Preparation of Three-Dimensional MF/Ti3C2Tx/PmPD by Interfacial Polymerization for Efficient Hexavalent Chromium Removal
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
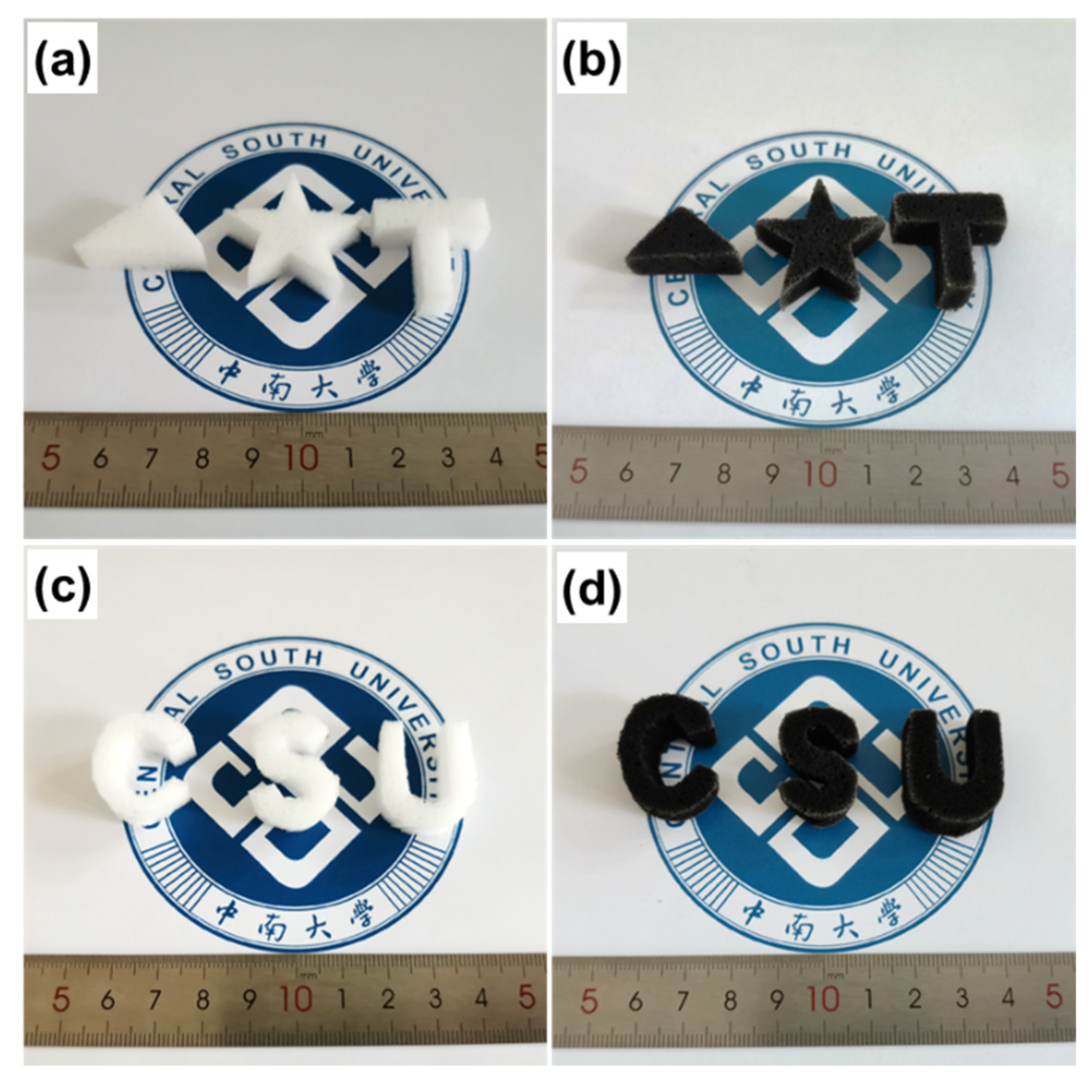
2.2. Preparation of MF/Ti3C2Tx/PmPD
2.3. Characterization
2.4. Batch Experiments
3. Results and Discussion
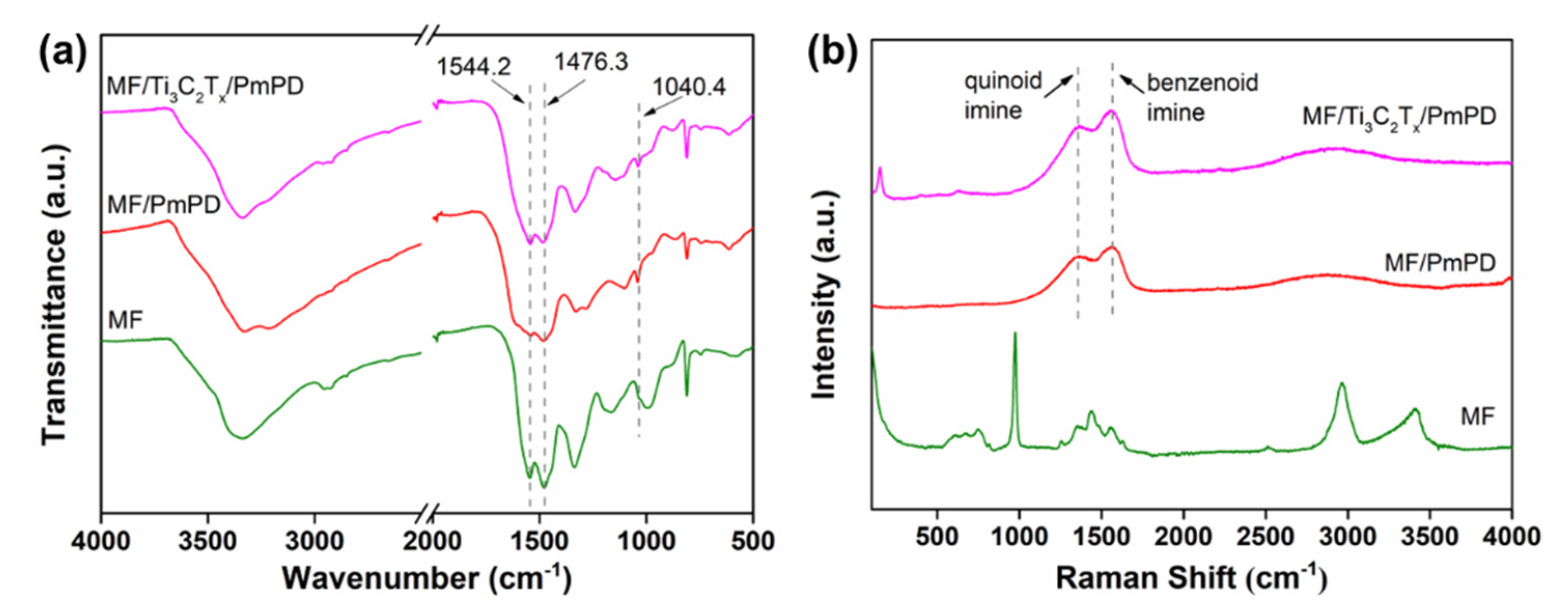
3.1. Material Characterization
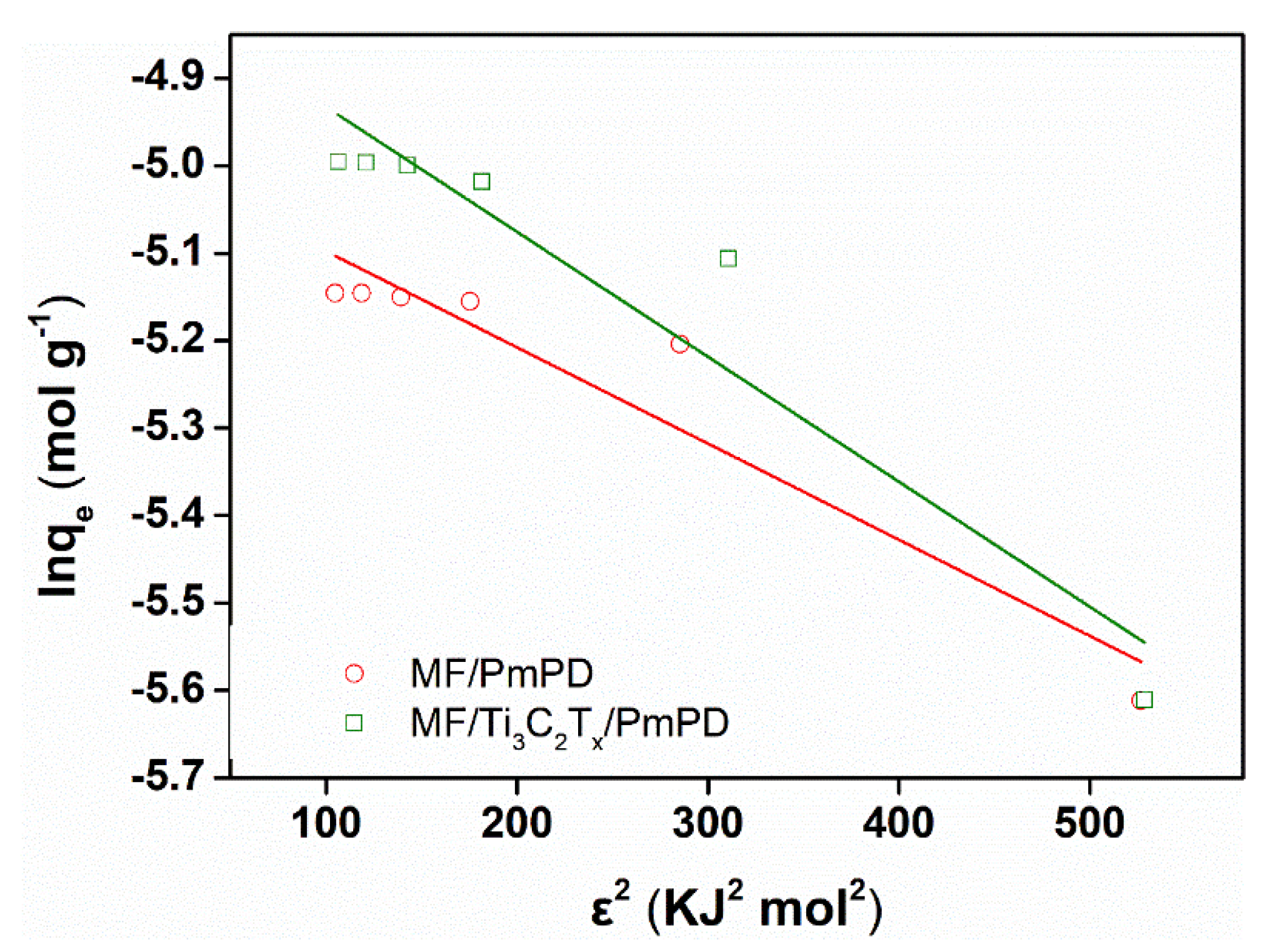
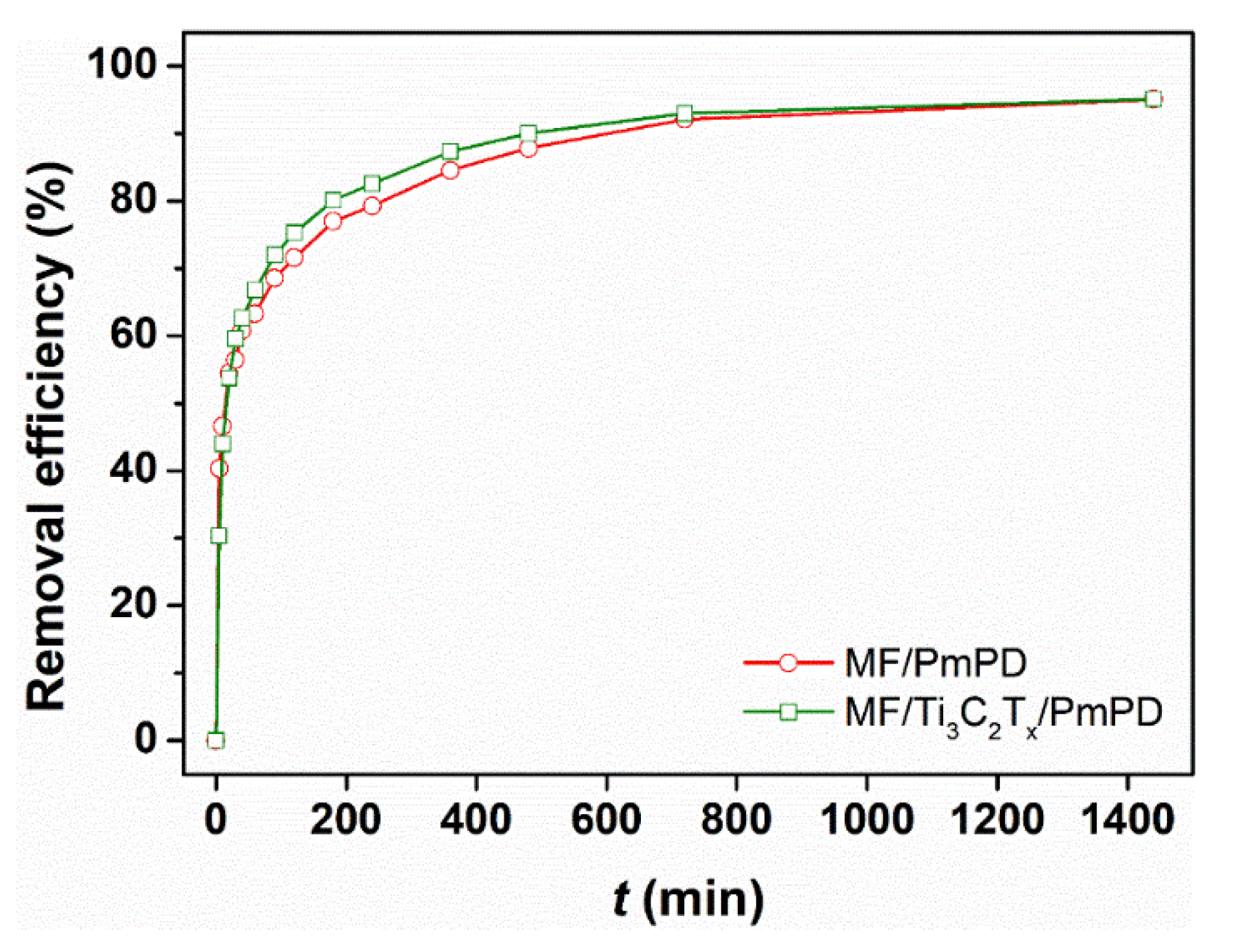
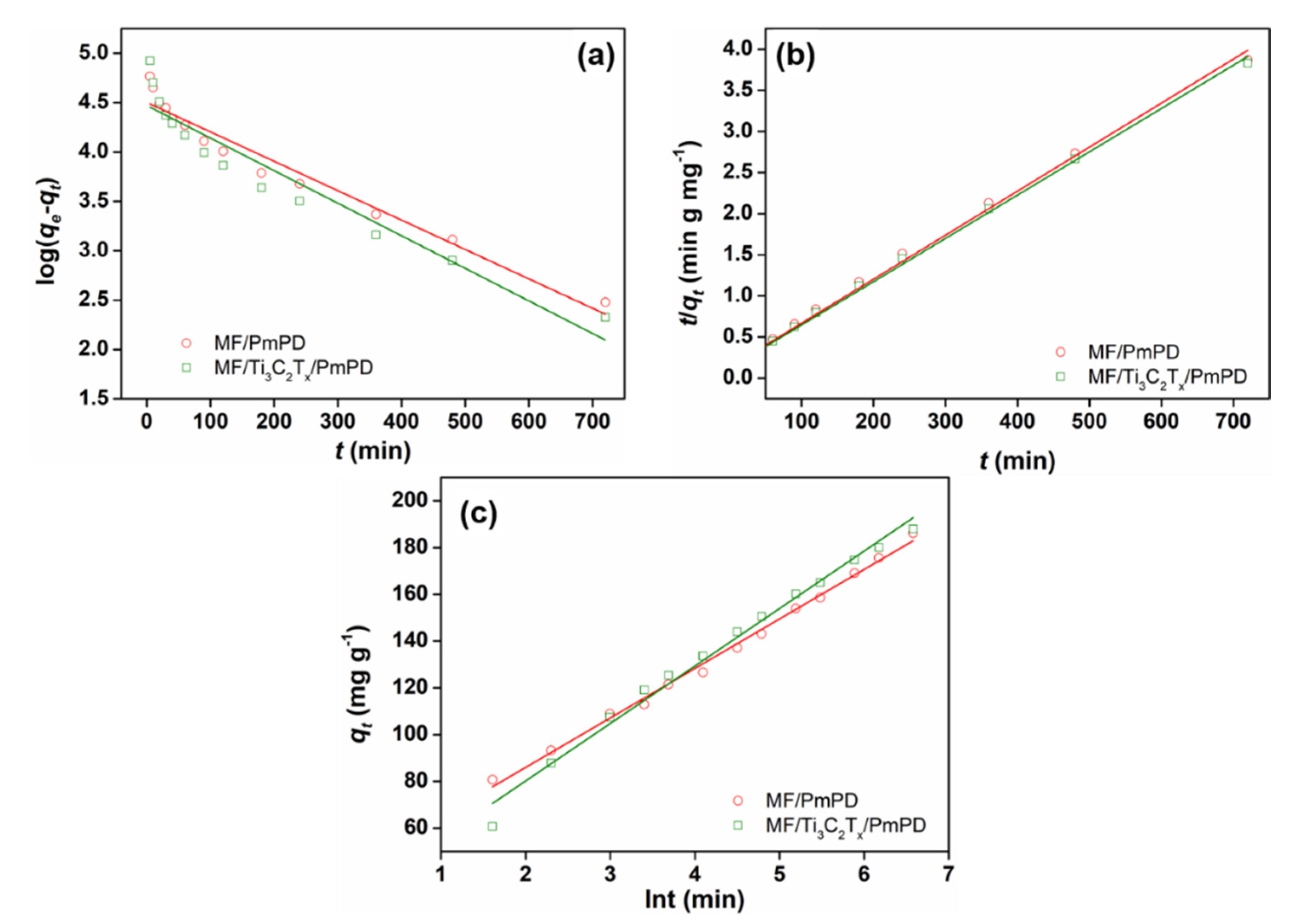
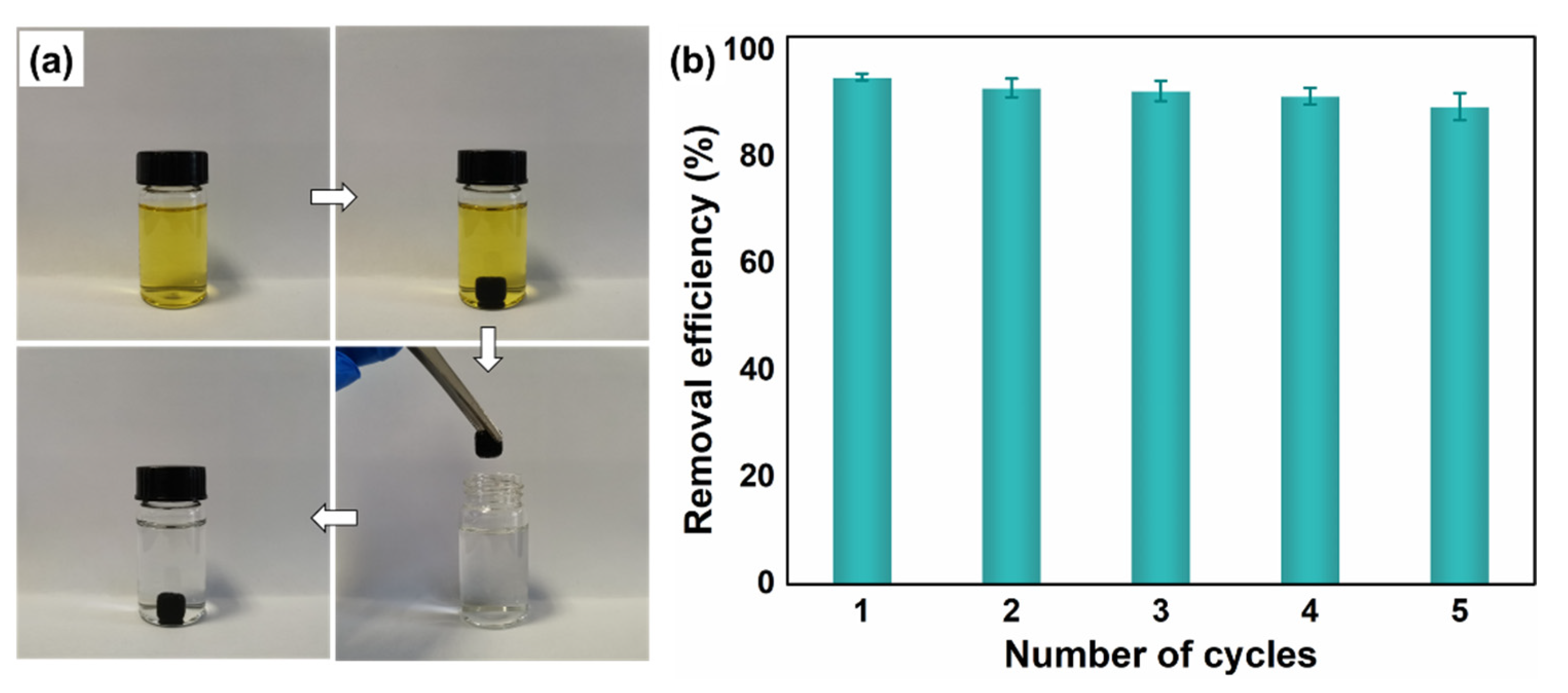
3.2. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; He, F.; Guan, Y. Immobilization of hexavalent chromium in contaminated soil by nano-sized layered double hydroxide intercalated with diethyldithiocarbamate: Fraction distribution, plant growth, and microbial evolution. J. Hazard. Mater. 2022, 430, 128382. [Google Scholar] [CrossRef]
- Luo, X.; Wu, C.; Lin, Y.; Li, W.; Deng, M.; Tan, J.; Xue, S. Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. J. Environ. Sci. 2023, 125, 662–677. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Wang, Z.; Gong, J.; Gao, J.; Tang, S.; Ma, S.; Duan, Z. Heavy metal pollution in agricultural soils of a typical volcanic area: Risk assessment and source appointment. Chemosphere 2022, 304, 135340. [Google Scholar] [CrossRef]
- Aryee, A.; Dovi, E.; Li, Q.; Han, R.; Li, Z.; Qu, L. Magnetic biocomposite based on peanut husk for adsorption of hexavalent chromium, Congo red and phosphate from solution: Characterization, kinetics, equilibrium, mechanism and antibacterial studies. Chemosphere 2022, 287, 132030. [Google Scholar] [CrossRef]
- Kong, L.; Yan, R.; Liu, M.; Xu, J.; Hagio, T.; Ichino, R.; Li, L.; Cao, X. Simultaneous reduction and sequestration of hexavalent chromium by magnetic beta-Cyclodextrin stabilized Fe3S4. J. Hazard. Mater. 2022, 431, 128592. [Google Scholar] [CrossRef]
- Fang, L.; Ding, L.; Ren, W.; Hu, H.; Huang, Y.; Shao, P.; Yang, L.; Shi, H.; Ren, Z.; Han, K.; et al. High exposure effect of the adsorption site significantly enhanced the adsorption capacity and removal rate: A case of adsorption of hexavalent chromium by quaternary ammonium polymers (QAPs). J. Hazard. Mater. 2021, 416, 125829. [Google Scholar] [CrossRef]
- Kekes, T.; Kolliopoulos, G.; Tzia, C. Hexavalent chromium adsorption onto crosslinked chitosan and chitosan/β-cyclodextrin beads: Novel materials for water decontamination. J. Environ. Chem. Eng. 2021, 9, 105581. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhang, L.; Yang, Y.; Liu, X. Diethylenetriaminepentaacetic acid-thiourea-modified magnetic chitosan for adsorption of hexavalent chromium from aqueous solutions. Carbohydr. Polym. 2021, 274, 118555. [Google Scholar] [CrossRef]
- Emenike, E.; Adeniyi, A.; Omuku, P.; Okwu, K.; Iwuozor, K. Recent advances in nano-adsorbents for the sequestration of copper from water. J. Water Process Eng. 2022, 47, 102715. [Google Scholar] [CrossRef]
- Emamy, F.; Bumajdad, A.; Lukaszewicz, J. Adsorption of hexavalent chromium and divalent lead ions on the nitrogen-enriched chitosan-based activated carbon. Nanomaterials 2021, 11, 1907. [Google Scholar] [CrossRef]
- Samuel, M.S.; Datta, S.; Chandrasekar, N.; Balaji, R.; Selvarajan, E.; Vuppala, S. Biogenic synthesis of iron oxide nanoparticles using enterococcus faecalis: Adsorption of hexavalent chromium from aqueous solution and In vitro cytotoxicity analysis. Nanomaterials 2021, 11, 3290. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Zhang, X.; Guo, H.; Cao, X.; Lou, Z.; Zhang, W.; Wang, C. A novel lignin hydrogel supported nZVI for efficient removal of Cr(VI). Chemosphere 2022, 301, 134781. [Google Scholar] [CrossRef]
- Safarzadeh, H.; Peighambardoust, S.J.; Mousavi, S.H.; Foroutan, R.; Mohammadi, R.; Peighambardoust, S.H. Adsorption ability evaluation of the poly(methacrylic acid-co-acrylamide)/cloisite 30B nanocomposite hydrogel as a new adsorbent for cationic dye removal. Environ. Res. 2022, 212, 113349. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.; Shao, Y.; Jin, X.; Qi, X.; Yang, J.; Zhou, Z.; Wang, Y. Melamine foam/reduced graphene oxide supported form-stable phase change materials with simultaneous shape memory property and light-to-thermal energy storage capability. Chem. Eng. J. 2020, 379, 122373. [Google Scholar] [CrossRef]
- Pi, X.; Wang, A.; Fan, R.; Zhou, X.; Sui, W.; Yang, Y. Metal-organic complexes@melamine foam template strategy to prepare three-dimensional porous carbon with hollow spheres structures for efficient organic vapor and small molecule gas adsorption. Inorg. Chem. 2020, 59, 5983–5992. [Google Scholar] [CrossRef]
- Wu, H.; Deng, S.; Shao, Y.; Yang, J.; Qi, X.; Wang, Y. Multiresponsive shape-adaptable phase change materials with cellulose nanofiber/graphene nanoplatelet hybrid-coated melamine foam for light/electro-to-thermal energy storage and utilization. ACS Appl. Mater. Inter. 2019, 11, 46851–46863. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, B.; Hu, X.; Peng, B.; Deng, Z. Temperature control of mussel-inspired chemistry toward hierarchical superhydrophobic surfaces for oil/water separation. Adv. Mater. Interfaces. 2017, 4, 1600727. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Qiu, X.; Liu, X.; Dapaah, M.F.; Niu, Q.; Cheng, L. Removal of chromium(VI) by nanoscale zero-valent iron supported on melamine carbon foam. Nanomaterials 2022, 12, 1866. [Google Scholar] [CrossRef]
- Lei, M.; Ge, F.; Ren, S.; Gao, X.; Zheng, H. A water-stable Cd-MOF and corresponding MOF@melamine foam composite for detection and removal of antibiotics, explosives, and anions. Sep. Purif. Technol. 2022, 286, 120433. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Tao, T.; Ren, Z.; Zeng, J.; Yu, J.; Umeyama, T.; Ohara, T.; Imahori, H. Highly cost-efficient sorption and desorption of mercury ions onto regenerable poly(m-phenylenediamine) microspheres with many active groups. Chem. Eng. J. 2020, 391, 123515. [Google Scholar] [CrossRef]
- Xiong, T.; Yuan, X.Z.; Wang, H.; Jiang, L.; Wu, Z.; Wang, H.; Cao, X. Integrating the (311) facet of MnO2 and the fictional groups of poly(m-phenylenediamine) in core-shell MnO2@poly(m-phenylenediamine) adsorbent to remove Pb ions from water. J. Hazard. Mater. 2020, 389, 122154. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, J.; Chen, M.; Jin, Y.; Ma, Y.; Ou, G.; Wei, Z. ZIF-67 derived magnetic nanoporous carbon coated by poly(m-phenylenediamine) for hexavalent chromium removal. Sep. Purif. Technol. 2021, 277, 119436. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Dai, T.; He, X.M.; Chen, M.; Liu, C.M.; Liang, R.; Chen, J. Preparation of pectin/poly(m-phenylenediamine) microsphere and its application for Pb2+ removal. Carbohydr. Polym. 2021, 260, 117811. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zou, Y.; Xu, H.; Wu, L.; Mai, Y. Efficient electrocatalytic upgradation of furan-based biomass: Key roles of a two-dimensional mesoporous poly(m-phenylenediamine)-graphene heterostructure and a ternary electrolyte. Macromolecules 2022, 55, 1445–1456. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, L.; Jin, L.; Huang, L.; He, Y.; Tang, J.; Yang, W.; Wang, H. In-situ functionalization of poly(m-phenylenediamine) nanoparticles on bacterial cellulose for chromium removal. Chem. Eng. J. 2018, 344, 441–452. [Google Scholar] [CrossRef]
- Jin, L.; Chai, L.; Ren, L.; Jiang, Y.; Yang, W.; Wang, S.; Liao, Q.; Wang, H.; Zhang, L. Enhanced adsorption-coupled reduction of hexavalent chromium by 2D poly(m-phenylenediamine)-functionalized reduction graphene oxide. Environ. Sci. Pollut. Res. 2019, 26, 31099–31110. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Wang, M.; He, Y.J.; Chai, L.; Huang, J.; Wang, H.; Wu, X.; Lai, Y. Highly flexible and porous nanoparticle-loaded films for dye removal by graphene oxide-fungus interaction. ACS Appl. Mater. Int. 2016, 8, 34638–34647. [Google Scholar] [CrossRef]
- Jin, L.; Chai, L.; Yang, W.; Wang, H.; Zhang, L. Two-dimensional titanium carbides (Ti3C2Tx) functionalized by Poly(m-phenylenediamine) for efficient adsorption and reduction of hexavalent chromium. Int. J. Environ. Res. Public Health 2019, 17, 167. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z. Interaction of norfloxacin and hexavalent chromium with ferrihydrite nanoparticles: Synergistic adsorption and antagonistic aggregation behavior. Chemosphere 2022, 299, 134386. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Zhu, H.; Wu, X.; Dai, L.; Chen, R.; van der Bruggen, B. Recovery of trivalent and hexavalent chromium from chromium slag using a bipolar membrane system combined with oxidation. J. Colloid Interface Sci. 2022, 619, 280–288. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, L.; Yan, Y.; Li, H.; Yu, Q.; Jiao, B.; Li, D. Rapid Cr(VI) reduction structure in chromium contaminated soil: The UV-assisted electrokinetic circulation of background iron. Sci. Total Environ. 2022, 822, 153508. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, S.; Anbuselvan, N.M.; Venkatachalam, P.; Shanmugam, S.R.; Selvasembian, R. Waste tire particles as efficient materials towards hexavalent chromium removal: Characterisation, adsorption behaviour, equilibrium, and kinetic modelling. Chemosphere 2022, 295, 133797. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Shin, M.; Kim, H.J. Interface engineering of MIL-88 derived MnFe-LDH and MnFe2O3 on three-dimensional carbon nanofibers for the efficient adsorption of Cr(VI), Pb(II), and As(III) ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Wang, W.; Gao, P.; Yang, C.; Zhao, Z.; Zhen, S.; Zhou, Y.; Zhang, T. Separable and reactivated magnetic mZVAl/nFe3O4 composite induced by ball milling for efficient adsorption-reduction-sequestration of aqueous Cr(VI). Sep. Purif. Technol. 2022, 288, 120689. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Yan, Z.; He, Y.; Lan, J.; Hou, H. Photoreductive synthesis of nanoscale zero-valent iron rod assisted by phosphotungstic acid over graphite carbon nitride and its enhanced removal of Cr(VI) from water. Appl. Surf. Sci. 2022, 582, 152479. [Google Scholar] [CrossRef]
- Shen, H.; Chen, L.; Zhou, C.; Du, J.; Lu, C.; Yang, H.; Tan, L.; Zeng, X.; Dong, L. Immobilizing Fe0 nanoparticles on covalent organic framework towards enhancement of Cr(VI) removal by adsorption and reduction synergistic effect. Sep. Purif. Technol. 2022, 290, 120883. [Google Scholar] [CrossRef]
- Wen, J.; Fu, W.; Ding, S.; Zhang, Y.; Wang, W. Pyrogallic acid modified nanoscale zero-valent iron efficiently removed Cr(VI) by improving adsorption and electron selectivity. Chem. Eng. J. 2022, 443, 136510. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, D.; Zhang, A.; Tian, J.; Zheng, Y.; Zhao, W.; Cui, L.; Liu, J. Synthesis of polypyrrole coated melamine foam by in-situ interfacial polymerization method for highly compressible and flexible supercapacitor. J. Colloid Interface Sci. 2019, 557, 617–627. [Google Scholar] [CrossRef]
- Zhao, X.; Zha, X.J.; Pu, J.H.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Yang, W. Macroporous three-dimensional MXene architectures for highly efficient solar steam generation. J. Mater. Chem. A 2019, 7, 10446–10455. [Google Scholar] [CrossRef]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manage. 2018, 206, 872–889. [Google Scholar] [CrossRef]
- Pinto, J.; Magri, D.; Valentini, P.; Palazon, F.; Heredia-Guerrero, J.A.; Lauciello, S.; Barroso-Solares, S.; Ceseracciu, L.; Pompa, P.; Athanassiou, A.; et al. Antibacterial melamine foams decorated with in situ synthesized silver nanoparticles. ACS Appl. Mater. Int. 2018, 10, 16095–16104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Liu, J.; Zhao, S.; Xie, X.; Liu, L.; Yang, R.; Koratkar, N.; Yu, Z. Multifunctional and water-resistant MXene-decorated polyester textiles with outstanding electromagnetic interference shielding and joule heating performances. Adv. Funct. Mater. 2019, 29, 1806819. [Google Scholar] [CrossRef]
- Mohammadi, A.V.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 22123–22133. [Google Scholar]
- Wang, H.; Gang, H.; Wei, D.; He, Y.; Issaka Alhassan, S.; Yan, L.; Wu, B.; Cao, Y.; Jin, L.; Huang, L. Bismuth−titanium alloy nanoparticle@porous carbon composite as efficient and stable Cl-storage electrode for electrochemical desalination. Sep. Purif. Technol. 2022, 296, 121375. [Google Scholar] [CrossRef]
- Ren, Q.; Dai, T.; Jin, X.; Wu, D.; Wang, C.; Li, J.; Zhu, S. Solution processed coating of polyolefin on melamine foams to fabricate tough oil superabsorbents. Macromol. Mater. Eng. 2018, 303, 1800436. [Google Scholar] [CrossRef]
- Jin, L.; Huang, L.; Ren, L.; He, Y.; Tang, J.; Wang, S.; Yang, W.; Wang, H.; Chai, L. Preparation of stable and high-efficient poly(m-phenylenediamine)/reduced graphene oxide composites for hexavalent chromium removal. J. Mater. Sci. 2019, 54, 383–395. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, N.; Han, G.; Li, M.; Xiao, Y.; Li, H. The properties of highly compressible electrochemical capacitors based on polypyrrole/melamine sponge-carbon fibers. J. Alloys Compd. 2019, 786, 668–676. [Google Scholar] [CrossRef]
- Peng, C.; Yu, J.; Chen, S.; Wang, L. High-performance supercapacitor based on ultralight and elastic three-dimensional carbon foam/reduced graphene/polyaniline nanocomposites. Chin. Chem. Lett. 2019, 30, 1137–1140. [Google Scholar] [CrossRef]
- Liu, Q.; Zang, L.; Qiao, X.; Qiu, J.; Wang, X.; Hu, L.; Yang, J.; Yang, C. Compressible all-In-one supercapacitor with adjustable output voltage based on polypyrrole-coated melamine foam. Adv. Electron. Mater. 2019, 5, 1900724. [Google Scholar] [CrossRef]
- Wang, X.; Han, Z.; Liu, Y.; Wang, Q. Micro-nano surface structure construction and hydrophobic modification to prepare efficient oil-water separation melamine formaldehyde foam. Appl. Surf. Sci. 2020, 505, 144577. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface functionalization of bamboo leave mediated synthesized SiO2 nanoparticles: Study of adsorption mechanism, isotherms and enhanced adsorption capacity for removal of Cr(VI) from aqueous solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Su, F.; Wang, Y.; Peng, L.; Liu, D. Adsorption behaviour of microplastics on the heavy metal Cr(VI) before and after ageing. Chemosphere 2022, 302, 134865. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, C.; Li, W.; Liu, H.; Liu, L.; Deng, S.; Li, Y. Effective removal of Cr(VI) from aqueous solution through adsorption and reduction by magnetic S-doped Fe-Cu-La trimetallic oxides. J. Environ. Chem. Eng. 2022, 10, 107433. [Google Scholar] [CrossRef]
- Bouaouina, K.; Barras, A.; Bezzi, N.; Amin, M.A.; Szunerits, S.; Boukherroub, R. Adsorption-reduction of Cr(VI) onto unmodified and phytic acid-modified carob waste: Kinetic and isotherm modeling. Chemosphere 2022, 297, 134188. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Ma, S.; Zhao, W.; Xie, W.; Ma, J.; Yao, Y.; Wei, W. Comparing the influence of humic/fulvic acid and tannic acid on Cr(VI) adsorption onto polystyrene microplastics: Evidence for the formation of Cr(OH)3 colloids. Chemosphere 2022, 307, 135697. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Li, H.; Huang, Z.; Qin, X.; Huang, J.; Zhang, X.; Li, X.; Lu, Q. A novel biochar-copolymer composite for rapid Cr(VI) removal: Adsorption-reduction performance and mechanism. Sep. Purif. Technol. 2022, 295, 121275. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Tian, Y.; Kong, L.; Cai, G.; Zhang, H.; Zhang, J.; Zuo, W.; Wen, B. Shapeable amino-functionalized sodium alginate aerogel for high-performance adsorption of Cr(VI) and Cd(II): Experimental and theoretical investigations. Chem. Eng. J. 2022, 446, 137430. [Google Scholar] [CrossRef]





| Adsorbents | Langmuir | |||
|---|---|---|---|---|
| qm | KL | R2 | ||
| MF/PmPD | 303.29 | 0.37 | 0.9989 | |
| MF/Ti3C2Tx/PmPD | 352.15 | 0.24 | 0.9974 | |
| Adsorbents | Freundlich | |||
| n | KF | R2 | ||
| MF/PmPD | 12.86 | 187.29 | 0.7850 | |
| MF/Ti3C2Tx/PmPD | 10.19 | 191.61 | 0.8175 | |
| Adsorbents | R–P model | |||
| K | α | β | R2 | |
| MF/PmPD | 106.61 | 0.36 | 0.99 | 0.9998 |
| MF/Ti3C2Tx/PmPD | 89.84 | 0.27 | 0.99 | 0.9997 |
| Adsorbents | Temkin model | |||
| KT | B1 | R2 | ||
| MF/PmPD | 3241.58 | 21.31 | 0.8330 | |
| MF/Ti3C2Tx/PmPD | 236.00 | 30.34 | 0.8531 | |
| Adsorbents | qm (mg/g) | β (mol2/kJ) | E (KJ/mol) | R2 |
|---|---|---|---|---|
| MF/PmPD | 356.67 | 0.11 × 10−2 | 21.32 | 0.9560 |
| MF/Ti3C2Tx/PmPD | 432.30 | 0.14 × 10−2 | 18.70 | 0.8926 |
| Adsorbents | Pseudo-First-Order | Pseudo-Second-Order | Enlovich Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | αE | βE | R2 | |
| MF/PmPD | 90.15 | 0.20 × 10−2 | 0.9553 | 186.92 | 0.22 × 10−3 | 0.9960 | 166.12 | 0.47 × 10−1 | 0.9952 |
| MF/Ti3C2Tx/PmPD | 87.43 | 0.33 × 10−2 | 0.9115 | 189.75 | 0.23 × 10−3 | 0.9979 | 86.71 | 0.40 × 10−1 | 0.9881 |
| Adsorption Stage | MF/PmPD | MF/Ti3C2Tx/PmPD | |
|---|---|---|---|
| Stage 1 | ki,1 (mg g−1 min−0.5) | 12.58 | 20.53 |
| C1 | 52.89 | 17.83 | |
| R2 | 0.9976 | 0.9265 | |
| Stage 2 | ki,2 (mg g−1 min−0.5) | 5.10 | 5.71 |
| C2 | 86.98 | 87.11 | |
| R2 | 0.9888 | 0.9669 | |
| Stage 3 | ki,3 (mg g−1 min−0.5) | 2.44 | 2.10 |
| C3 | 121.52 | 133.10 | |
| R2 | 0.9933 | 0.9803 | |
| Boyd model | Intercept | 0.29 | 0.32 |
| R2 | 0.9553 | 0.9115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Pan, Q.; Li, X.; Su, C.; Wang, Z.; Wang, H.; Huang, L. Preparation of Three-Dimensional MF/Ti3C2Tx/PmPD by Interfacial Polymerization for Efficient Hexavalent Chromium Removal. Nanomaterials 2022, 12, 2838. https://doi.org/10.3390/nano12162838
Jin L, Pan Q, Li X, Su C, Wang Z, Wang H, Huang L. Preparation of Three-Dimensional MF/Ti3C2Tx/PmPD by Interfacial Polymerization for Efficient Hexavalent Chromium Removal. Nanomaterials. 2022; 12(16):2838. https://doi.org/10.3390/nano12162838
Chicago/Turabian StyleJin, Linfeng, Qinglin Pan, Xiaorui Li, Changqing Su, Zhongyu Wang, Haiying Wang, and Lei Huang. 2022. "Preparation of Three-Dimensional MF/Ti3C2Tx/PmPD by Interfacial Polymerization for Efficient Hexavalent Chromium Removal" Nanomaterials 12, no. 16: 2838. https://doi.org/10.3390/nano12162838






