Hybrid Nanofluid Thermal Conductivity and Optimization: Original Approach and Background
Abstract
:1. Introduction
2. Nanofluid Thermal Conductivity Models
2.1. Effective Medium Theory
2.2. Brownian Models
2.3. Nanolayer Models
2.4. Aggregation Models
2.5. Molecular Dynamics Simulations
2.6. Thermal Conductivity Models for Hybrid Nanofluids
3. Comprehensive Thermal Conductivity Model for General N Hybrid Nanofluids
4. Nanofluid Viscosity Models
5. Optimization of Nanofluids for Heat Transfer Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Symbol | Definition |
|---|---|
| Number of data points | |
| Predicted value | |
| Measured value | |
| Thermal conductivity (W/m·K) | |
| Volume concentration of nanoparticles | |
| Shape factor | |
| Sphericity | |
| Reynolds number | |
| Prandtl number | |
| Specific heat (J/kg·K) | |
| Dynamic viscosity (kg/m·s) | |
| Density (kg/m3) | |
| Temperature (°C) | |
| Freezing temperature (K) | |
| Reference temperature (°C) | |
| Brownian velocity (m/s) | |
| Boltzmann’s constant (1.380649 × 10−23 m2 kg s−2 K−1) | |
| Diameter (m) | |
| Radius (m) | |
| Mean free path of base fluid molecule (m) | |
| Empirical correlation factor from Koo and Kleinstreuer [30] or Vajjha and Das [31] | |
| Constant from Kumar et al. [33], taken as 2.95 in this paper | |
| Ratio of equivalent concentration including the nanolayer the to actual concentration | |
| Nanolayer thickness (m) | |
| Ratio of nanolayer to particle thermal conductivity | |
| Fractal dimension | |
| Aggregate distribution function | |
| Equivalent matrix thickness (m) | |
| Ratio of particle radius to equivalent matrix thickness | |
| Ratio of equivalent thermal thickness to particle radius | |
| Dimensionless thermal conductivity parameter | |
| Surface area (m2) | |
| Volume (m3) | |
| General enhancement term | |
| Number of distinct nanoparticles in suspension | |
| Average distance between nanoparticles (m) | |
| Empirical correlation factor from Hosseini et al. [13] or Klazly and Bognár [14] | |
| Empirical correlation factors from Klazly and Bognár [14] | |
| Subscripts | |
| Nanofluid | |
| Base fluid | |
| Particle | |
| Nanolayer | |
| Particle equivalent | |
| Aggregate | |
| Equivalent | |
| Cluster | |
| Hybrid nanofluid | |
| Ternary hybrid nanofluid | |
| hybrid nanofluid | |
| Corresponding to nanoparticle in hybrid nanofluid | |
| Abbreviations | |
| MAE | Mean absolute error (%) |
| EMT | Effective Medium Theory |
| CNT | Carbon nanotubes |
| SWCNT | Single-walled carbon nanotubes |
| MWCNT | Multi-walled carbon nanotubes |
| EG | Ethylene Glycol |
| PG | Propylene Glycol |
| MDS | Molecular Dynamics Simulation(s) |
| TC | Thermal conductivity |
| VSC | Viscosity |
| CNC | Cellulose nanocrystals |
Appendix B
| Source | Description | Model |
| Maxwell [14] | Modified Einstein model, assumes spherical particles. | |
| Hamilton and Crosser [15] | EMT, Modified Maxwell [14] model accounting for particle shape. | |
| Jeffery [16] | EMT, assumes static and homogeneous particles, and is a truncated infinite series. | |
| Timofeeva et al. [17] | EMT, as-sumes uses kp ≫ kbf, Al2O3–water data. | |
| Sundar et al. [18] | EMT, modified Timofeeva et al. [17] model using Fe3O4–water data. | |
| Corcione [19] | Brownian, empircal correlation, wide range of input data, considers Brownian motion through Reynolds number. | |
| Chon et al. [20] | Brownian, empirical correlation using Buckingham π Theorem and Al2O3–water data. | |
| Koo and Kleinstreuer [21] | Brownian, accounts for static and dynamic enhance-ment, β given in Table 2. | |
| Vajjha and Das [22] | Brownian, validity range extension of Koo and Kleinstreuer [21] model, β in Table 3. | |
| Patel et al. [23] | Brownian, non- linear regression analysis over wide range of data. | |
| Kumar et al. [24] | Brownian, combination of static and dynamic enhance- ment models. | |
| Leong et al. [25] | Nanolayer, assumes spherical particles and steady-state heat transfer, derived from cylindrical 2D heat conduction equation. | |
| Xie et al. [26] | Nanolayer, assumes knl varies linearly along the nanolayer thickness, utilizes order of magnitude analysis. | |
| Yu and Choi [27] | Nanolayer, modified Maxwell [14] model by replacing particle properties with effective ones. Assumes spherical particles in a homogeneous distribution. | |
| Tinga et al. [28] | Nanolayer, original model for prediction of dielectric constant of cellulose-air–water mixtures, assumes spherical particles. | |
| Prasher et al. [29] | Aggregation and Brownian, based on Maxwell [14] model, models clumping as a time-dependent process. | |
| Xuan et al. [30] | Aggregation and Brownian, considered stationary and dynamic enhancement mechanisms, assumes limited max cluster size. | |
| Feng et al. [31] | Aggregation and nanolayer, modified Yu and Choi [27] model, considers clusters as spherical 2D lattice. | |
| Wang et al. [32] | Aggregation, assumes a steady distribution of aggregates having varying sizes with 1.5 standard deviation, and geometric mean of particle radius equals the average radius. | |
| Evans et al. [33] | MDS, assumes stationary, non-aggregating particles, different levels of wetting used in simulations. | |
| Vladkov and Barrat [34] | MDS, considers effects of interfacial resistance, assumes interfacial thermal thickness to be unity. | |
| Lin et al. [35] | MDS, used Cu–EG simulations, considers both EMT and the nanolayer, assumes spherical particles. | |
| Mirmohammadi et al. [36] | MDS, simulated Ag–water nanofluid, accounts for particle shape. | |
| Chamkha et al. [37] | Hybrid EMT, modified Maxwell [14] model, particle properties replaced with averages. | |
| Devi and Devi [38] | Hybrid EMT, modified Hamilton and Crosser [15] model, treats base fluid as separate nanofluid. | |
| Chougule and Sahu [39] | Hybrid Brownian, accounts for particle size, similar technique to Kumar et al. [24] | |
| Present | Hybrid Brownian, utilizes work of Patel et al. [23], valid for higher-order nanofluids. |
| Source | Description | Model |
| Brinkman [48] | Modified Einstein model, assumes spherical particles. | |
| Meybodi et al. [49] | Empirical correlation based on data for oxide–water nanofluids. | |
| Masoumi et al. [50] | Brownian, assumes a homogeneous distribution of particles and no inter-particle interaction. | |
| Hosseini et al. [51] | Nanolayer, empirical correlation based on Al2O3–water nanofluid data. | |
| Klazly and Bognár [52] | Brownian and nanolayer, empirical correlation based on wide range of existing data. |
References
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Choi, S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab. (ANL): Argonne, IL, USA, 1995.
- Giwa, S.O.; Adegoke, K.A.; Sharifpur, M.; Meyer, J.P. Research trends in nanofluid and its applications: A bibliometric analysis. J. Nanoparticle Res. 2022, 24, 1–22. [Google Scholar] [CrossRef]
- Meyer, J.P.; Adio, S.A.; Sharifpur, M.; Nwosu, P.N. The viscosity of nanofluids: A review of the theoretical, empirical, and numerical models. Heat Transf. Eng. 2016, 37, 387–421. [Google Scholar] [CrossRef]
- Berezshnaya, O.V.; Gribkov, E.P.; Borovik, P.V.; Kassov, V.D. The finite element modulation of thermostressed state of coating formation at electric contact surfacing of “shaft” type parts. Adv. Mater. Sci. Eng. 2019, 2019, 7601792. [Google Scholar] [CrossRef]
- Jin, Y.; Xi, C.; Xue, P.; Zhang, C.; Wang, S.; Luo, J. Constitutive Model and Microstructure Evolution Finite Element Simulation of Multidirectional Forging for GH4169 Superalloy. Metals 2020, 10, 1695. [Google Scholar] [CrossRef]
- Nevskii, S.; Sarychev, V.; Konovalov, S.; Granovskii, A.; Gromov, V. Wave instability on the interface coating/substrate material under heterogeneous plasma flows. J. Mater. Res. Technol. 2020, 9, 539–550. [Google Scholar] [CrossRef]
- Jehhef, K.A.; Siba, M.A.A.A. Effect of surfactant addition on the nanofluids properties: A review. Acta Mech. Malays. 2019, 2, 1–19. [Google Scholar] [CrossRef]
- Said, Z.; Jamei, M.; Sundar, L.S.; Pandey, A.; Allouhi, A.; Li, C. Thermophysical properties of water, water and ethylene glycol mixture-based nanodiamond+ Fe3O4 hybrid nanofluids: An experimental assessment and application of data-driven approaches. J. Mol. Liq. 2022, 347, 117944. [Google Scholar] [CrossRef]
- Dong, F.; Wan, J.; Feng, Y.; Wang, Z.; Ni, J. Experimental study on thermophysical properties of propylene glycol-based graphene nanofluids. Int. J. Thermophys. 2021, 42, 1–18. [Google Scholar] [CrossRef]
- Mohd-Ghazali, N.; Estellé, P.; Halelfadl, S.; Maré, T.; Siong, T.C.; Abidin, U. Thermal and hydrodynamic performance of a microchannel heat sink with carbon nanotube nanofluids. J. Therm. Anal. Calorim. 2019, 138, 937–945. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.S.; Li, S.; Eastman, J.A. Measuring thermal conductivity of fluids containing oxide nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Aberoumand, H.; Javaherdeh, K. A complete experimental investigation on the rheological behavior of silver oil based nanofluid. Heat Transf.-Asian Res. 2017, 46, 294–304. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1873. [Google Scholar]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Jeffery, D. Conduction through a random suspension of spheres. Proc. R. Soc. Lond. A Math. Phys. Sci. 1973, 335, 355–367. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef]
- Sundar, L.; Singh, M.K.; Sousa, A.C. Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int. Commun. Heat Mass Transf. 2013, 44, 7–14. [Google Scholar] [CrossRef]
- Corcione, M. Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers. Manag. 2011, 52, 789–793. [Google Scholar] [CrossRef]
- Chon, C.H.; Kihm, K.D.; Lee, S.P.; Choi, S.U. Empirical correlation finding the role of temperature and particle size for nanofluid (al2o3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 153107. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanoparticle Res. 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]
- Patel, H.E.; Sundararajan, T.; Das, S.K. An experimental investigation into the thermal conductivity enhancement in oxide and metallic nanofluids. J. Nanoparticle Res. 2009, 12, 1015–1031. [Google Scholar] [CrossRef]
- Kumar, D.H.; Patel, H.E.; Kumar, V.R.R.; Sundararajan, T.; Pradeep, T.; Das, S.K. Model for Heat Conduction in Nanofluids. Phys. Rev. Lett. 2004, 93, 144301. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.; Yang, C.; Murshed, S. A model for the thermal conductivity of nanofluids—The effect of interfacial layer. J. Nanoparticle Res. 2006, 8, 245–254. [Google Scholar] [CrossRef]
- Xie, H.; Fujii, M.; Zhang, X. Effect of interfacial nanolayer on the effective thermal conductivity of nanoparticle-fluid mixture. Int. J. Heat Mass Transf. 2005, 48, 2926–2932. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell Model. J. Nanoparticle Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Tinga, W.R.; Voss, W.A.; Blossey, D.F. Generalized approach to multiphase dielectric mixture theory. J. Appl. Phys. 1973, 44, 3897–3902. [Google Scholar] [CrossRef]
- Prasher, R.; Phelan, P.E.; Bhattacharya, P. Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions (nanofluid). Nano Lett. 2006, 6, 1529–1534. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, B.; Xu, P.; Zou, M. The effective thermal conductivity of nanofluids based on the nanolayer and the aggregation of nanoparticles. J. Phys. D Appl. Phys. 2007, 40, 3164–3171. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhou, L.P.; Peng, X.F. A fractal model for predicting the effective thermal conductivity of liquid with suspension of nanoparticles. Int. J. Heat Mass Transf. 2003, 46, 2665–2672. [Google Scholar] [CrossRef]
- Evans, W.; Fish, J.; Keblinski, P. Role of brownian motion hydrodynamics on nanofluid thermal conductivity. Appl. Phys. Lett. 2006, 88, 093116. [Google Scholar] [CrossRef]
- Vladkov, M.; Barrat, J.L. Modeling transient absorption and thermal conductivity in a simple nanofluid. Nano Lett. 2006, 6, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Hsiao, P.Y.; Chieng, C.C. Roles of nanolayer and particle size on thermophysical characteristics of ethylene glycol-based copper nanofluids. Appl. Phys. Lett. 2011, 98, 153105. [Google Scholar] [CrossRef]
- Mirmohammadi, S.A.; Behi, M.; Gan, Y.; Shen, L. Particle-shape-, temperature-, and concentration-dependent thermal conductivity and viscosity of nanofluids. Phys. Rev. E 2019, 99, 043109. [Google Scholar] [CrossRef]
- Chamkha, A.J.; Miroshnichenko, I.V.; Sheremet, M.A. Numerical analysis of unsteady conjugate natural convection of hybrid water-based nanofluid in a semicircular cavity. J. Therm. Sci. Eng. Appl. 2017, 9, 041004. [Google Scholar] [CrossRef]
- Devi, S.A.; Devi, S.S.U. Numerical investigation of hydromagnetic hybrid Cu–Al2O3/water nanofluid flow over a permeable stretching sheet with suction. Int. J. Nonlinear Sci. Numer. Simul. 2016, 17, 249–257. [Google Scholar] [CrossRef]
- Chougule, S.S.; Sahu, S.K. Model of heat conduction in hybrid nanofluid. In Proceedings of the 2013 IEEE International Conference on Emerging Trends in Computing, Communication and Nanotechnology (ICECCN), Tirunelveli, India, 25–26 March 2013; IEEE: New York, NY, USA; pp. 337–341. [Google Scholar]
- Choy, T.C. Effective Medium Theory: Principles and Applications; Oxford University Press: Oxford, UK, 2015; Volume 165. [Google Scholar]
- Jang, S.P.; Choi, S.U. Role of brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Henderson, J.; van Swol, F. On the interface between a fluid and a planar wall. Mol. Phys. 1984, 51, 991–1010. [Google Scholar] [CrossRef]
- Gregory, J. Particle aggregation: Modeling and measurement. Nanoparticles Solids Solut. 1996, 18, 203–256. [Google Scholar]
- Keblinski, P.; Phillpot, S.; Choi, S.; Eastman, J. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Allen, M. Introduction to Molecular Dynamics Simulation. In Computational Soft Matter: From Synthetic Polymers to Proteins; John Von Neumann Institute for Computing (NIC): Juelich, Germany, 2004; Volume 23, pp. 1–28. [Google Scholar]
- Kshirsagar, D.P.; Venkatesh, M. A review on hybrid nanofluids for engineering applications. Mater. Today Proc. 2021, 44, 744–755. [Google Scholar] [CrossRef]
- Pourrajab, R.; Ahmadianfar, I.; Jamei, M.; Behbahani, M. A meticulous intelligent approach to predict thermal conductivity ratio of hybrid nanofluids for heat transfer applications. J. Therm. Anal. Calorim. 2021, 146, 611–628. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Meybodi, M.K.; Daryasafar, A.; Koochi, M.M.; Moghadasi, J.; Meybodi, R.B.; Ghahfarokhi, A.K. A novel correlation approach for viscosity prediction of water based nanofluids of Al2O3, TiO2, SiO2 and CuO. J. Taiwan Inst. Chem. Eng. 2016, 58, 19–27. [Google Scholar] [CrossRef]
- Masoumi, N.; Sohrabi, N.; Behzadmehr, A. A new model for calculating the effective viscosity of nanofluids. J. Phys. D Appl. Phys. 2009, 42, 055501. [Google Scholar] [CrossRef]
- Hosseini, S.; Moghadassi, A.; Henneke, D. A new dimensionless group model for determining the viscosity of nanofluids. J. Therm. Anal. Calorim. 2010, 100, 873–877. [Google Scholar] [CrossRef]
- Klazly, M.; Bognár, G. A novel empirical equation for the effective viscosity of nanofluids based on theoretical and empirical results. Int. Commun. Heat Mass Transf. 2022, 135, 106054. [Google Scholar] [CrossRef]
- Sharma, P.; Ramesh, K.; Parameshwaran, R.; Deshmukh, S.S. Thermal conductivity prediction of titania-water nanofluid: A case study using different machine learning algorithms. Case Stud. Therm. Eng. 2022, 30, 101658. [Google Scholar] [CrossRef]
- He, D.; Li, C.; Chen, Y.; Li, X. Prediction of thermal conductivity of hybrid nanofluids based on deep forest model. Heat Transf. Res. 2022, 53, 55–71. [Google Scholar] [CrossRef]
- Jamei, M.; Said, Z. Recent advances in the prediction of thermophysical properties of nanofluids using artificial intelligence. Hybrid Nanofluids 2022, 203–232. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Nwosu, P.N.; Meyer, J.; Sharifpur, M. A review and parametric investigation into nanofluid viscosity models. J. Nanotechnol. Eng. Med. 2014, 5, 031008. [Google Scholar] [CrossRef]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A brief review on viscosity of nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef]
- Pil Jang, S.; Choi, S.U. Effects of various parameters on nanofluid thermal conductivity. J. Heat Transf. 2006, 129, 617–623. [Google Scholar] [CrossRef]
- Liu, M.S.; Lin, M.C.C.; Huang, I.T.; Wang, C.C. Enhancement of thermal conductivity with CUO for Nanofluids. Chem. Eng. Technol. 2006, 29, 72–77. [Google Scholar] [CrossRef]
- Li, C.H.; Peterson, G.P. Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticle suspensions (nanofluids). J. Appl. Phys. 2006, 99, 084314. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. Exp. Therm. Fluid Sci. 2007, 31, 593–599. [Google Scholar] [CrossRef]
- Haddad, Z.; Abu-Nada, E.; Oztop, H.F.; Mataoui, A. Natural convection in nanofluids: Are the thermophoresis and Brownian motion effects significant in nanofluid heat transfer enhancement? Int. J. Therm. Sci. 2012, 57, 152–162. [Google Scholar] [CrossRef]
- Azizian, R.; Doroodchi, E.; Moghtaderi, B. Effect of nanoconvection caused by Brownian motion on the enhancement of thermal conductivity in nanofluids. Ind. Eng. Chem. Res. 2011, 51, 1782–1789. [Google Scholar] [CrossRef]
- Çolak, A.B. Experimental study for thermal conductivity of water-based zirconium oxide nanofluid: Developing optimal artificial neural network and proposing new correlation. Int. J. Energy Res. 2020, 45, 2912–2930. [Google Scholar] [CrossRef]
- Mintsa, H.A.; Roy, G.; Nguyen, C.T.; Doucet, D. New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 2009, 48, 363–371. [Google Scholar] [CrossRef]
- Yu, C.J.; Richter, A.; Datta, A.; Durbin, M.; Dutta, P. Molecular layering in a liquid on a solid substrate: An X-ray reflectivity study. Phys. B Condens. Matter 2000, 283, 27–31. [Google Scholar] [CrossRef]
- Yu, C.J.; Richter, A.; Datta, A.; Durbin, M.; Dutta, P. Observation of molecular layering in thin liquid films using X-ray reflectivity. Phys. Rev. Lett. 1999, 82, 2326. [Google Scholar] [CrossRef]
- Tillman, P.; Hill, J.M. Determination of nanolayer thickness for a nanofluid. Int. Commun. Heat Mass Transf. 2007, 34, 399–407. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Ellison, J.; Tschulik, K.; Stuart, E.J.; Jurkschat, K.; Omanovi´c, D.; Uhlemann, M.; Crossley, A.; Compton, R.G. Get more out of your data: A new approach to agglomeration and aggregation studies using nanoparticle impact experiments. ChemistryOpen 2013, 2, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Meakin, P. Fractal aggregates. Adv. Colloid Interface Sci. 1987, 28, 249–331. [Google Scholar] [CrossRef]
- Shima, P.D.; Philip, J.; Raj, B. Influence of aggregation on thermal conductivity in stable and unstable nanofluids. Appl. Phys. Lett. 2010, 97, 153113. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Liu, S.; Tang, Y.; Yin, Y. Effects of nanoparticle clustering and alignment on thermal conductivities of fe3o4 aqueous nanofluids. Appl. Phys. Lett. 2006, 89, 023123. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, L. Monodisperse magnetite nanofluids: Synthesis, aggregation, and thermal conductivity. J. Appl. Phys. 2010, 108, 114311. [Google Scholar] [CrossRef]
- Ju, Y.S.; Kim, J.; Hung, M.T. Experimental study of heat conduction in aqueous suspensions of aluminum oxide nanoparticles. J. Heat Transf. 2008, 130, 092403. [Google Scholar] [CrossRef]
- Bruggeman, V.D. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Sedeh, R.N.; Abdollahi, A.; Karimipour, A. Experimental investigation toward obtaining nanoparticles’ surficial interaction with basefluid components based on measuring thermal conductivity of nanofluids. Int. Commun. Heat Mass Transf. 2019, 103, 72–82. [Google Scholar] [CrossRef]
- Teng, T.P.; Hung, Y.H.; Teng, T.C.; Mo, H.E.; Hsu, H.G. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl. Therm. Eng. 2010, 30, 2213–2218. [Google Scholar] [CrossRef]
- Kanti, P.; Sharma, K.; Yashawantha, K.M.; Jamei, M.; Said, Z. Properties of water-based fly ash-copper hybrid nanofluid for solar energy applications: Application of RBF model. Sol. Energy Mater. Sol. Cells 2022, 234, 111423. [Google Scholar] [CrossRef]
- Asadi, A.; Bakhtiyari, A.N.; Alarifi, I.M. Predictability evaluation of support vector regression methods for thermophysical properties, heat transfer performance, and pumping power estimation of MWCNT/ZnO–engine oil hybrid nanofluid. Eng. Comput. 2021, 37, 3813–3823. [Google Scholar] [CrossRef]
- Malika, M.; Sonawane, S.S. Application of RSM and ANN for the prediction and optimization of thermal conductivity ratio of water based Fe2O3 coated SiC hybrid nanofluid. Int. Commun. Heat Mass Transf. 2021, 126, 105354. [Google Scholar] [CrossRef]
- Moghadam, I.P.; Afrand, M.; Hamad, S.M.; Barzinjy, A.A.; Talebizadehsardari, P. Curve-fitting on experimental data for predicting the thermal-conductivity of a new generated hybrid nanofluid of graphene oxide-titanium oxide/water. Phys. A Stat. Mech. Its Appl. 2020, 548, 122140. [Google Scholar] [CrossRef]
- Urmi, W.; Rahman, M.; Hamzah, W. An experimental investigation on the thermophysical properties of 40% ethylene glycol based TiO2-Al2O3 hybrid nanofluids. Int. Commun. Heat Mass Transf. 2020, 116, 104663. [Google Scholar] [CrossRef]
- Kazemi, I.; Sefid, M.; Afrand, M. Improving the thermal conductivity of water by adding mono & hybrid nano-additives containing graphene and silica: A comparative experimental study. Int. Commun. Heat Mass Transf. 2020, 116, 104648. [Google Scholar]
- Asadikia, A.; Mirjalily, S.A.A.; Nasirizadeh, N.; Kargarsharifabad, H. Characterization of thermal and electrical properties of hybrid nanofluids prepared with multi-walled carbon nanotubes and Fe2O3 nanoparticles. Int. Commun. Heat Mass Transf. 2020, 117, 104603. [Google Scholar] [CrossRef]
- Ji, W.; Yang, L.; Chen, Z.; Mao, M.; Huang, J.N. Experimental studies and ANN predictions on the thermal properties of TiO2-Ag hybrid nanofluids: Consideration of temperature, particle loading, ultrasonication and storage time. Powder Technol. 2021, 388, 212–232. [Google Scholar] [CrossRef]
- Öcal, S.; Gökçek, M.; Çolak, A.B.; Korkanç, M. A comprehensive and comparative experimental analysis on thermal conductivity of TiO2-CaCO3/water hybrid nanofluid: Proposing new correlation and artificial neural network optimization. Heat Transf. Res. 2021, 52, 55–79. [Google Scholar] [CrossRef]
- Al-Oran, O.; Lezsovits, F. Experimental Study of Thermal Conductivity and Viscosity of Water-Based MWCNT-Y2O3 Hybrid Nanofluid with Surfactant. J. Eng. Thermophys. 2022, 31, 98–110. [Google Scholar] [CrossRef]
- Nfawa, S.R.; Basri, A.A.; Masuri, S.U.; Abu Talib, A.R. Novel use of MgO nanoparticle additive for enhancing the thermal conductivity of CuO/water nanofluid. Case Stud. Therm. Eng. 2021, 27, 101279. [Google Scholar] [CrossRef]
- Giwa, S.; Momin, M.; Nwaokocha, C.; Sharifpur, M.; Meyer, J. Influence of nanoparticles size, per cent mass ratio, and temperature on the thermal properties of water-based MgO–ZnO nanofluid: An experimental approach. J. Therm. Anal. Calorim. 2021, 143, 1063–1079. [Google Scholar] [CrossRef]
- Esfe, M.H.; Alirezaie, A.; Toghraie, D. Thermal conductivity of ethylene glycol based nanofluids containing hybrid nanoparticles of SWCNT and Fe3O4 and its price-performance analysis for energy management. J. Mater. Res. Technol. 2021, 14, 1754–1760. [Google Scholar] [CrossRef]
- Kamel, M.S.; Al-Oran, O.; Lezsovits, F. Thermal conductivity of Al2O3 and CeO2 nanoparticles and their hybrid based water nanofluids: An experimental study. Period. Polytech. Chem. Eng. 2021, 65, 50–60. [Google Scholar] [CrossRef]
- Hamid, K.; Azmi, W.; Nabil, M.; Mamat, R. Improved thermal conductivity of TiO2–SiO2 hybrid nanofluid in ethylene glycol and water mixture. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 257, p. 012067. [Google Scholar]
- Ramadhan, A.I.; Azmi, W.H.; Mamat, R. Experimental investigation of thermo-physical properties of tri-hybrid nanoparticles in water-ethylene glycol mixture. Walailak J. Sci. Technol. (WJST) 2021, 18, 9335. [Google Scholar] [CrossRef]
- Adun, H.; Kavaz, D.; Dagbasi, M.; Umar, H.; Wole-Osho, I. An experimental investigation of thermal conductivity and dynamic viscosity of Al2O3-ZnO-Fe3O4 ternary hybrid nanofluid and development of machine learning model. Powder Technol. 2021, 394, 1121–1140. [Google Scholar] [CrossRef]
- Dezfulizadeh, A.; Aghaei, A.; Joshaghani, A.H.; Najafizadeh, M.M. An experimental study on dynamic viscosity and thermal conductivity of water-Cu-SiO2-MWCNT ternary hybrid nanofluid and the development of practical correlations. Powder Technol. 2021, 389, 215–234. [Google Scholar] [CrossRef]
- Babu, J.R.; Kumar, K.K.; Rao, S.S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Shah, T.R.; Ali, H.M. Applications of hybrid nanofluids in solar energy, practical limitations and challenges: A critical review. Sol. Energy 2019, 183, 173–203. [Google Scholar] [CrossRef]
- Apmann, K.; Fulmer, R.; Soto, A.; Vafaei, S. Thermal Conductivity and Viscosity: Review and Optimization of Effects of Nanoparticles. Materials 2021, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gupta, M.; Katyal, P. Experimental Investigation on Viscosity of CuO/Water Nanofluid. Int. J. Res. Eng. Appl. Manag. 2018, 4, 271–277. [Google Scholar] [CrossRef]
- Ebrahimnia-Bajestan, E.; Moghadam, M.C.; Niazmand, H.; Daungthongsuk, W.; Wongwises, S. Experimental and numerical investigation of nanofluids heat transfer characteristics for application in solar heat exchangers. Int. J. Heat Mass Transf. 2016, 92, 1041–1052. [Google Scholar] [CrossRef]
- Ahammed, N.; Asirvatham Lazarus, G.; Wongwises, S. Effect of volume concentration and temperature on viscosity and surface tension of graphene-water nanofluid for heat transfer applications. J. Therm. Anal. Calorim. 2015, 123, 1399–1409. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Jing, Y.; Qu, P.; Wu, Z. Effect of particle size on the heat transfer performance of SiO2-water nanofluids. J. Phys. Chem. 2021, 125, 13590–13600. [Google Scholar] [CrossRef]
- Timofeeva, E.; Smith, D.; Yu, W.; France, D.; Singh, D.; Routbort, J. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-based alpha-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Onsager, L. Electric moments of molecules in liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Kanti, P.; Sharma, K.V.; CG, R.; Azmi, W. Experimental determination of thermophysical properties of Indonesian fly-ash nanofluid for heat transfer applications. Part. Sci. Technol. 2021, 39, 597–606. [Google Scholar] [CrossRef]
- Hou, X.; Wang, R.T.; Huang, S.; Wang, J.C. Thermoelectric Generation and Thermophysical Properties of Metal Oxide Nanofluids. J. Mar. Sci. Technol. 2021, 29, 8. [Google Scholar] [CrossRef]
- Dong, J.; Zheng, Q.; Xiong, C.; Sun, E.; Chen, J. Experimental investigation and application of stability and thermal characteristics of SiO2-ethylene-glycol/water nanofluids. Int. J. Therm. Sci. 2022, 176, 107533. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Z. Experiment and artificial neural network prediction of thermal conductivity and viscosity for alumina-water nanofluids. Materials 2017, 10, 552. [Google Scholar] [CrossRef]
- Hussein, A.M.; Sharma, K.; Bakar, R.; Kadirgama, K. The effect of nanofluid volume concentration on heat transfer and friction factor inside a horizontal tube. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.; Casanova, C.; Páramo, R.; Barbés, B.; Legido, J.; Piñeiro, M. A study on stability and thermophysical properties (density and viscosity) of Al2 O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Mohammed, H.A.; Ramasamy, D.; Samykano, M.; Saidur, R. Analysis of efficiency enhancement of flat plate solar collector using crystal nano-cellulose (CNC) nanofluids. Sustain. Energy Technol. Assess. 2021, 45, 101049. [Google Scholar] [CrossRef]
- Abdolbaqi, M.K.; Azmi, W.; Mamat, R.; Sharma, K.; Najafi, G. Experimental investigation of thermal conductivity and electrical conductivity of BioGlycol–water mixture based Al2O3 nanofluid. Appl. Therm. Eng. 2016, 102, 932–941. [Google Scholar] [CrossRef]
- Lahari, M.C.; Sai, P.S.T.; Sharma, K.; Narayanaswamy, K.; Bee, P.H.; Devaraj, S. Analysis of parallel flow heat exchanger using SiO2 nanofluids in the laminar flow region. J. Phys. Conf. Ser. IOP Publ. 2021, 2070, 012230. [Google Scholar] [CrossRef]
- Bahiraei, M.; Hosseinalipour, S.M.; Zabihi, K.; Taheran, E. Using neural network for determination of viscosity in water-TiO2 nanofluid. Adv. Mech. Eng. 2012, 4, 742680. [Google Scholar] [CrossRef]


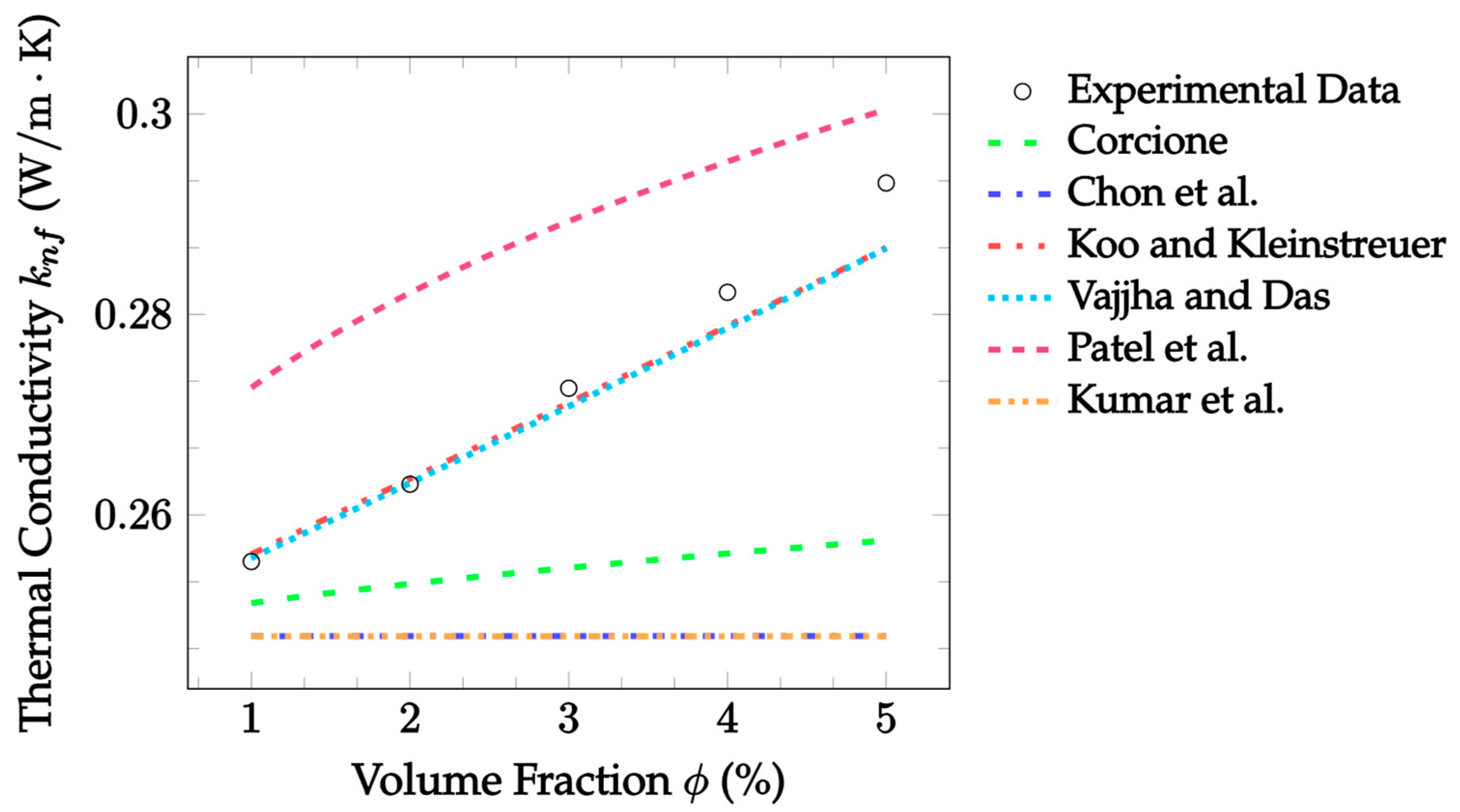
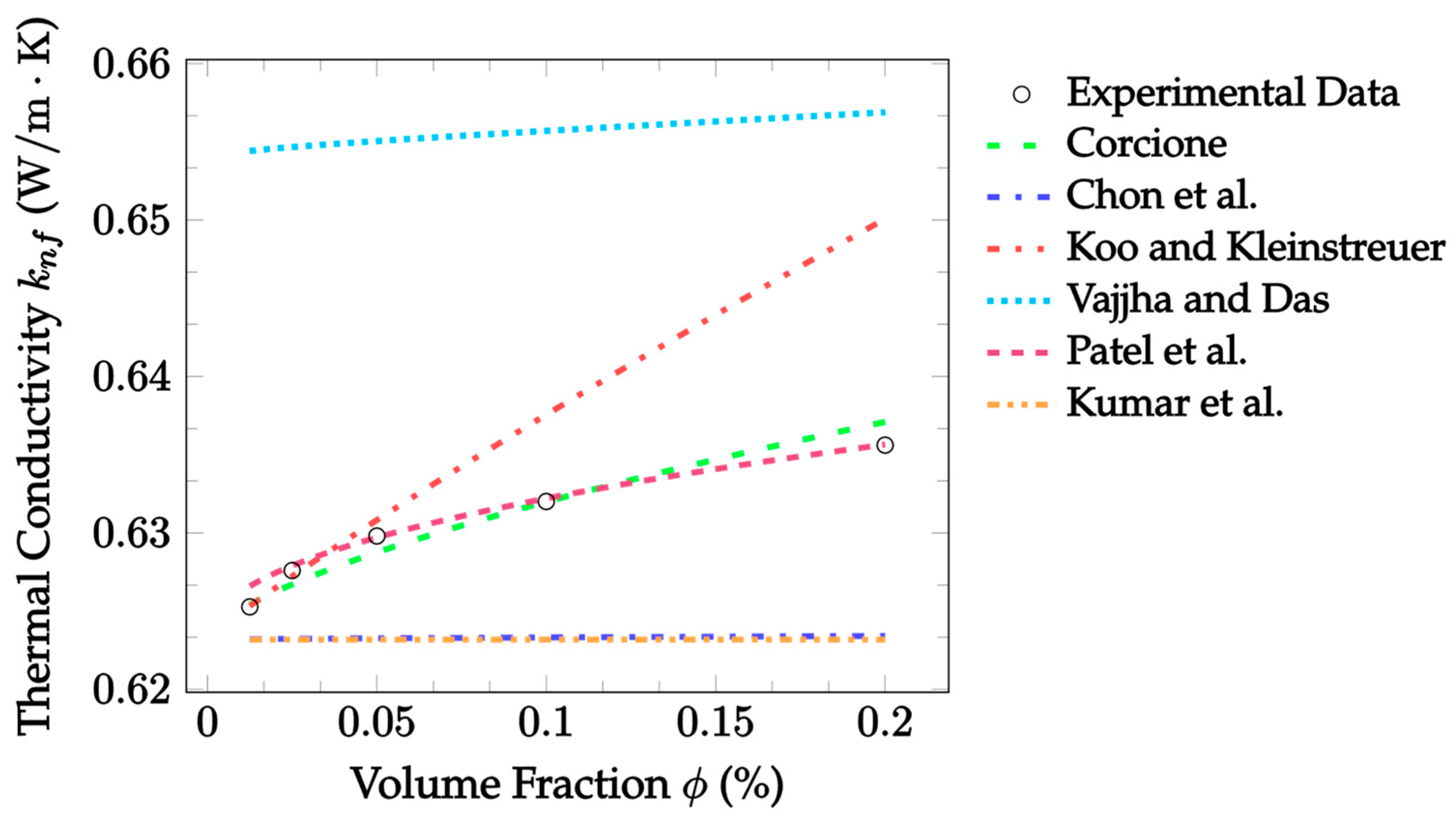
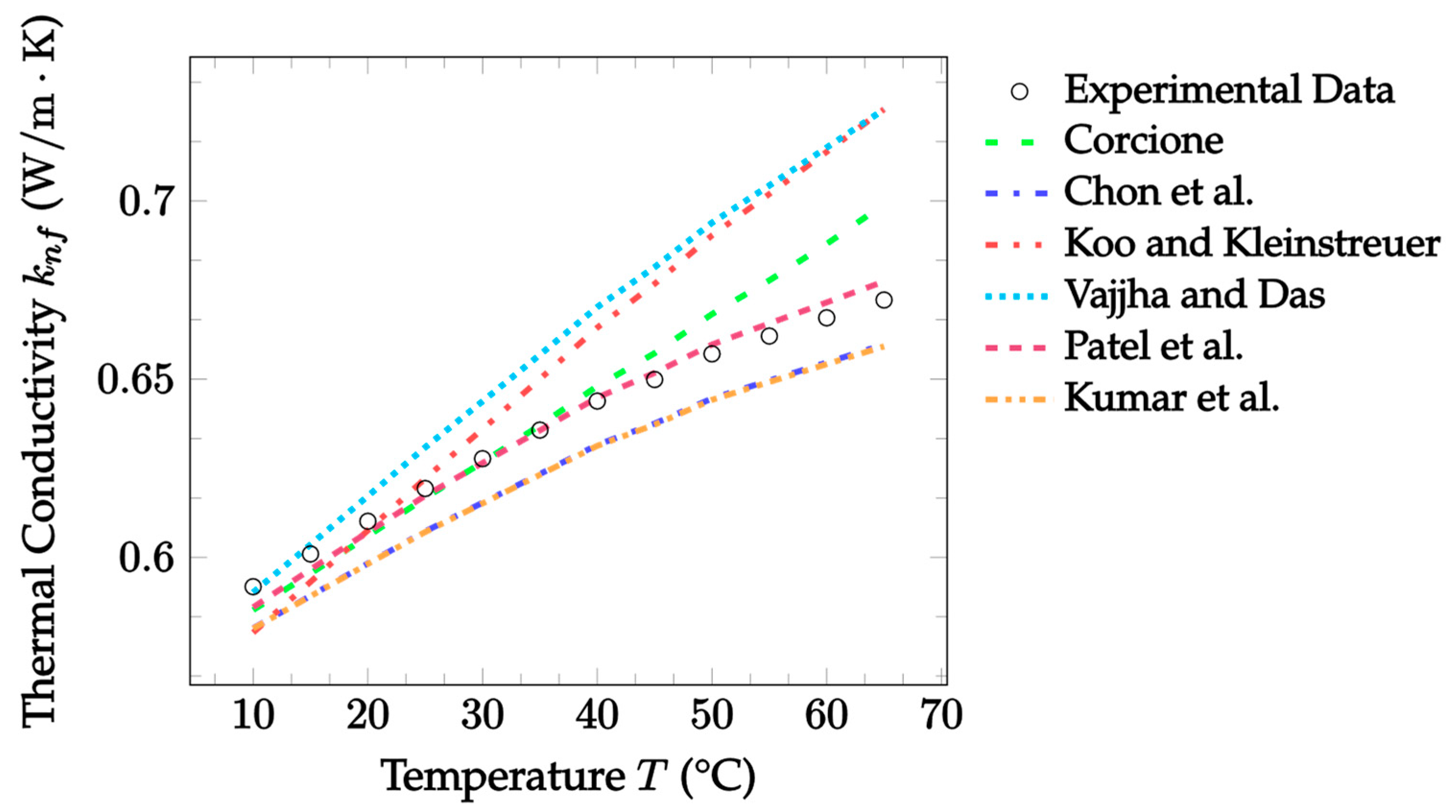
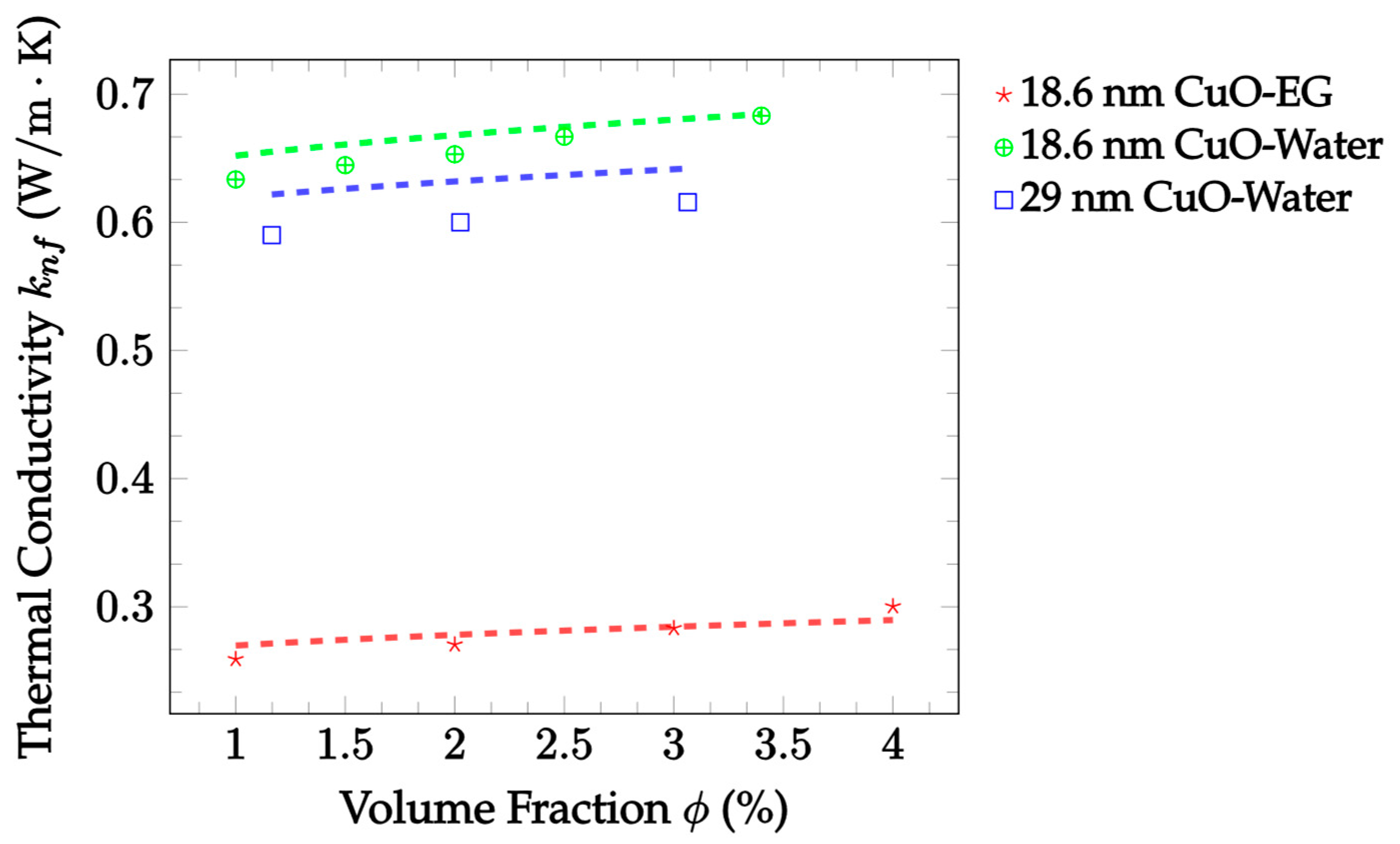
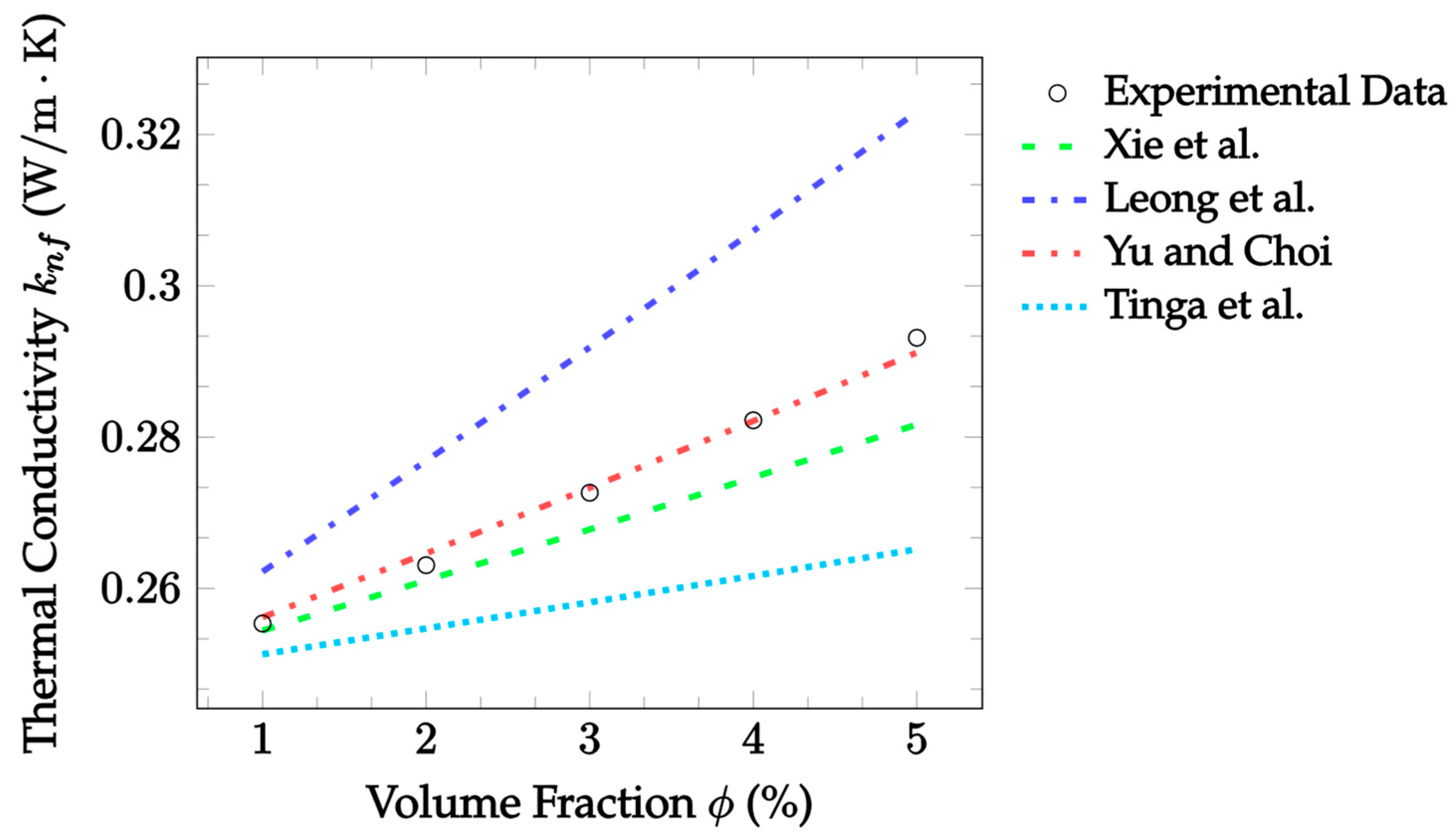
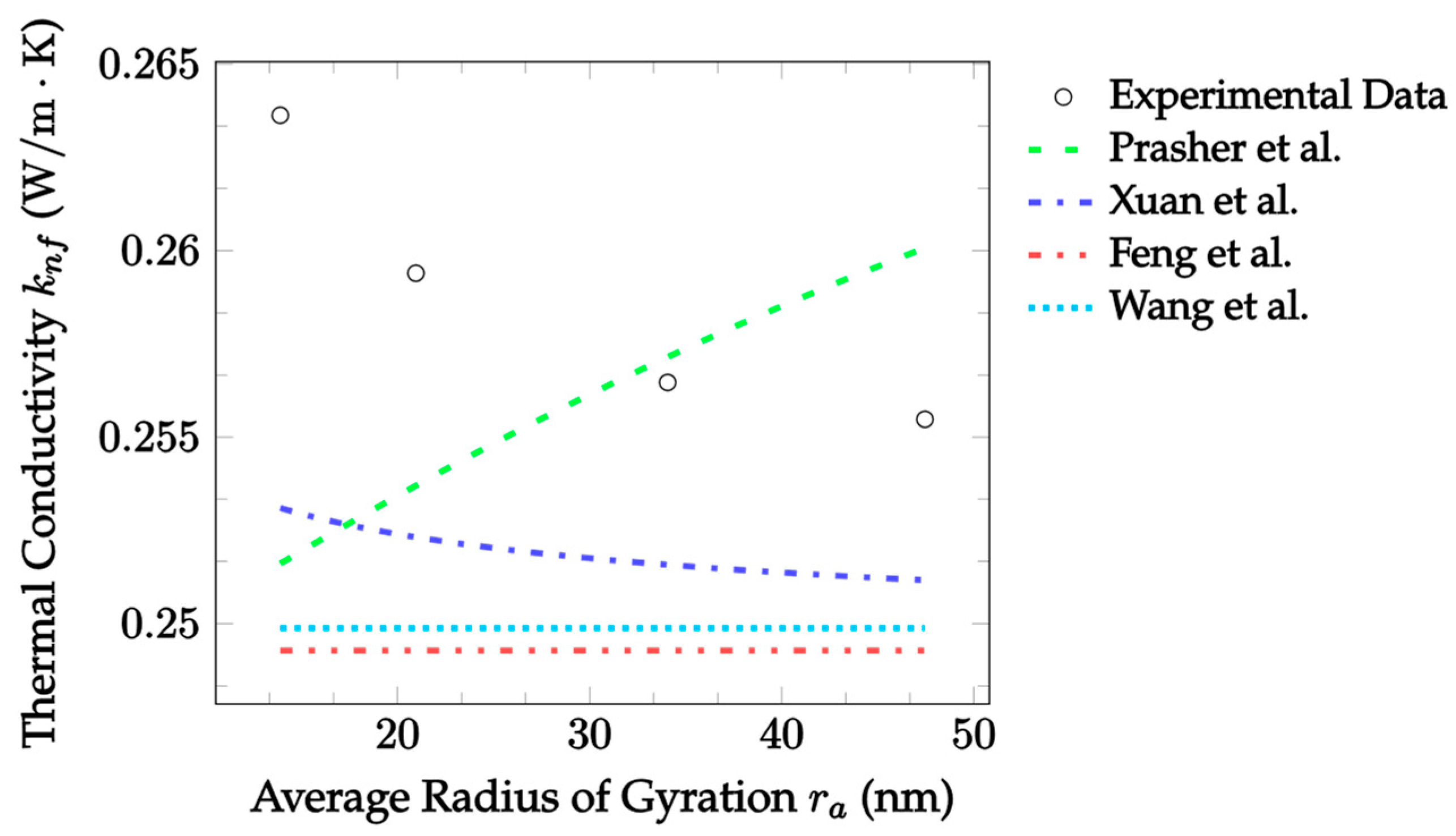
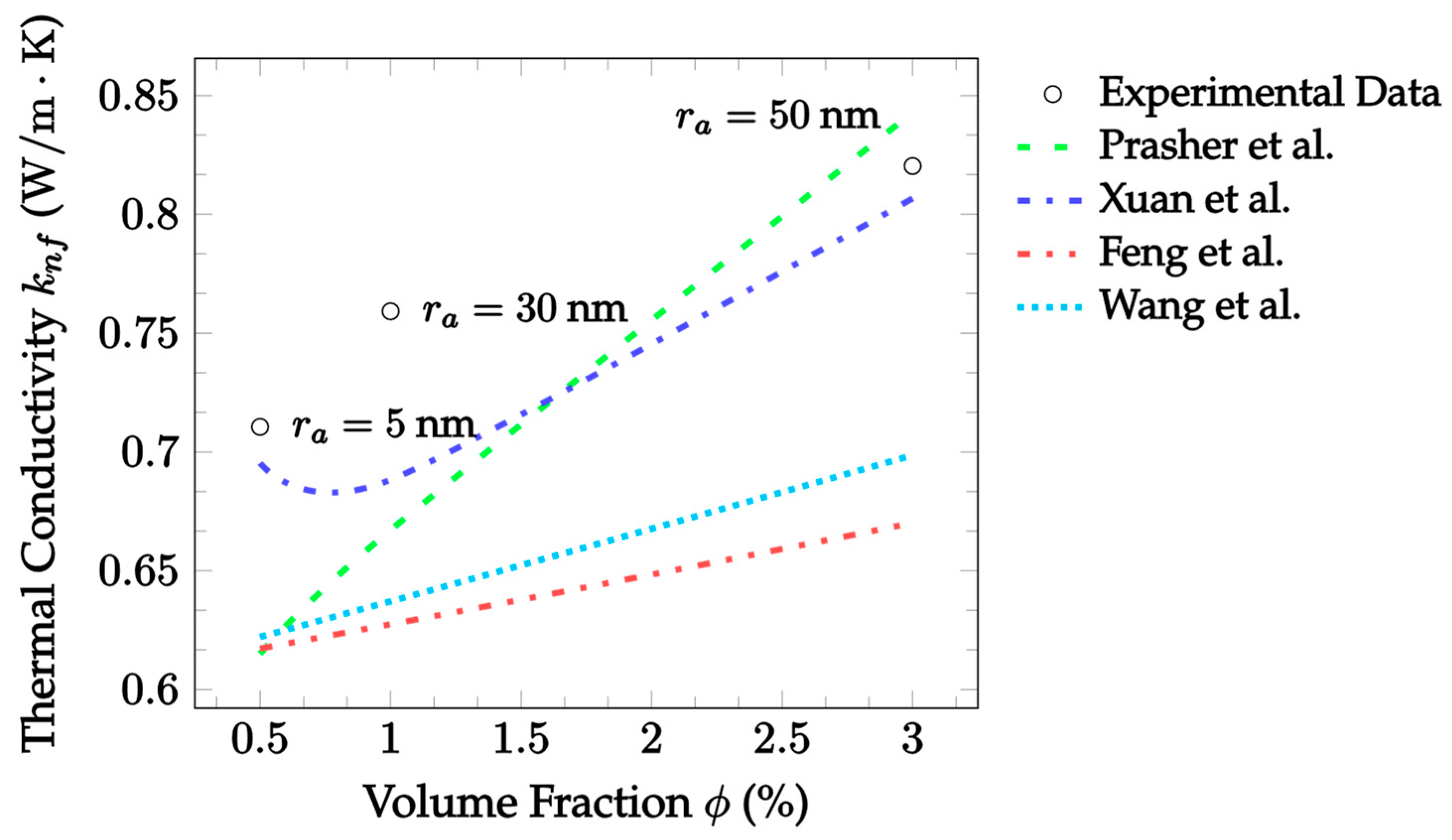
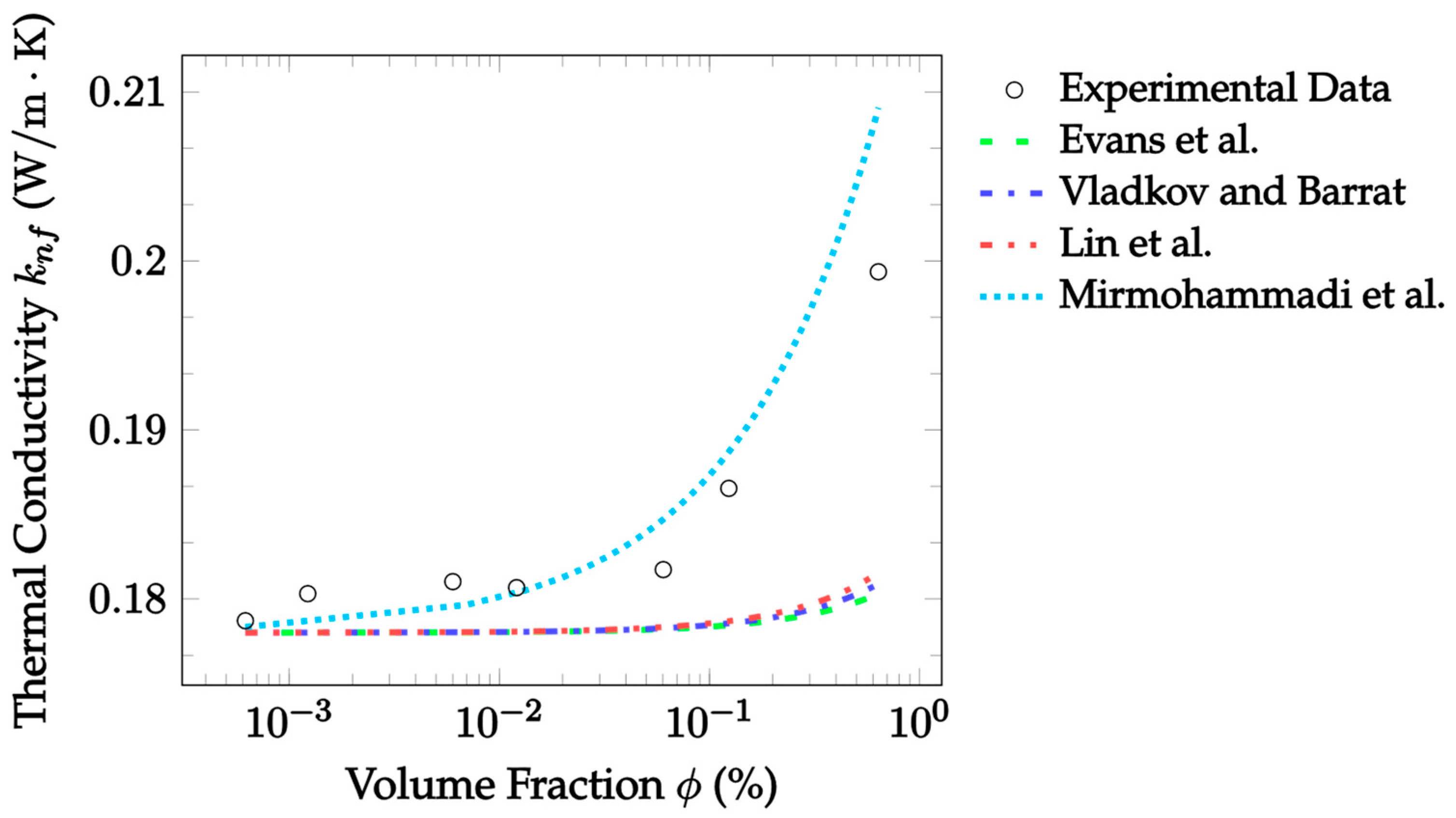
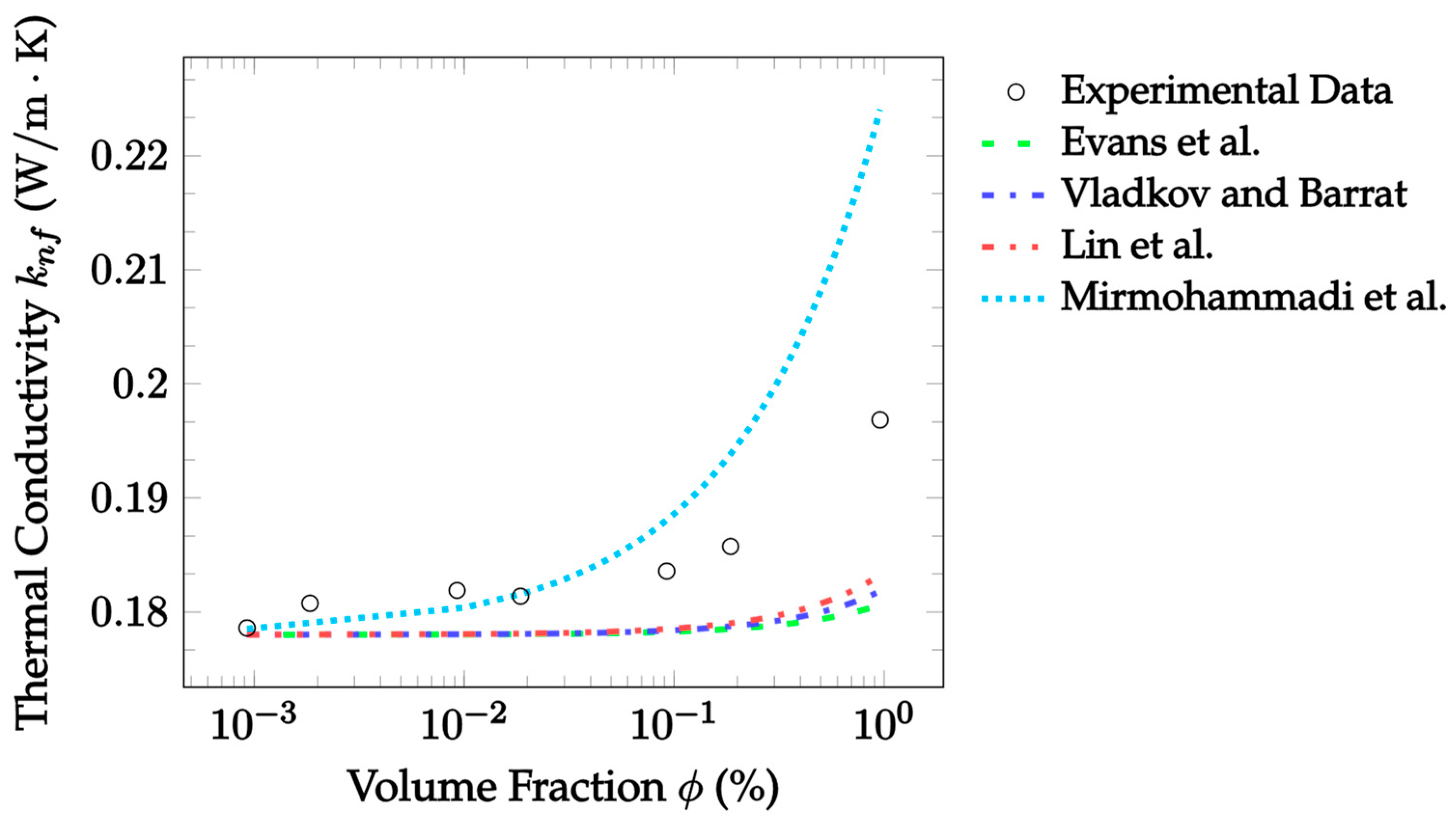
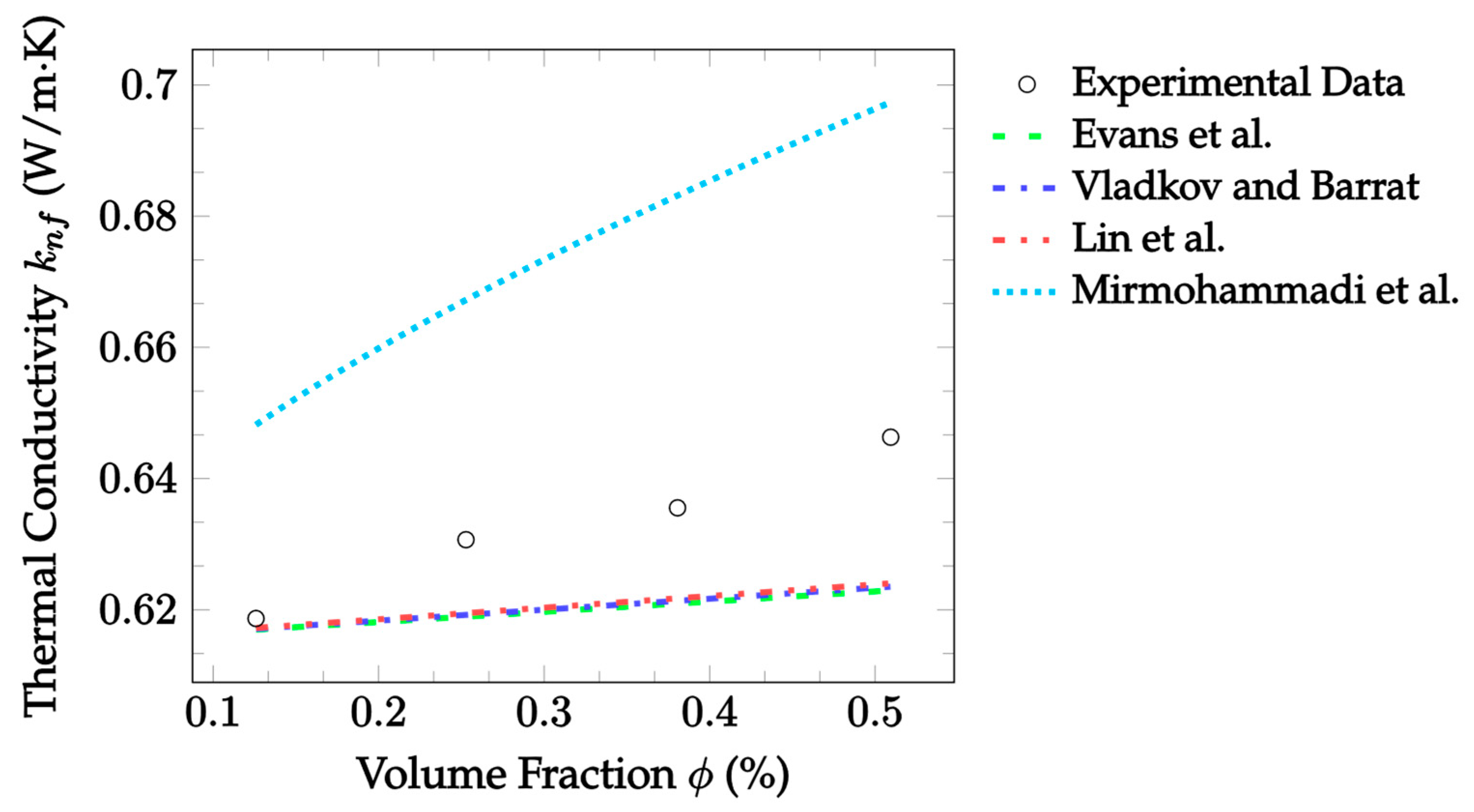
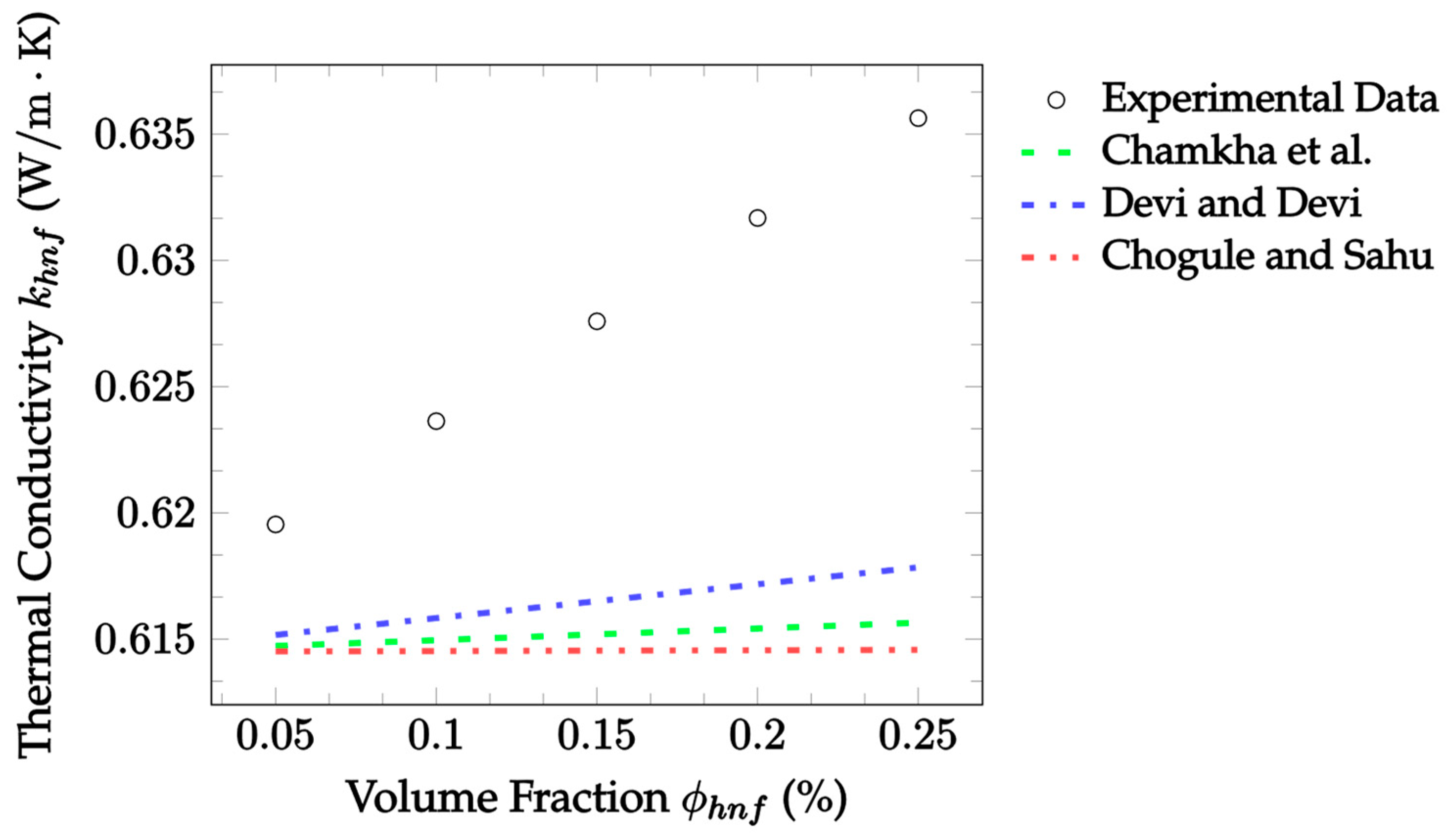
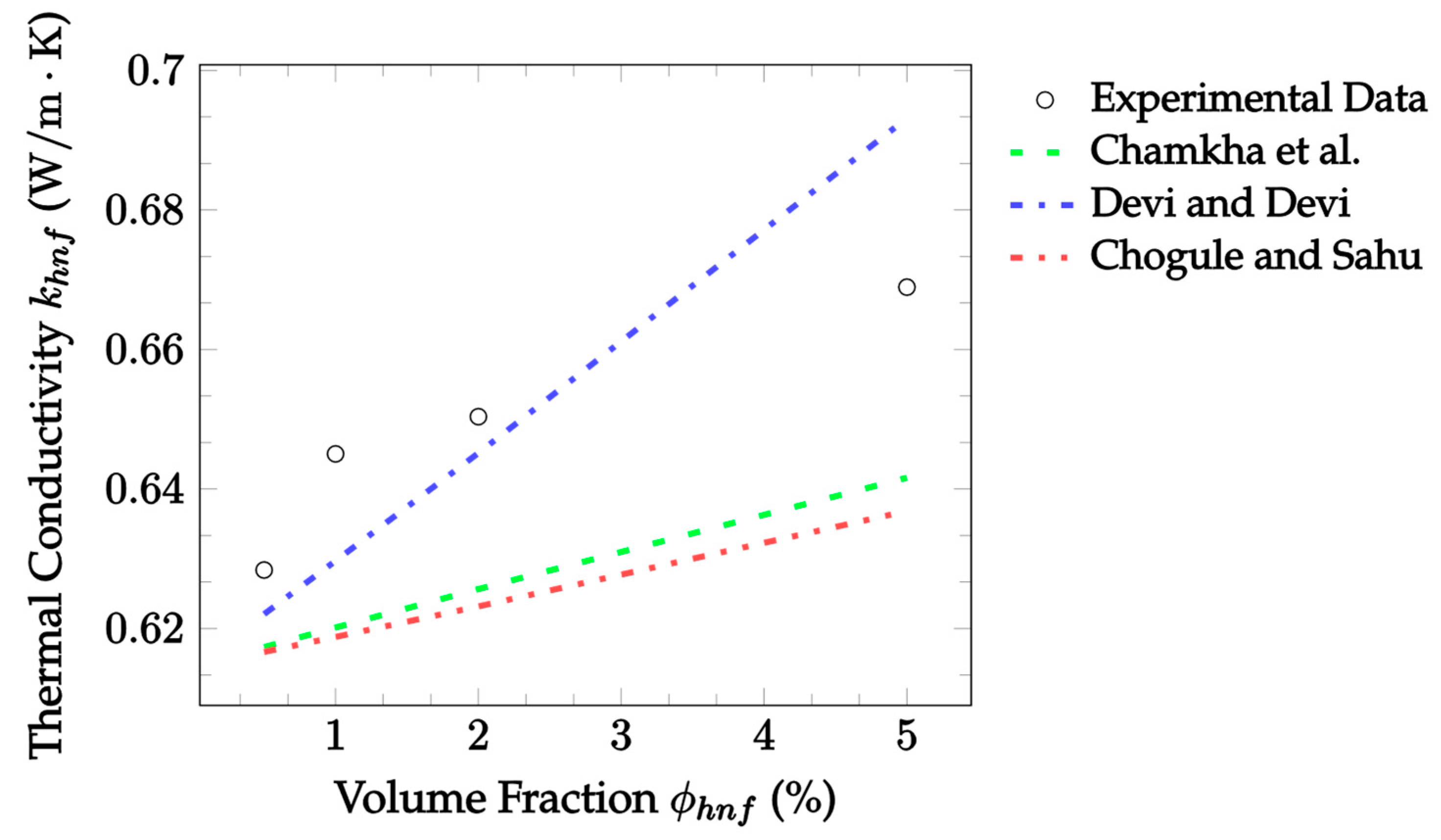
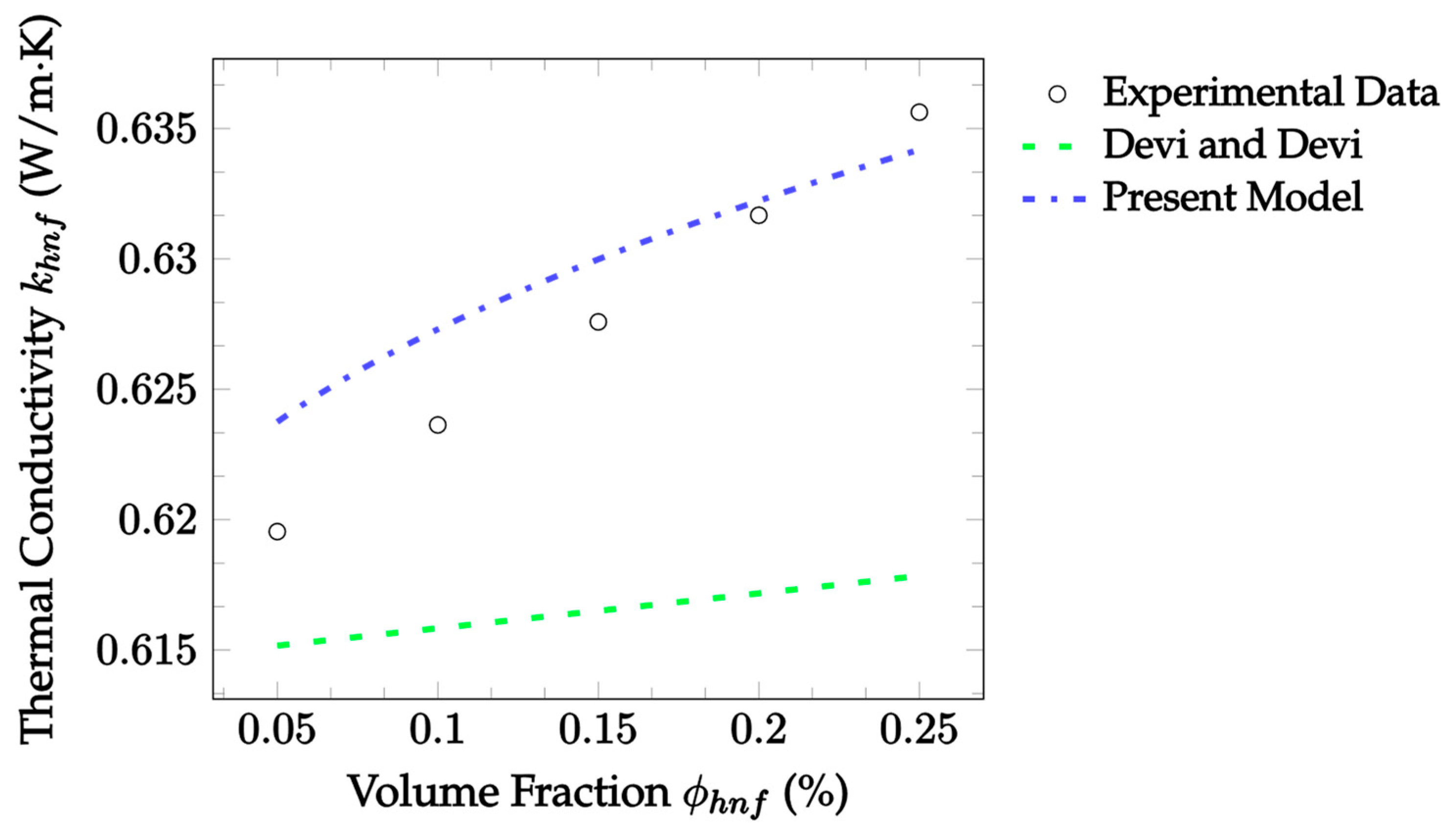
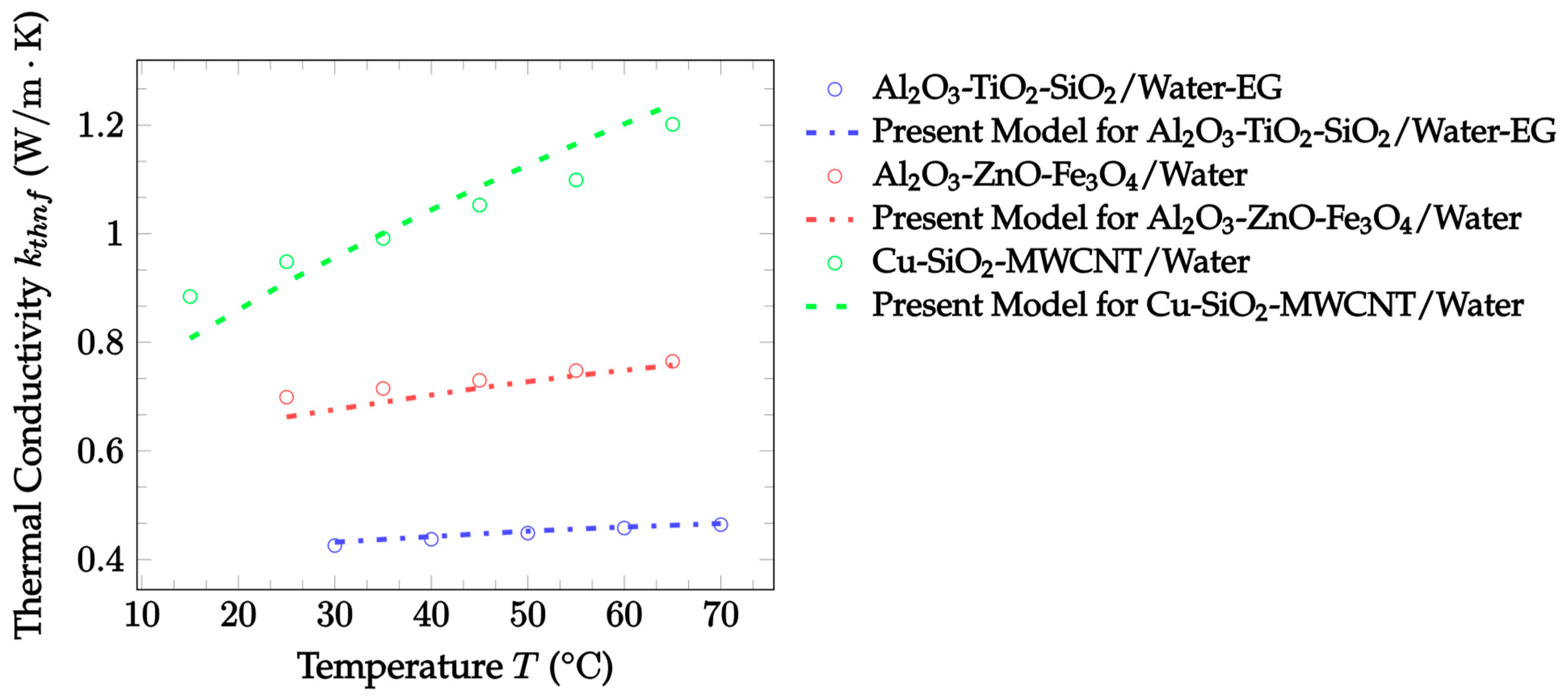
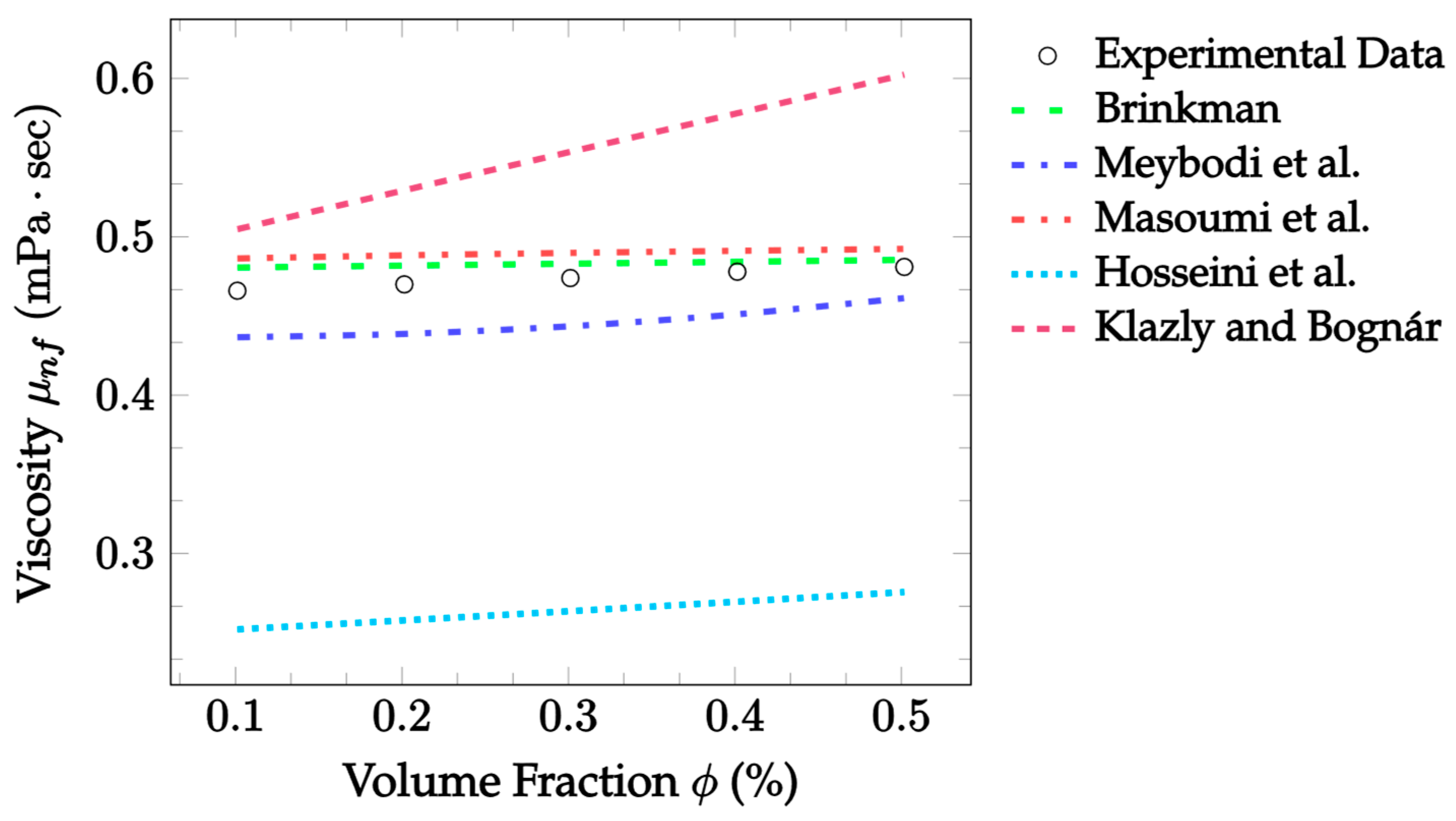

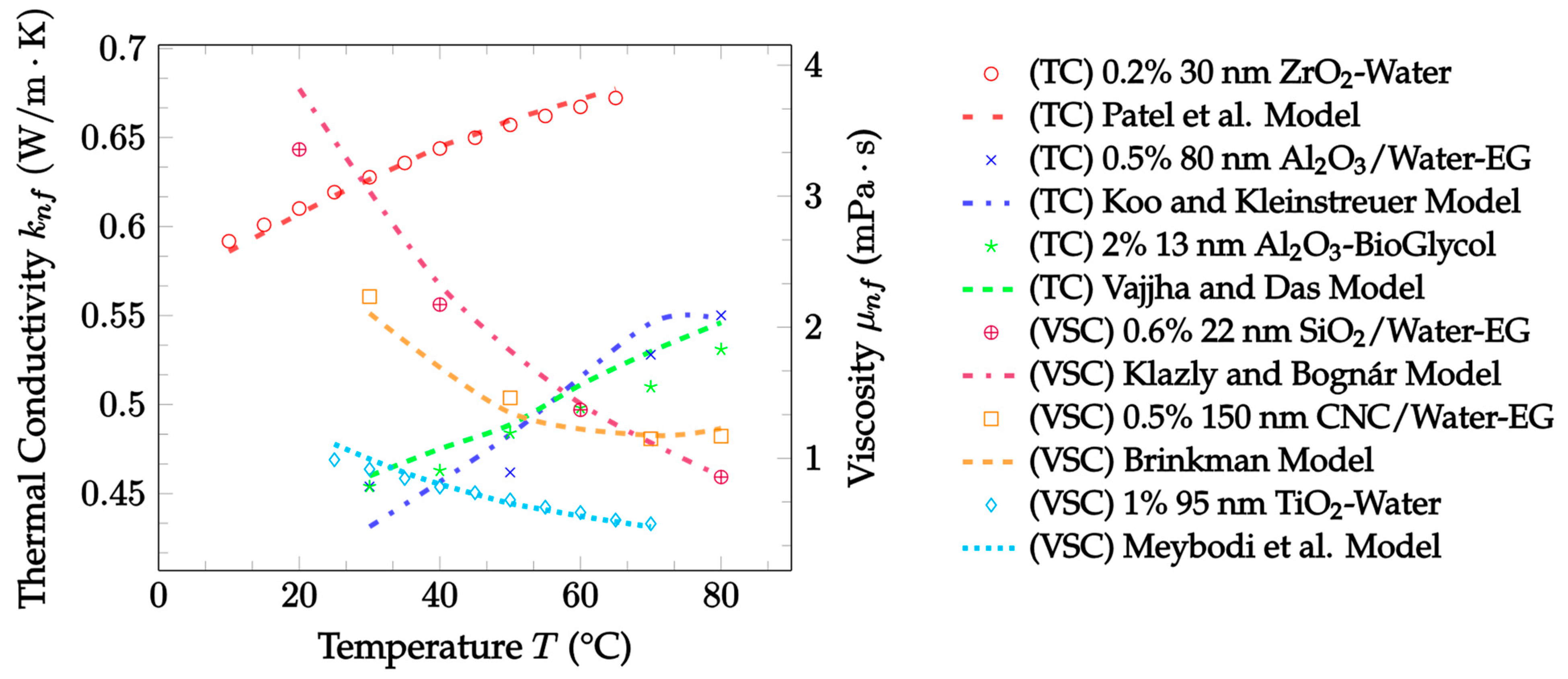
| Dataset | Maxwell [14] | Hamilton and Crosser [15] | Timofeeva et al. [17] | Jeffery [16] | Sundar et al. [18] |
|---|---|---|---|---|---|
| [12] * | 1.626 | 1.626 | 1.719 | 2.216 | 3.698 |
| [12] | 1.684 | 1.684 | 1.253 | 0.844 | 5.086 |
| [12] | 0.933 | 0.933 | 1.072 | 0.681 | 6.524 |
| , [12] | 4.205 | 4.205 | 4.114 | 3.750 | 8.522 |
| , [59] | 1.501 | 1.501 | 1.612 | 2.012 | 3.127 |
| [59] ** | 1.379 | 1.379 | 0.941 | 0.547 | 4.824 |
| [60] | 4.229 | 4.229 | 4.172 | 3.671 | 9.436 |
| [61] | 8.984 | 8.984 | 8.343 | 10.193 | 4.433 |
| [61] | 15.482 | 15.482 | 14.917 | 13.673 | 22.661 |
| [62] | 15.854 | 13.505 | 15.860 | 15.850 | 16.571 |
| Dataset | Corcione [19] | Chon et al. [20] | Koo and Kleinstreuer [21] | Vajjha and Das [22] | Patel et al. [23] | Kumar et al. [24] |
|---|---|---|---|---|---|---|
| Al2O3–water, 300 K, 24.4 nm, 1–4.3% [12] † | 4.051 | 4.831 | 8.456 | 5.519 | 5.243 | 6.318 |
| CuO–water, 300 K, 18.6 nm, 1–3.4% [12] | 2.180 | 6.223 | 14.88 | 2.847 | 1.804 | 6.994 |
| Al2O3–EG, 300 K, 24.4 nm, 1–5% [12] * | 6.675 | 9.063 | 0.884 | 0.867 | 5.456 | 9.067 |
| CuO–EG, 300 K, 18.6 nm, 1–4%, [12] † | 8.370 | 10.65 | 3.545 | 4.021 | 2.719 | 10.651 |
| Al2O3–water, 22 °C, 36 nm, 1–3% [66] | 3.178 | 3.137 | 5.614 | 4.237 | 4.278 | 3.990 |
| Al2O3–water, 22 °C, 47 nm, 1–3% [66] | 1.720 | 3.171 | 5.202 | 3.959 | 3.882 | 3.952 |
| CuO–water, 22 °C, 29 nm, 1–3% [66] † | 4.316 | 1.984 | 11.96 | 6.063 | 4.997 | 2.572 |
| ZrO2–water, 10 °C, 30 nm, 0.0125–0.2% [65] | 0.653 | 1.078 | 1.172 | 0.541 | 0.496 | 1.085 |
| ZrO2–water, 35 °C, 30 nm, 0.0125–0.2% [65] ** | 0.114 | 1.072 | 0.676 | 4.017 | 0.0624 | 1.088 |
| ZrO2–water, 65 °C, 30 nm, 0.0125–0.2% [65] | 1.832 | 1.052 | 2.994 | 8.578 | 0.493 | 1.088 |
| ZrO2–water, 10–65 °C, 30 nm, 0.2% [65] *** | 1.371 | 1.922 | 3.441 | 3.807 | 0.461 | 1.964 |
| Dataset | Leong et al. [25] | Xie et al. [26] | Yu and Choi [27] | Tinga et al. [28] |
|---|---|---|---|---|
| TiO2–water, 15 °C, 21 nm [70] | 1.154 | 3.351 | 2.681 | 4.144 |
| TiO2–water, 25 °C, 21 nm [70] | 4.156 | 6.179 | 5.558 | 6.910 |
| TiO2–water, 35 °C, 21 nm, [70] | 1.644 | 1.658 | 1.298 | 3.538 |
| CuO–water, 22 °C, 29 nm [66] | 26.65 | 9.634 | 13.42 | 2.328 |
| Al2O3–water, 22 °C, 36 nm, [66] | 35.18 | 12.20 | 16.22 | 2.320 |
| Al2O3–water, 22 °C, 47 nm [66] | 41.689 | 13.721 | 17.681 | 5.240 |
| Al2O3–water, 27 °C, 24.4 nm [12] | 8.654 | 0.650 | 2.476 | 2.727 |
| CuO–water, 27 °C, 18.6 nm [12] | 2.874 | 2.478 | 0.817 | 3.965 |
| Al2O3–EG, 27 °C, 24.4 nm [12] * | 6.808 | 1.900 | 0.369 | 5.382 |
| CuO–EG, 27 °C, 18.6 nm [12] | 1.769 | 5.158 | 3.121 | 7.034 |
| Dataset | Prasher et al. [29] | Xuan et al. [30] | Feng et al. [31] | Wang et al. [32] |
|---|---|---|---|---|
| CuO–EG, 25 °C, 10 nm, 0.18%, 14–48 m, [73] * | 2.204 | 2.578 | 3.643 | 3.409 |
| Fe3O4–water, 25 °C, 9.8 nm, 1–3%, 5–50 nm [74] ** | 9.442 | 4.377 | 16.27 | 14.45 |
| Fe3O4-Toulene, 24 °C, 12.4 nm, 0.01–0.15%, 15–39 nm [75] | 4.573 | 2.675 | 5.063 | 4.950 |
| Al2O3–water, 25 °C, 30 nm, 5%, 68–74 nm [76] | 87.23 | 39.31 | 9.829 | 18.41 |
| Al2O3–water, 50 °C, 30 nm, 5%, 68–74 nm [76] | 82.02 | 44.53 | 7.686 | 16.13 |
| Dataset | Evans et al. [33] | Vladkov and Barrat [34] | Lin et al. [35] | Mirmohammadi et al. [36] |
|---|---|---|---|---|
| [78] * | 2.947 | 2.891 | 2.827 | 1.409 |
| −0.626% [78] | 10.518 | 10.471 | 10.041 | 6.459 |
| −0.625% [78] | 15.629 | 15.589 | 15.540 | 11.140 |
| 0.957% [78] ** | 2.979 | 2.863 | 2.724 | 3.249 |
| 0.957% [78] | 9.456 | 9.356 | 9.235 | 3.458 |
| Al2O3–water, 30 °C, 20 nm, 0.125–0.511% [79] | 7.464 | 7.223 | 7.009 | 4.844 |
| Al2O3–water, 30 °C, 50 nm, 0.125–0.511% [79] | 3.327 | 3.203 | 3.104 | 6.761 |
| Al2O3–water, 30 °C, 100 nm, 0.125–0.511% [79] *** | 2.013 | 1.957 | 1.906 | 6.490 |
| Al2O3–water, 23 °C, 40 nm, 2.5–10.0% [17] | 2.084 | 1.946 | 3.325 | 49.539 |
| Al2O3–EG, 23 °C, 40 nm, 1.0–10.0% [17] | 1.913 | 1.484 | 2.568 | 43.430 |
| Dataset | Chamkha et al. [37] | Devi and Devi [38] | Chougule and Sahu [39] |
|---|---|---|---|
| 50:50 69 nm TiO2-40 nm CaCO3/Water, 30 °C, 0.05–0.25% [88] * | 1.972 | 1.763 | 3.730 |
| 80:20 29 nm Fly Ash-50 nm Cu/Water, 30 °C, 0.5–4% [80] | 19.793 | 18.948 | 20.069 |
| 80:20 37.5 nm Y2O3-50 nm MWCNT/Water, 30 °C, 0.01–0.2% [89] | 1.929 | 2.028 | 1.896 |
| 10:1 15 nm TiO2-15 nm Ag/Water, 30 °C, 0.5–5% [87] ** | 3.377 | 1.952 | 2.074 |
| 80:20 40 nm CuO-40 nm MgO/Water, 30 °C, 0.25–1.25% [90] | 4.642 | 3.304 | 5.096 |
| 60:40 20 nm MgO-20 nm ZnO/Water, 0.1%, 20–50 °C [91] | 16.693 | 16.532 | 16.681 |
| 60:40 24.24 nm Fe3O4-1.1 nm SWCNT/EG, 50 °C, 0.01–0.5% [92] | 14.538 | 11.000 | 14.765 |
| 50:50 20 nm Al2O3-50 nm CeO2, 50 °C, 0.01–5% [93] | 4.934 | 4.650 | 5.004 |
| 40:25 50 nm TiO2-30 nm SiO2/60:40 Water–EG, 50 °C, 0.5–3% [94] | 10.104 | 8.302 | 11.111 |
| Dataset | Devi and Devi [38] | Present Model |
|---|---|---|
| 50:50 69 nm TiO2-40 nm CaCO3/Water, 30 °C, 0.05–0.25% [88] * | 1.763 | 0.393 |
| 80:20 29 nm Fly Ash-50 nm Cu/Water, 30 °C, 0.5–4% [80] | 18.95 | 11.31 |
| 80:20 37.5 nm Y2O3-50 nm MWCNT/Water, 30 °C, 0.01–0.2% [89] | 2.028 | 0.397 |
| 10:1 15 nm TiO2-15 nm Ag/Water, 30 °C, 0.5–5% [87] | 1.952 | 7.522 |
| 80:20 40 nm CuO-40 nm MgO/Water, 30 °C, 0.25–1.25% [90] | 3.304 | 2.263 |
| 60:40 20 nm MgO-20 nm ZnO/Water, 0.1%, 20–50 °C [91] | 16.53 | 12.65 |
| 60:40 24.24 nm Fe3O4-1.1 nm SWCNT/EG, 50 °C, 0.01–0.5% [92] | 11.00 | 10.15 |
| 50:50 20 nm Al2O3-50 nm CeO2, 50 °C, 0.01–5% [93] | 4.650 | 1.044 |
| 40:25 50 nm TiO2-30 nm SiO2/60:40 Water–EG, 50 °C, 0.5–3% [94] | 8.302 | 1.868 |
| Dataset | Brinkman [48] | Meybodi et al. [49] | Masoumi et al. [50] | Hosseini et al. [51] | Klazly and Bognár [52] |
|---|---|---|---|---|---|
| TiO2–water, 21 nm, 40 °C, 1–5% [108] | 47.91 | 21.78 | 21.95 | 24.48 | 30.48 |
| Fly ash–water, 14 nm, 60 °C, 0.1–0.5% [107] * | 1.960 | 13.34 | 5.820 | 44.34 | 16.80 |
| Graphene-PG, 5 nm, 50 °C, 0.02–0.1% [10] | 9.883 | 27.95 | 22.06 | 41.02 | 9.130 |
| SiO2–EG/Water, 15 nm, 50 °C, 0.5–3% [109] | 25.59 | 17.88 | 20.44 | 32.27 | 30.08 |
| SWCNT–water, 9.2 nm, 20 °C, 0.006–0.56% [11] | 17.83 | 21.34 | 14.89 | 33.81 | 13.23 |
| Al2O3–water, 30 nm, 25 °C, 1.3–5.9% [110] | 30.40 | 22.65 | 24.84 | 59.80 | 67.34 |
| TiO2–water, 30 nm, 25 °C, 1–2.5% [111] | 8.414 | 5.635 | 6.207 | 57.48 | 59.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohld, J.; Beck, J.; Inman, K.; Palmer, M.; Cummings, M.; Fulmer, R.; Vafaei, S. Hybrid Nanofluid Thermal Conductivity and Optimization: Original Approach and Background. Nanomaterials 2022, 12, 2847. https://doi.org/10.3390/nano12162847
Wohld J, Beck J, Inman K, Palmer M, Cummings M, Fulmer R, Vafaei S. Hybrid Nanofluid Thermal Conductivity and Optimization: Original Approach and Background. Nanomaterials. 2022; 12(16):2847. https://doi.org/10.3390/nano12162847
Chicago/Turabian StyleWohld, Jake, Joshua Beck, Kallie Inman, Michael Palmer, Marcus Cummings, Ryan Fulmer, and Saeid Vafaei. 2022. "Hybrid Nanofluid Thermal Conductivity and Optimization: Original Approach and Background" Nanomaterials 12, no. 16: 2847. https://doi.org/10.3390/nano12162847
APA StyleWohld, J., Beck, J., Inman, K., Palmer, M., Cummings, M., Fulmer, R., & Vafaei, S. (2022). Hybrid Nanofluid Thermal Conductivity and Optimization: Original Approach and Background. Nanomaterials, 12(16), 2847. https://doi.org/10.3390/nano12162847




