.-F.; Cao, C.-S.; Zhao, B. Applications of MOFs: Recent advances in photocatalytic hydrogen production from water. Coord. Chem. Rev. 2019, 390, 50–75. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Santaclara, J.G.; Kapteijn, F.; Gascon, J.; van der Veen, M.A. Understanding metal-organic frameworks for photocatalytic solar fuel production. CrystEngComm 2017, 19, 4118–4125. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Su, R.; Lv, G.; Wang, Z.; Gao, B.; Zhou, W. Enhanced degradation of bisphenol F in a porphyrin-MOF based visible-light system under high salinity conditions. Chem. Eng. J. 2022, 428, 132106. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Guo, Q. CdSe QDs@ Fe-based metal organic framework composites for improved photocatalytic RhB degradation under visible Light. Microporous Mesoporous Mater. 2021, 324, 111291. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Q.; Chen, P.; Chen, L.; Ding, F.; Tang, J.; Li, Y.; Au, C.-T.; Yin, S.-F. Copper-mediated metal-organic framework as efficient photocatalyst for the partial oxidation of aromatic alcohols under visible-light irradiation: Synergism of plasmonic effect and schottky junction. Appl. Catal. B Environ. 2019, 248, 380–387. [Google Scholar] [CrossRef]
- Kamandi, R.; Mahmoodi, N.M.; Kazemeini, M. Graphitic carbon nitride nanosheet/metal-organic framework heterostructure: Synthesis and pollutant degradation using visible light. Mater. Chem. Phys. 2021, 269, 124726. [Google Scholar] [CrossRef]
- Hou, W.; Chen, M.; Chen, C.; Wang, Y.; Xu, Y. Increased production of H2 under visible light by packing CdS in a Ti, Zr-based metal organic framework. J. Colloid Interface Sci. 2021, 604, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.Y.; Chen, Y.; Liang, Y.N.; Gao, Y.H.; Shi, J.L.; Zhang, H.L.; Li, Y. Modifying Ag3VO4 with metal-organic frameworks for enhanced photocatalytic activity under visible light. Mater. Chem. Phys. 2020, 239, 122078. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal organic framework G-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- He, Y.; Wang, D.; Li, X.; Fu, Q.; Yin, L.; Yang, Q.; Chen, H. Photocatalytic degradation of tetracycline by metal-organic frameworks modified with Bi2WO6 nanosheet under direct sunlight. Chemosphere 2021, 284, 131386. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, D.; Shen, T.; Hou, X.; Zhu, M.; Liu, S.; Hu, Q. Titanium dioxide/magnetic metal-organic framework preparation for organic pollutants removal from water under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124484. [Google Scholar] [CrossRef]
- Xia, Q.; Huang, B.; Yuan, X.; Wang, H.; Wu, Z.; Jiang, L.; Xiong, T.; Zhang, J.; Zeng, G.; Wang, H. Modified stannous sulfide nanoparticles with metal-organic framework: Toward efficient and enhanced photocatalytic reduction of chromium (VI) under visible light. J. Colloid Interface Sci. 2018, 530, 481–492. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, S.; Zhao, Y.F.; Bedia, J.; Rodriguez, J.J.; Belver, C. UiO-66-based metal organic frameworks for the photodegradation of acetaminophen under simulated solar irradiation. J. Environ. Chem. Eng. 2021, 9, 106087. [Google Scholar] [CrossRef]
- Parnicka, P.; Lisowski, W.; Klimczuk, T.; Łuczak, J.; Żak, A.; Zaleska-Medynska, A. Visible-light-driven lanthanide-organic-frameworks modified TiO2 photocatalysts utilizing up-conversion effect. Appl. Catal. B Environ. 2021, 291, 120056. [Google Scholar] [CrossRef]
- Tang, T.; Jin, X.; Tao, X.; Huang, L.; Shang, S. Low-crystalline Ce-based bimetallic MOFs synthesized via DBD plasma for excellent visible photocatalytic performance. J. Alloy. Compd. 2022, 895, 162452. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Guo, W.; Wang, Z.; Shi, Y.; Yu, Y.; Wu, L. Functionalized UiO-66(Ce) for photocatalytic organic transformation: The role of active sites modulated by ligand functionalization. Catal. Sci. Technol. 2022, 12, 1812–1823. [Google Scholar] [CrossRef]
- Somnath; Ahmad, M.; Siddiqui, K.A. Synthesis of mixed ligand 3D cobalt MOF: Smart responsiveness towards photocatalytic dye degradation in environmental contaminants. J. Mol. Struct. 2022, 1265, 133399. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, X.; Ma, S.; Xia, X.; Shi, Y.; Yang, J. Preparation and application of bimetallic mixed ligand MOF photocatalytic materials. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128108. [Google Scholar] [CrossRef]
- Li, M.; Yuan, J.; Wang, G.; Yang, L.; Shao, J.; Li, H.; Lu, J. One-step construction of Ti-in bimetallic MOFs to improve synergistic effect of adsorption and photocatalytic degradation of bisphenol A. Sep. Purif. Technol. 2022, 298, 121658. [Google Scholar] [CrossRef]
- Han, W.; Shao, L.-H.; Sun, X.-J.; Liu, Y.-H.; Zhang, F.-M.; Wang, Y.; Dong, P.-Y.; Zhang, G.-L. Constructing Cu ion sites in MOF/COF heterostructure for noble-metal-free photoredox catalysis. Appl. Catal. B Environ. 2022, 317, 121710. [Google Scholar] [CrossRef]
- Ye, Z.; Feng, S.; Wu, W.; Zhou, Y.; Wang, Y.; Dai, X.; Cao, X. Synthesis of double MOFs composite material for visible light photocatalytic degradation of tetracycline. Solid State Sci. 2022, 127, 106842. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A. Chemosphere 2020, 243, 125378. [Google Scholar] [CrossRef]
- Guo, J.; Liang, Y.; Liu, L.; Hu, J.; Wang, H.; An, W.; Cui, W. Noble-metal-free CdS/Ni-MOF composites with highly efficient charge separation for photocatalytic H2 evolution. Appl. Surf. Sci. 2020, 522, 146356. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Li, Y.; Xu, X.; Du, Y.; Jiang, Y.; Lin, K. A novel heterostructure coupling MOF-derived fluffy porous indium oxide with g-C3N4 for enhanced photocatalytic activity. Mater. Res. Bull. 2021, 133, 111078. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Liu, C.; Zhang, Y.; Meng, X.; Dai, L.; Wang, W.; Yu, H.; Ni, Y. A self-cleaning and photocatalytic cellulose-fiber-supported “Ag@AgCl@MOF-Cloth” membrane for complex wastewater remediation. Carbohydr. Polym. 2020, 247, 116691. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, J.; Mao, M.; Li, L.; Li, X. Protonated G-C3N4 cooperated with Co-MOF doped with Sm to construct 2D/2D heterojunction for integrated dye-sensitized photocatalytic H2 evolution. J. Colloid Interface Sci. 2021, 583, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, B.; Li, X.; Wang, X.; Huang, K.; Chen, Z. MOF-derived magnetically recoverable Z-scheme ZnFe2O4/Fe2O3 perforated nanotube for efficient photocatalytic ciprofloxacin removal. Chem. Eng. J. 2022, 430, 132728. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Peng, D.; Sheng, D.; Zhang, Y.; Tian, Y.; Ma, D. MOF derived carbon modified porous TiO2 mixed-phase junction with efficient visible-light photocatalysis for cyclohexane oxidation. Mater. Res. Bull. 2021, 146, 111602. [Google Scholar] [CrossRef]
- Xia, Z.; Shi, B.; Zhu, W.; Lü, C. Temperature-responsive polymer-tethered Zr-porphyrin MOFs encapsulated carbon dot nanohybrids with boosted visible-light photodegradation for organic contaminants in water. Chem. Eng. J. 2021, 426, 131794. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, R.; Lou, J.; Yuan, J.; Xu, J.; Fan, X. Novel marigold-like CuO@Cu-Based MOFs composite photocatalyst for high-performance removal of alkylphenol ethoxylate under visible light. J. Environ. Chem. Eng. 2021, 9, 106434. [Google Scholar] [CrossRef]
- Prakash Tripathy, S.; Subudhi, S.; Das, S.; Kumar Ghosh, M.; Das, M.; Acharya, R.; Acharya, R.; Parida, K. Hydrolytically stable citrate capped Fe3O4@UiO-66-NH2 MOF: A hetero-structure composite with enhanced activity towards Cr (VI) adsorption and photocatalytic H2 evolution. J. Colloid Interface Sci. 2022, 606, 353–366. [Google Scholar] [CrossRef]
- Thi, Q.V.; Tamboli, M.S.; Thanh Hoai Ta, Q.; Kolekar, G.B.; Sohn, D. A nanostructured MOF/reduced graphene oxide hybrid for enhanced photocatalytic efficiency under solar Light. Mater. Sci. Eng. B 2020, 261, 114678. [Google Scholar] [CrossRef]
- Tang, B.; Dai, Y.; Sun, Y.; Chen, H.; Wang, Z. Graphene and MOFs co-modified composites for high adsorption capacity and photocatalytic performance to remove pollutant under both UV- and visible-light irradiation. J. Solid State Chem. 2020, 284, 121215. [Google Scholar] [CrossRef]
- Qian, J.-F.; Yue, H.-D.; Qiu, P.-X.; Liang, Q.; Hang, M.-T.; He, M.-Y.; Bu, Y.-F.; Chen, Q.; Zhang, Z.-H. Anions mediated amino-type Cd-MOFs catalysts for efficient photocatalytic hydrogen evolution. J. Solid State Chem. 2021, 304, 122632. [Google Scholar] [CrossRef]
- Peña-Velasco, G.; Hinojosa-Reyes, L.; Morán-Quintanilla, G.A.; Hernández-Ramírez, A.; Villanueva-Rodríguez, M.; Guzmán-Mar, J.L. Synthesis of heterostructured catalyst coupling MOF derived Fe2O3 with TiO2 for enhanced photocatalytic activity in anti-inflammatory drugs mixture degradation. Ceram. Int. 2021, 47, 24632–24640. [Google Scholar] [CrossRef]
- Hou, C.; Chen, W.; Fu, L.; Zhang, S.; Liang, C.; Wang, Y. Efficient degradation of perfluorooctanoic acid by electrospun lignin-based bimetallic MOFs nanofibers composite membranes with peroxymonosulfate under solar light irradiation. Int. J. Biol. Macromol. 2021, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, F.; Lin, R.; Wang, J.; Li, C.; Li, Z.; Jiang, J.; Xiong, Y. Enabling photocatalytic hydrogen production over Fe-based MOFs by refining band structure with dye sensitization. Chem. Eng. J. 2022, 429, 132217. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, C.; Hu, C.; Liu, B. Facile fabrication of BiOIO3/MIL-88B heterostructured photocatalysts for removal of pollutants under visible light irradiation. J. Colloid Interface Sci. 2022, 607, 595–606. [Google Scholar] [CrossRef]
- Pattappan, D.; Kavya, K.V.; Vargheese, S.; Kumar, R.T.R.; Haldorai, Y. Graphitic carbon Nitride/NH2-MIL-101(Fe) composite for environmental remediation: Visible-light-assisted photocatalytic degradation of acetaminophen and reduction of hexavalent chromium. Chemosphere 2022, 286, 131875. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Rao, Z.; Tang, Z.; Shi, G.; Wang, Y.; Lu, G.; Xie, X.; Chen, D.; Sun, J. One-pot synthesis of the MIL-100 (Fe) MOF/MOX homojunctions with tunable hierarchical pores for the photocatalytic removal of BTXS. Appl. Catal. B Environ. 2022, 303, 120885. [Google Scholar] [CrossRef]
- Abdollahi, B.; Najafidoust, A.; Abbasi Asl, E.; Sillanpaa, M. Fabrication of ZiF-8 metal organic framework (MOFs)-based CuO-ZnO photocatalyst with enhanced solar-light-driven property for degradation of organic dyes. Arab. J. Chem. 2021, 14, 103444. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wu, G.; Guo, J.; Yin, X.; Mu, M. Construction of 2D Co-TCPP MOF decorated on B-TiO2−X nanosheets: Oxygen vacancy and 2D-2D heterojunctions for enhancing visible Light-driven photocatalytic degradation of bisphenol A. J. Environ. Chem. Eng. 2021, 9, 106723. [Google Scholar] [CrossRef]
- Qiu, J.-L.; Su, J.; Muhammad, N.; Zheng, W.-T.; Yue, C.-L.; Liu, F.-Q.; Zuo, J.-L.; Ding, Z.-J. Facile encapsulating Ag nanoparticles into a tetrathiafulvalene-based Zr-MOF for enhanced photocatalysis. Chem. Eng. J. 2022, 427, 131970. [Google Scholar] [CrossRef]
- Xu, M.; Sun, C.; Zhao, X.; Jiang, H.; Wang, H.; Huo, P. Fabricated hierarchical CdS/Ni-MOF heterostructure for promoting photocatalytic reduction of CO2. Appl. Surf. Sci. 2022, 576, 151792. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Alharbi, N.S.; Chen, C. Fabrication of a novel Co/Ni-MOFs@BiOI composite with boosting photocatalytic degradation of methylene blue under visible light. J. Environ. Chem. Eng. 2021, 9, 106194. [Google Scholar] [CrossRef]
- Govindaraju, S.; Arumugasamy, S.K.; Chellasamy, G.; Yun, K. Zn-MOF decorated bio activated carbon for photocatalytic degradation, oxygen evolution and reduction catalysis. J. Hazard. Mater. 2022, 421, 126720. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.Z.; Yang, Z.; Khalil, A.M.E.; Hussain, S.; Awan, S.U.; Jia, Q.; Fischer, R.A.; Zhu, Y.; Xia, Y. Metal-organic framework derived multi-functionalized and co-doped TiO2/C nanocomposites for excellent visible-light photocatalysis. J. Mater. Sci. Technol. 2022, 101, 49–59. [Google Scholar] [CrossRef]
- Sonowal, K.; Nandal, N.; Basyach, P.; Kalita, L.; Jain, S.L.; Saikia, L. Photocatalytic reduction of CO2 to methanol using Zr (IV)-based MOF composite with g-C3N4 quantum dots under visible light irradiation. J. CO2 Util. 2022, 57, 101905. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Kim, K.-H.; Kwon, E.E.; Younis, S.A. Metal-organic frameworks for photocatalytic detoxification of chromium and uranium in water. Coord. Chem. Rev. 2021, 447, 214148. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Yao, J.; Gao, J. Recent progress in 2D metal-organic framework photocatalysts: Synthesis, photocatalytic mechanism and applications. J. Phys. Energy 2021, 3, 032010. [Google Scholar] [CrossRef]
- Yu, J.; Park, J.; Van Wyk, A.; Rumbles, G.; Deria, P. Excited-state electronic properties in Zr-based metal-organic frameworks as a function of a topological network. J. Am. Chem. Soc. 2018, 140, 10488–10496. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Z.; Yu, G.; Wu, H.; Chen, H.; Zhou, L.; Zhang, Y.; Su, Y.; Tan, S.; Yang, L.; et al. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 2021, 272, 129501. [Google Scholar] [CrossRef]
- Mukherjee, D.; van der Bruggen, B.; Mandal, B. Advancements in visible light responsive MOF composites for photocatalytic decontamination of textile wastewater: A review. Chemosphere 2022, 295, 133835. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-D.; Jiang, H.-L. Metal-organic frameworks for photocatalysis and photothermal catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Kwon, E.E.; Qasim, M.; Kim, K.-H.; Kim, T.; Kukkar, D.; Dou, X.; Ali, I. Metal-organic framework as a photocatalyst: Progress in modulation strategies and environmental/energy applications. Prog. Energy Combust. Sci. 2020, 81, 100870. [Google Scholar] [CrossRef]
- Ramyashree, M.S.; Shanmuga Priya, S.; Freudenberg, N.C.; Sudhakar, K.; Tahir, M. Metal-organic framework-based photocatalysts for carbon dioxide reduction to methanol: A review on progress and application. J. CO2 Util. 2021, 43, 101374. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. A Critical review in recent developments of metal-organic-frameworks (MOFs) with band engineering alteration for photocatalytic CO2 reduction to solar fuels. J. CO2 Util. 2021, 43, 101381. [Google Scholar] [CrossRef]
- Sun, D.; Ye, L.; Li, Z. Visible-light-assisted aerobic photocatalytic oxidation of amines to imines over NH2-MIL-125(Ti). Appl. Catal. B Environ. 2015, 164, 428–432. [Google Scholar] [CrossRef]
- Chen, S.; Yang, F.; Gao, H.; Wang, J.; Chen, X.; Zhang, X.; Li, J.; Li, A. Construction of dual ligand Ti-based MOFs with enhanced photocatalytic CO2 reduction performance. J. CO2 Util. 2021, 48, 101528. [Google Scholar] [CrossRef]
- Behera, P.; Subudhi, S.; Tripathy, S.P.; Parida, K. MOF derived nano-materials: A recent progress in strategic fabrication, characterization and mechanistic insight towards divergent photocatalytic applications. Coord. Chem. Rev. 2022, 456, 214392. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, W.; Liu, N.; Huang, H.T.; Karuppasamy, L.; Yang, H.J.; Liu, C.H.; Wu, J.J. Synthesis of MOF/MoS2 composite photocatalysts with enhanced photocatalytic performance for hydrogen evolution from water splitting. Int. J. Hydrog. Energy, 2021; in press. [Google Scholar] [CrossRef]
- Khosroshahi, N.; Bakhtian, M.; Safarifard, V. Mechanochemical synthesis of ferrite/MOF nanocomposite: Efficient photocatalyst for the removal of meropenem and hexavalent chromium from water. J. Photochem. Photobiol. A Chem. 2022, 431, 114033. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, G.; Liu, H.; Li, Y.; Jin, Z.; Ma, Q. Regular octahedron Cu-MOFs modifies Mn0.05Cd0.95S nanoparticles to form a S-scheme heterojunction for photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 7230–7240. [Google Scholar] [CrossRef]
- Bao, Y.; Song, S.; Yao, G.; Jiang, S. S-scheme photocatalytic systems. Solar RRL 2021, 5, 2100118. [Google Scholar] [CrossRef]
- Ma, X.; Xiong, Y.; Liu, Y.; Han, J.; Duan, G.; Chen, Y.; He, S.; Mei, C.; Jiang, S.; Zhang, K. When MOFs meet wood: From opportunities toward applications. Chem, 2022; in press. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
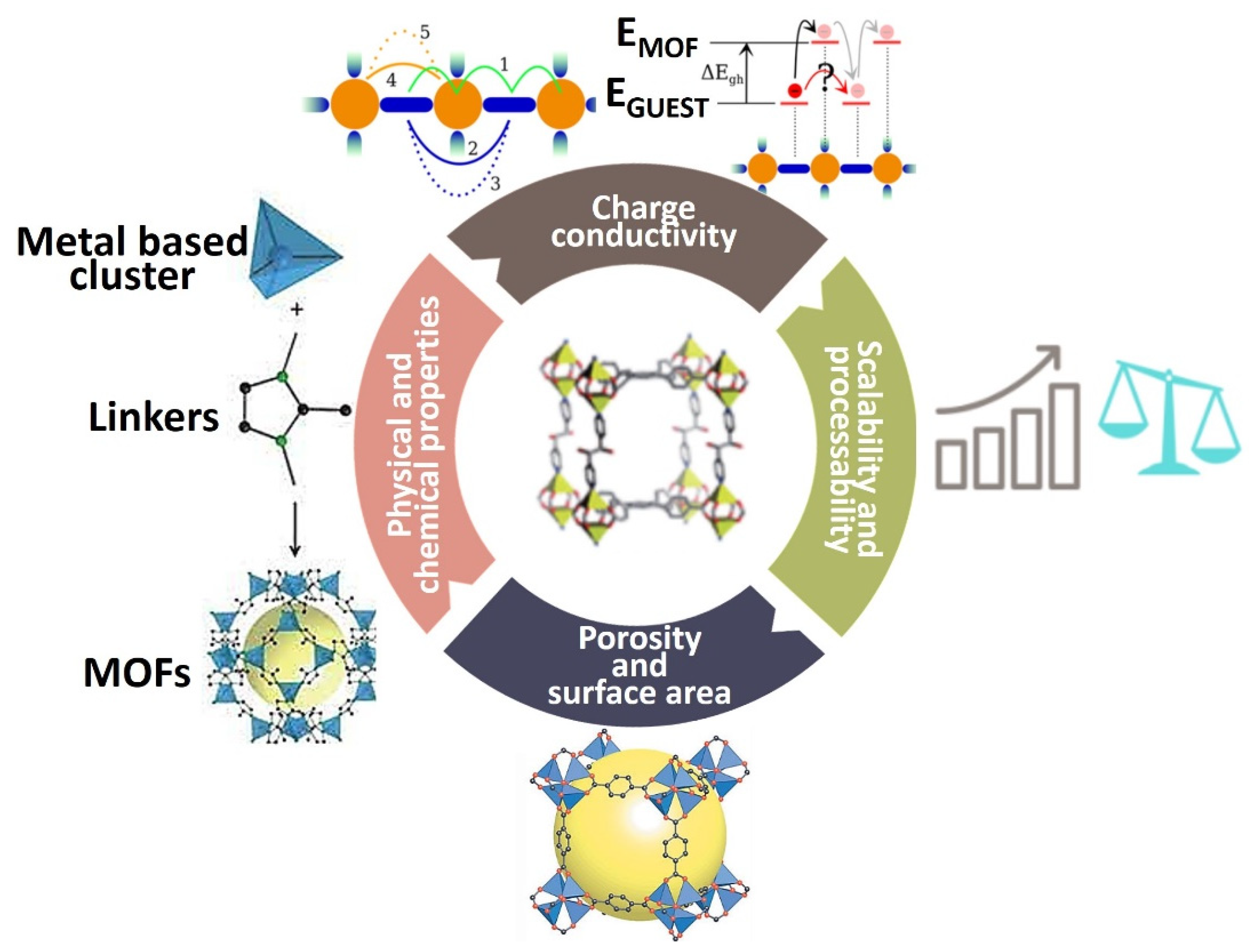
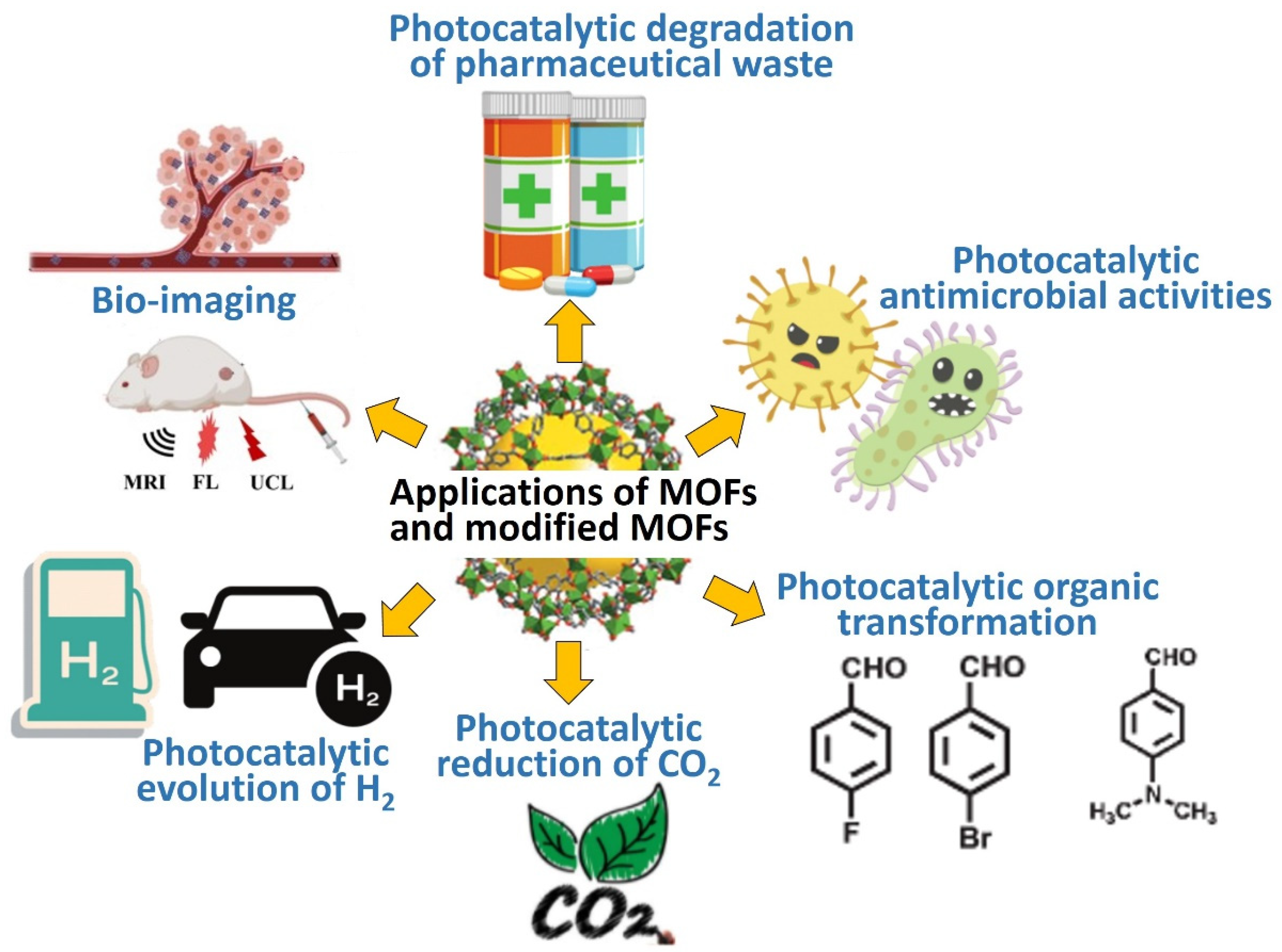

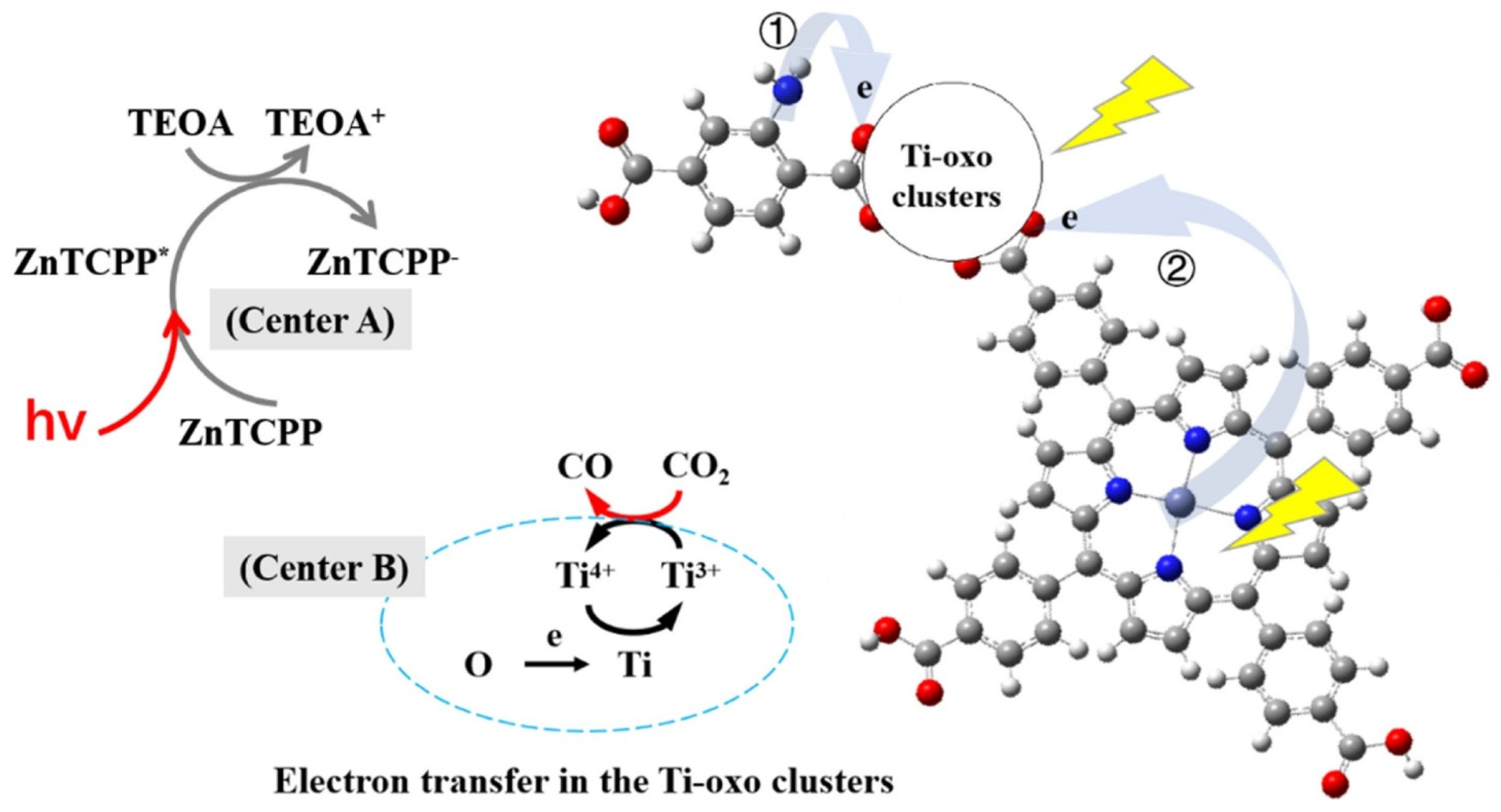
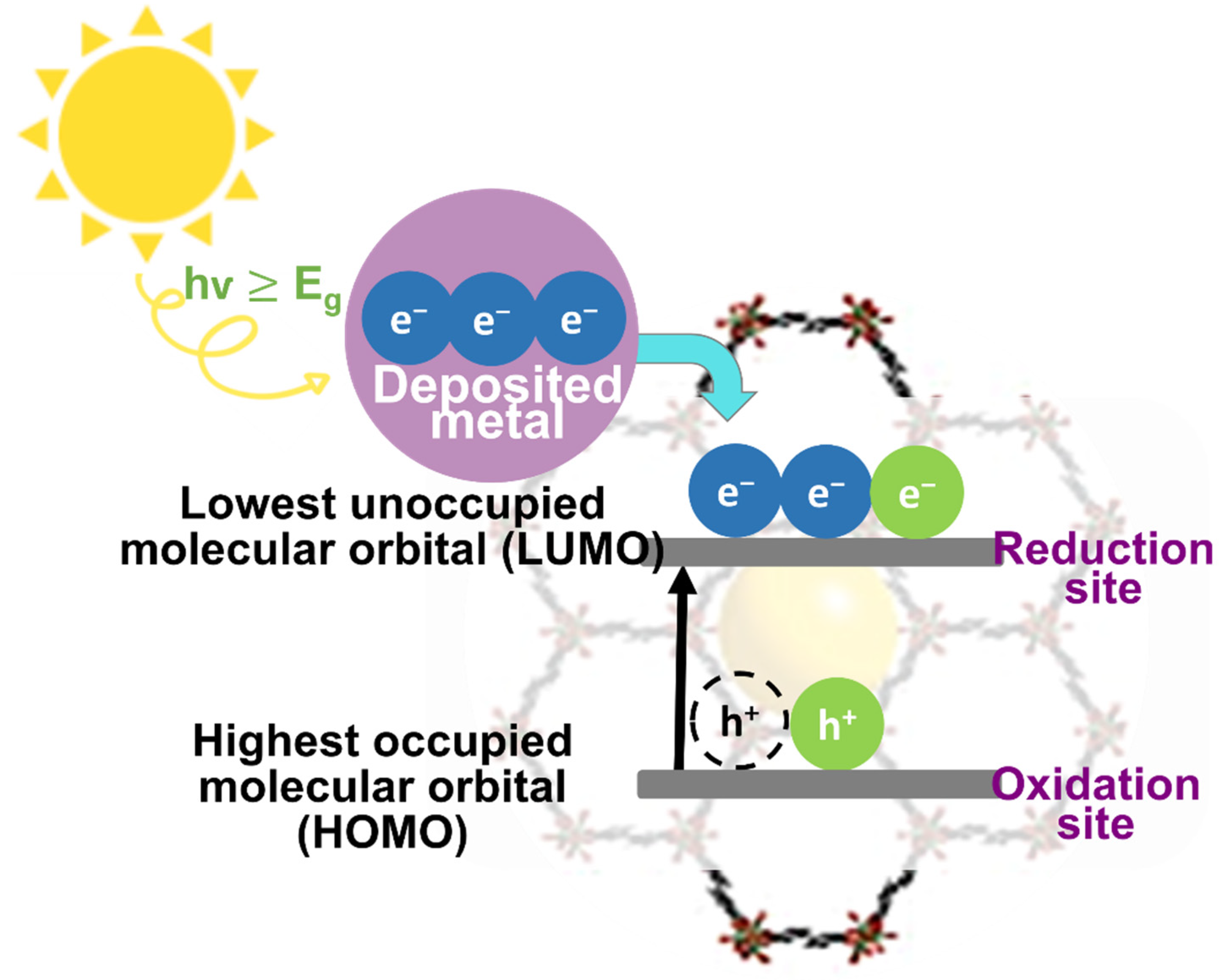
| No. | Materials | Synthesis Methods | Average Particle Size | Ref. |
|---|---|---|---|---|
| 1 | PEGylated iron trimesate (materials institute Lavoisier) MIL-100 (Fe) | Hydrothermal | 129–174 nm | [52] |
| 2 | Rare-earth 3D cluster-based MOFs | Hydrothermal | 0.4–2.7 μm | [54] |
| 3 | 2D-MOFs with Ni(II), Cu(II), and Co(II) | Hydrothermal | - | [55] |
| 4 | Zn4O(BDS)3 MOFs | Solvothermal | 807 nm | [49] |
| 5 | Cu(II)-based MOFs | Solvothermal | 50 nm | [57] |
| 6 | Zr-based MOFs | Microwave | Less than 100 nm | [51] |
| 7 | Cu-BTC MOFs | Room temperature template method | - | [50] |
| 8 | UiO-66 MOFs | Vapor-assisted | 200 nm | [58] |
| 9 | Copper dicarboxylate MOFs | Chemical vapor deposition | - | [59] |
| 10 | Zn-based MOFs | Sonochemical | - | [61] |
| No. | MOFs-Based Heterostructures | Synthesis Method | Band Gap Energy | Photocatalytic Activities | Photocatalytic Performance | Ref. |
|---|---|---|---|---|---|---|
| 1 | BiPO4/Fe-metal organic framework composite | Solvothermal method | Pristine NH2-MIL-53(Fe): 2.11 eV BiPO4: 3.75 eV | Photocatalytic degradation of tetracycline hydrochloride, Indigo Carmine and reduction of 4-nitrophenol | Tetracycline hydrochloride: degraded 80% within 120 min Indigo Carmine: degraded 94% within 120 min 4-nitrophenol: 95% reduction efficiency within 12 min | [66] |
| 2 | CdSe QDs@ Fe-based metal-organic framework composites | Heated to reflux in oil bath | CdSe: 1.97 eV Fe-BDC: 2.71 eV | Photocatalytic degradation of rhodamine B | Almost completely degraded ~99.8% after 240 min | [75] |
| 3 | Copper-mediated metal-organic framework | Advanced double-solvent approach followed by one-step reduction | - | Photocatalytic oxidation of aromatic alcohols | Up to 69.4% conversion | [76] |
| 4 | Graphitic carbon nitride nanosheet/metal-organic framework | Hydrothermal | g-C3N4: 2.72 eV MIL-101(Fe): 2.55 eV g-C3N4/MIL-101(Fe): 2.05 eV | Photocatalytic degradation of rhodamine B | MIL-101(Fe)/g-C3N4: 99.3% Bare g-C3N4: 40% Bare MIL-101(Fe): 73% | [77] |
| 5 | CdS in a Ti, Zr-based metal-organic framework | Stepwise precipitation | P415: 3.47 eV P415-NH2: 2.30 eV CdS: 2.41 eV | Photocatalytic production of H2 | The rate of H2 production: 5.8 μmol min−1g−1 | [78] |
| 6 | Hydrogels encapsulating Gold/metal-organic frameworks nanocomposites | Schiff base reaction and radical polymerization | ZIF-8: 3.19 eV | Photocatalytic antibacterial and wound healing activities | Au@ZIF-8@GCOA: up to 99.1% for E. coli and 99.6% for S. aureus after 20 min of irradiation | [35] |
| 7 | Modified Ag3VO4 with metal-organic frameworks | Facile two-step method | Ag3VO4: 2.0 eV 20%AZ: 2.11 eV 40%AZ: 2.21 eV 60%AZ: 2.25 eV 80%AZ: 2.83 eV ZIF-8: 5.06 eV | Photocatalytic degradation of rhodamine B | 60%AZ composites exhibited the highest, about 4.2 times of pure Ag3VO and 22.8 times of bare ZIF-8, respectively | [79] |
| 8 | Metal-organic framework g-C3N4/MIL-53(Fe) heterojunctions | Solvothermal method | MIL-53(Fe): 2.72 eV CMFe-3: 2.51 eV | Photocatalytic reduction of Cr(VI) | g-C3N4/MIL-53(Fe) showed about 2.1 and 2.0 times higher photocatalytic efficiency for the reduction of Cr(VI) in comparison to pure g-C3N4 and MIL-53(Fe), respectively. | [80] |
| 9 | Metal-organic frameworks modified with Bi2WO6 nanosheet | Hydrothermal method | - | Photocatalytic degradation of tetracycline | 12%MIL/BWO achieved the highest removal efficiency of about 92.4% within 120 min | [81] |
| 10 | Titanium dioxide/magnetic metal-organic framework | Hydrothermal method | TiO2: 3.1 eV TiO2/magnetic MIL-101(Cr): 1.61 eV | Photocatalytic degradation of acid red 1 | TiO2/magnetic MIL-101(Cr) showed 90% degradation of acid red 1 | [82] |
| 11 | Modified stannous sulfide nanoparticles with a metal-organic framework | Deposition method at room temperature | pure MIL-53(Fe): 1.6 eV SnS: 0.87 eV | Photocatalytic reduction of chromium (VI) | 71.3% of Cr(VI) removal is achieved after 60 min | [83] |
| 12 | UiO-66-based metal-organic frameworks | Solvothermal method | UiO-66: 3.82 eV UiO-66-NH2: 2.63 eV UiO-66-(OH)2: 2.50 eV | Photocatalytic degradation of acetaminophen | 90% after 6 h | [84] |
| 13 | Lanthanide-organic-frameworks modified TiO2 | Solvothermal method | TiO2: ~3.2 eV Ln(ndc) MOF: ~2.9 eV | Photocatalytic degradation of phenol | 87.5% after 60 min | [85] |
| 14 | Fe/Ce-based bimetallic MOF | Dielectric barrier discharge plasma | Ce-MOF: 3.01 eV Fe/Ce-MOF-1: 1.90 eV Fe/Ce-MOF-2: 1.97 eV Fe/Ce-MOF-3: 1.75 eV | Photocatalytic degradation of methyl orange | Fe/Ce-MOF-2 could degrade 93% methyl orange in 30 min under visible light | [86] |
| No. | Synthesized Materials | Band Gap Energy | Applications | Source of Light | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 1 | Sm doped 2D Co-MOF/ protonated-g-C3N4 | g-C3N4: 2.82 eV Protonated-g-C3N4: 2.70 eV 2D Co-MOF: 3.12 eV | Photocatalytic evolution of hydrogen | 300 W Xe lamp with an AM 1.5G filter | The H2 evolution rate of 5% Sm doped with the highest activity has reached 73.42 μmolh−1. | [98] |
| 2 | MOF-derived magnetically recoverable ZnFe2O4/Fe2O3 perforated nanotube | Fe2O3: 2.10 eV ZnFe2O4: 2.24 eV | Photocatalytic removal of ciprofloxacin | 300 W Xe lamp with AM 1.5 filter | ZFF-2 exhibits the best photocatalytic ciprofloxacin degradation performance with a degradation percentage of 96.5% and a TOC removal percentage up to 89% under light irradiation for 180 min | [99] |
| 3 | MOF derived carbon modified porous TiO2 | C-TiO2-3: 2.45 eV C-TiO2-4: 2.85 eV C-TiO2-5: 2.94 eV C-TiO2-6: 2.37 eV C-TiO2-7: 2.93 eV | Photocatalytic oxidation of cyclohexane | 100 W mercury lamp with a UV cutoff filter (λ ≥ 420 nm) | C-TiO2-4 catalyst, the yield of cyclohexanol and cyclohexanone could reach 13.18 and 107.07 μmol respectively, and the selectivity to cyclohexanone reaches 89.0%. | [100] |
| 4 | Polymer-tethered Zr-porphyrin MOFs encapsulated carbon dot nanohybrids | PCN-222: 1.75 eV Citric acid derived QDs@PCN-222: 1.64 eV Nitrogen-doped CDs@PCN-222: 1.59 eV Sulfur- doped CDs@PCN-222: 1.66 eV | Photocatalytic degradation of rhodamine B and tetracycline | 300 W UV lamp with a 420 filter | The removal efficiency of rhodamine B and tetracycline reached almost 100% and 90.93%, respectively under 20 min visible light irradiation | [101] |
| 5 | Marigold-like CuO@Cu-based MOFs composite | CuO: 1.41 eV Cu-H3BTC MOF: 2.0 eV | Photocatalytic removal of alkylphenol ethoxylate | 300 W Xe lamp with a 420 nm cut-off filter | CuO@Cu-H3BTC MOF composite exhibited up to 79% removal of alkylphenol polyethoxylate within 120 min of visible light irradiation | [102] |
| 6 | Citrate capped Fe3O4@ UiO-66-NH2 MOF | Citrate capped Fe3O4: 1.45 eV UiO-66-NH2: 2.67 eV | Adsorption of Cr (VI) and photocatalytic evolution of H2 | 300 W Xe lamp | MU-2 showed a maximum monolayer adsorption capacity of 743 mg g−1 which followed pseudo-second-order kinetics. In addition, the synthesized composite material displayed enhanced activity towards photocatalytic H2 evolution with a maximum evolution rate of 417 μmolh−1 with an apparent conversion efficiency of 3.12%. | [103] |
| 7 | MOF/ reduced graphene oxide hybrid | Not calculated | Photocatalytic degradation of methylene blue, rhodamine B and methyl orange | Solar simulator with wavelengths in the range of 300–1900 nm | MOF-5@rGO photocatalytic degradation efficiency reached 93% after 20 min illumination | [104] |
| 8 | ZIF-8, UiO-66 and Cu-BTC are adopted to combine with rGO and TiO2 | Uio-66-rGO/TiO2: 2.6 eV Cu-BTC-rGO/TiO2: 2.80 eV ZIF-8-rGO/TiO2: 2.90 eV | Photocatalytic degradation of rhodamine B | 500 W Xe lamp and a cutoff filter (λ > 400 nm) | Degradation rate constants of rhodamine B for UiO-66-RGO/TiO2 reached 1.65 × 10−1min−1 and 1.12 × 10−1 min−1 under UV- and visible-light irradiation, respectively. | [105] |
| 9 | Cd-MOFs | Not calculated | Photocatalytic evolution of H2 | 300 W Xe lamp | The highest H2 production rate reached 17,242 μmolg−1 h−1 | [106] |
| 10 | Co/Ni-MOFs@ BiOI composite | Not calculated | Photocatalytic degradation of crystal violet, rhodamine 6G, malachite green, Congo red and methyl orange | 300 W PLS- SXE with a cut-off filter (λ = 420 nm) | The removal efficiency of Co/[email protected]% followed the order of MG > MB > MO > CR > CV > R6G, and the degradation rate constants (k, min−1) were 0.04705, 0.00627, 0.00681, 0.00695, 0.00347, 0.00066, respectively. | [91] |
| 11 | MIL-101(Fe) derived Fe2O3 with TiO2 | Fe2O3: 1.7–2.0 eV TiO2: 3.17 eV FeTi-3%: 2.94 eV | Photocatalytic degradation of a mixture of nonsteroidal anti-inflammatory drugs namely ibuprofen and naproxen | Philips 25 W/m2, λ = 365–700 nm | The material exhibited up to 91% and 100% degradation of ibuprofen and naproxen within 240 and 15 min of reaction, respectively. | [107] |
| 12 | Ti-MOF/ plasmonic Ag nanoparticle/ NiFe layered double hydroxide | Ti-MOF: 2.53 eV NiFeLDH: 2.19 eV Ti-MOF/ NiFeLDH: 2.41 eV Ti-MOF/Ag/ NiFeLDH-1: 2.17 eV Ti-MOF/Ag/ NiFeLDH-2: 2.09 eV Ti-MOF/Ag/ NiFeLDH-3: 2.12 eV Ti-MOF/Ag/ NiFeLDH-4: 2.15 eV | Photocatalytic degradation of antibiotics, levofloxacin and rhodamine B dye | 300 W Xe With a cut-off filter of λ > 420 nm | Ti-MOF/Ag/NiFeLDH composite displayed outstanding photocatalytic degradation up to 95% for rhodamine B removal within 50 min and 92% levofloxacin degradation efficiency in 70 min | [34] |
| 13 | Dye sensitized Fe-MOF nanosheets | Not calculated | Photocatalytic reduction of CO2 | 300 W Xe lamp with a 420 nm cut-off filter | The synthesized material exhibited a significant photocatalytic CO production rate of 1120 μmol g−1 h−1 | [40] |
| 14 | Lignin-based bimetallic MOFs nanofibers composite membranes with peroxymonosulfate | 2.66 eV | Photocatalytic degradation of perfluorooctanoic acid | 9 W UV lamp with the wavelength of photoexcitation is 185 nm and under solar light | Lignin/PVA/bimetallic-MOFs showed outstanding performance up to 89.6% degradation of perfluorooctanoic acid within 3 h | [108] |
| 15 | Pt photo deposited on MIL-53(Fe) | Not calculated | Photocatalytic evolution of H2 | 300 W Xe lamp | Deposition of Pt nanoparticles on Fe-MOFs can lower the overpotential for H2 evolution toward further enhanced photocatalytic activity. | [109] |
| 16 | BiOIO3/ MIL-88B | BiOIO3: 2.88 eV MIL-88B: 2.31 eV BMIL-5: 2.48 eV | Photocatalytic degradation of Reactive Blue 19 and tetracycline hydrochloride | 300 W Xe lamp | The BiOIO3/MIL-88B composites exhibited an excellent removal rate for Reactive Blue 19 and tetracycline hydrochloride under visible light irradiation, which was approximately 3.28 and 4.22 times higher than the pristine BiOIO3, respectively. | [110] |
| 17 | Porphyrin- Zr MOF | Not calculated | Photocatalytic degradation of bisphenol F | 500 W Xe lamp with a 420 nm cut off filter | The material could achieve over 78% BPF removal (with/without salt) after 8 cycles. | [83] |
| 18 | Graphitic carbon nitride/NH2-MIL-101(Fe) | Fe-MOF: 1.64 eV g-C3N4: 2.77 eV Composite: 1.90 eV | Photocatalytic degradation of acetaminophen and reduction of Cr(VI) | Solar light (60,000 lux) | The composite showed the highest degradation of acetaminophen 94% at pH 7 and Cr(VI) reduction efficiency of 91% at pH 2 | [111] |
| 19 | MIL-100 (Fe) MOF/MOX homojunction | Not calculated | Photocatalytic oxidation of gaseous benzene, toluene, xylenes and styrene | 250 W Xe lamp | MIL-100(Fe) MOF/MOX homojunction showed up to 23%, 41%, 82%, 79% and 83% photocatalytic oxidation of benzene, toluene, p-xylene, m-xylene and styrene, respectively | [112] |
| 20 | CuO-ZnO/ ZiF-8 MOF | CuO-ZnO: 2.89 eV CuO-ZnO/ZiF-8(20): 1.96 eV ZiF-8: 5.34 eV | Photocatalytic degradation of acid orange 7 | 400 W halogen lamp | CuO-ZnO/ZiF-8(20) showed the highest photocatalytic degradation of 98.1% acid orange 7 in 100 min | [113] |
| 21 | Co-TCPP MOF@B- TiO2−X | pure B-TiO2−x: 2.71 eV Co-TCPP MOF: 2.55 eV BTC-10%: 2.24 eV | Photocatalytic degradation of bisphenol A | A 300W Xe lamp with a 420nm cut- off filter | Co-TCPP MOF@B-TiO2−X exhibited remarkable photocatalytic degradation of bisphenol A up to 97% within 120 min irradiation | [114] |
| 22 | Ag@Tetra thiafulvalene-based Zr-MOF | Not calculated | Photocatalytic degradation of sulfamethoxazole | 500 W Xe lamp | Ag NPs@Zr-TTFTB were found to efficiently remove sulfamethoxazole (k = 0.009 min−1) | [115] |
| 23 | CdS/Ni-MOF heterostructure | CdS: 2.0 eV Ni-MOF: 3.3 eV | Photocatalytic reduction of CO2 | 300 W Xe lamp | 20%-CdS/Ni-MOF showed the best photocatalytic reduction performance, the yield of CO reached 7.47 μmol/g in the 4th hour, which was nearly 16 times and 7 times that of Ni-MOF and CdS | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Rahman, A.; Matussin, S.N. Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts. Nanomaterials 2022, 12, 2820. https://doi.org/10.3390/nano12162820
Khan MM, Rahman A, Matussin SN. Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts. Nanomaterials. 2022; 12(16):2820. https://doi.org/10.3390/nano12162820
Chicago/Turabian StyleKhan, Mohammad Mansoob, Ashmalina Rahman, and Shaidatul Najihah Matussin. 2022. "Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts" Nanomaterials 12, no. 16: 2820. https://doi.org/10.3390/nano12162820







