NaCl-Assisted Chemical Vapor Deposition of Large-Domain Bilayer MoS2 on Soda-Lime Glass
Abstract
:1. Introduction
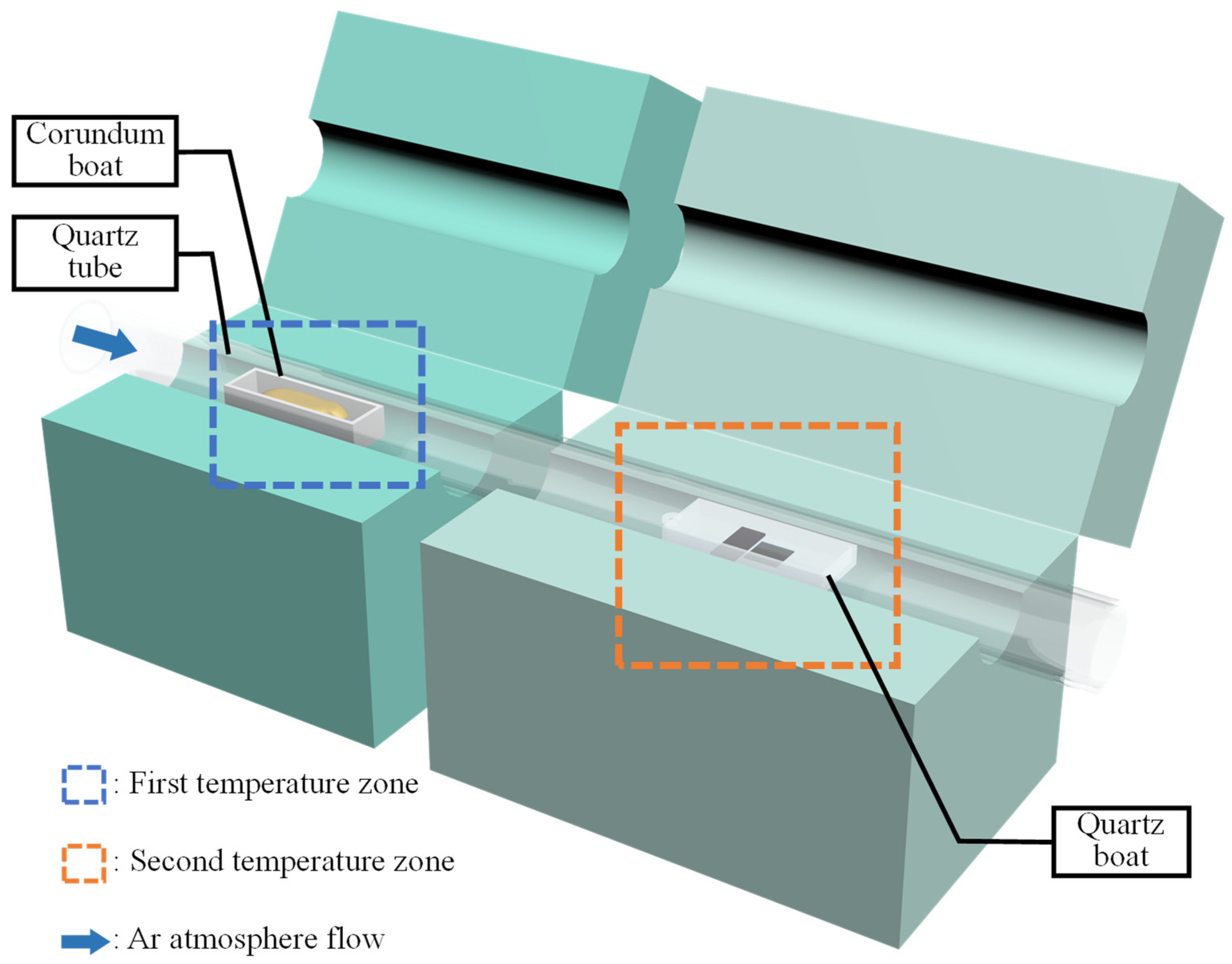
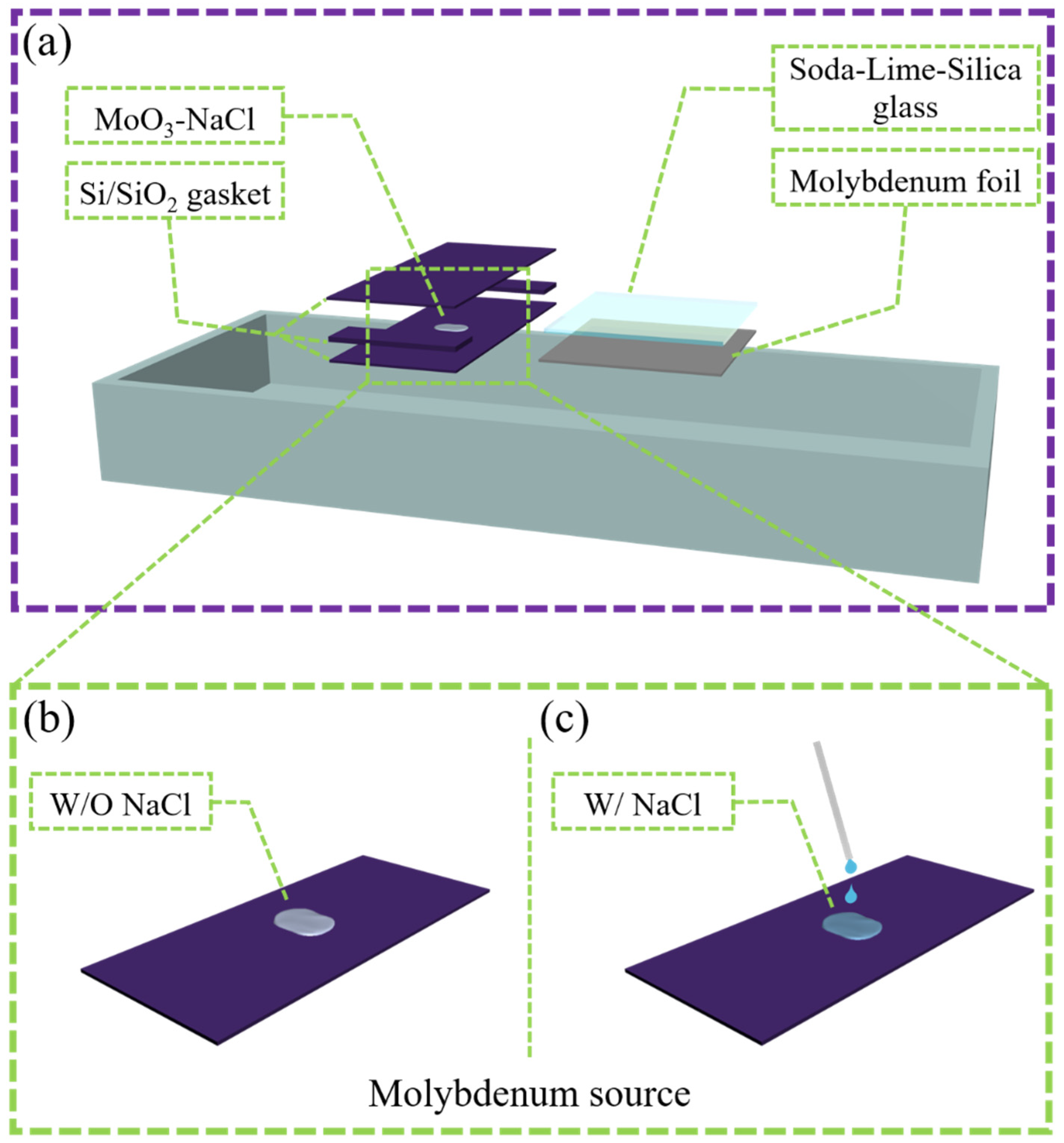
2. Experiments and Methods
3. Results and Discussion
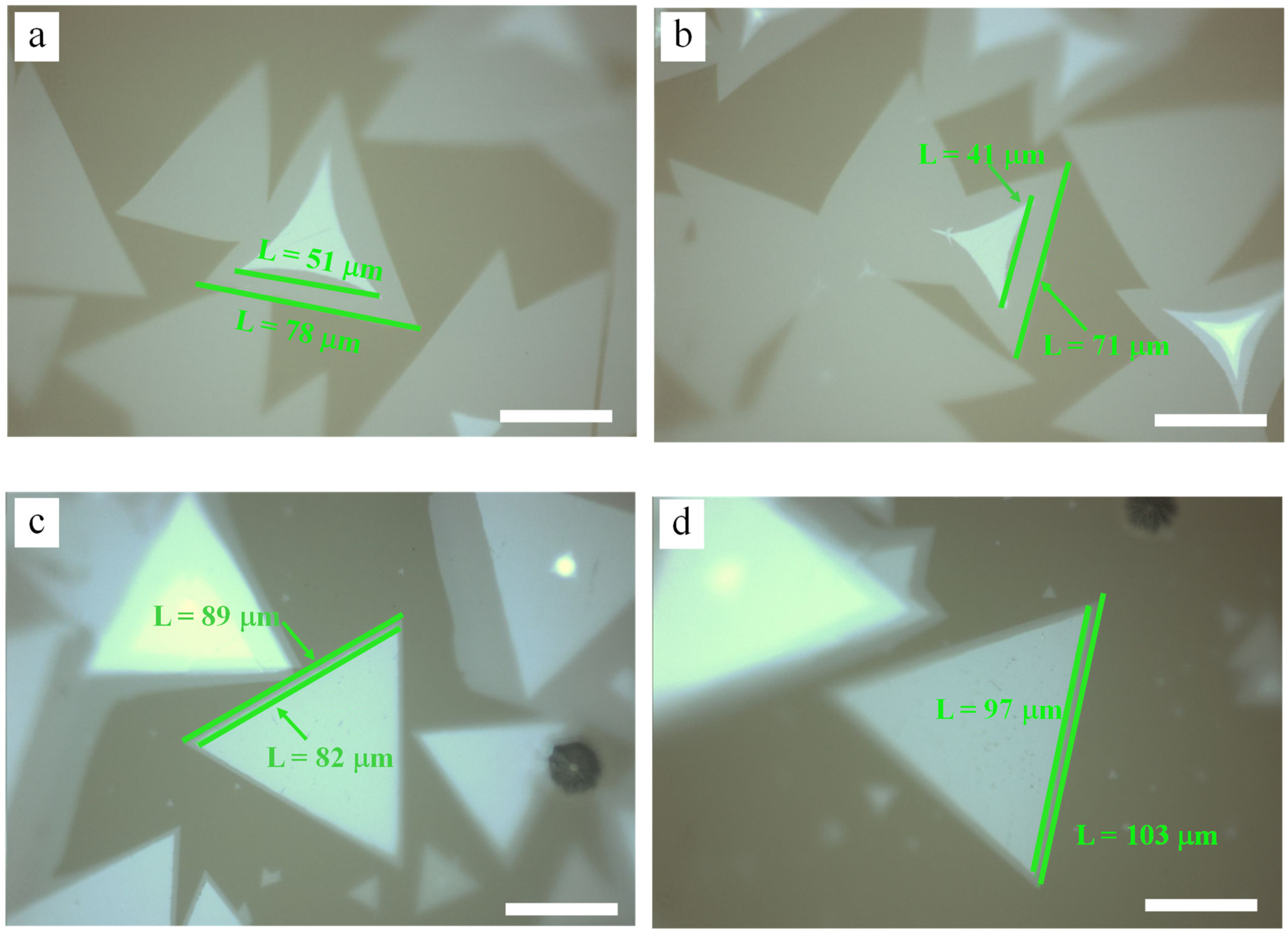
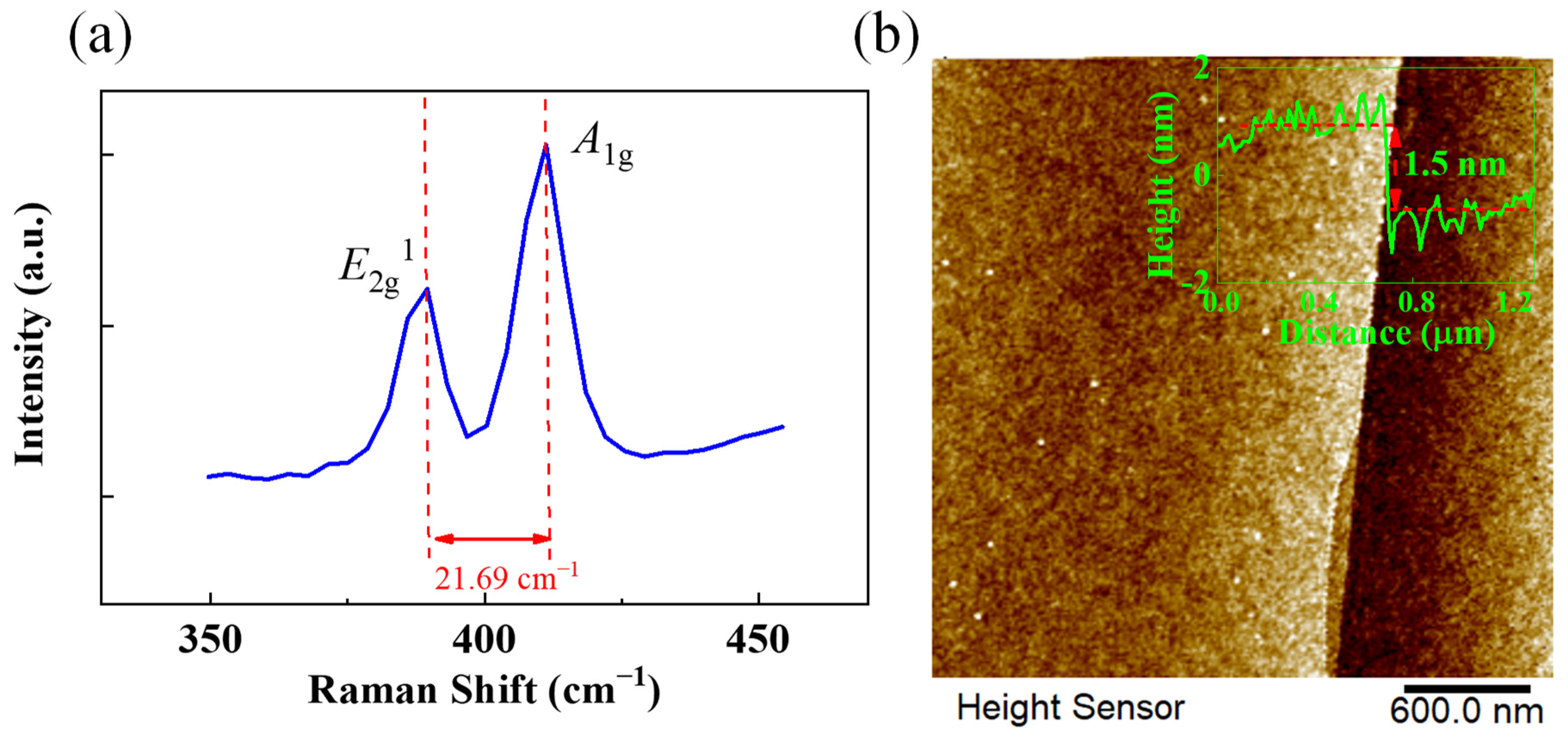
3.1. NaCl-Assisted Growth of Bilayer MoS2
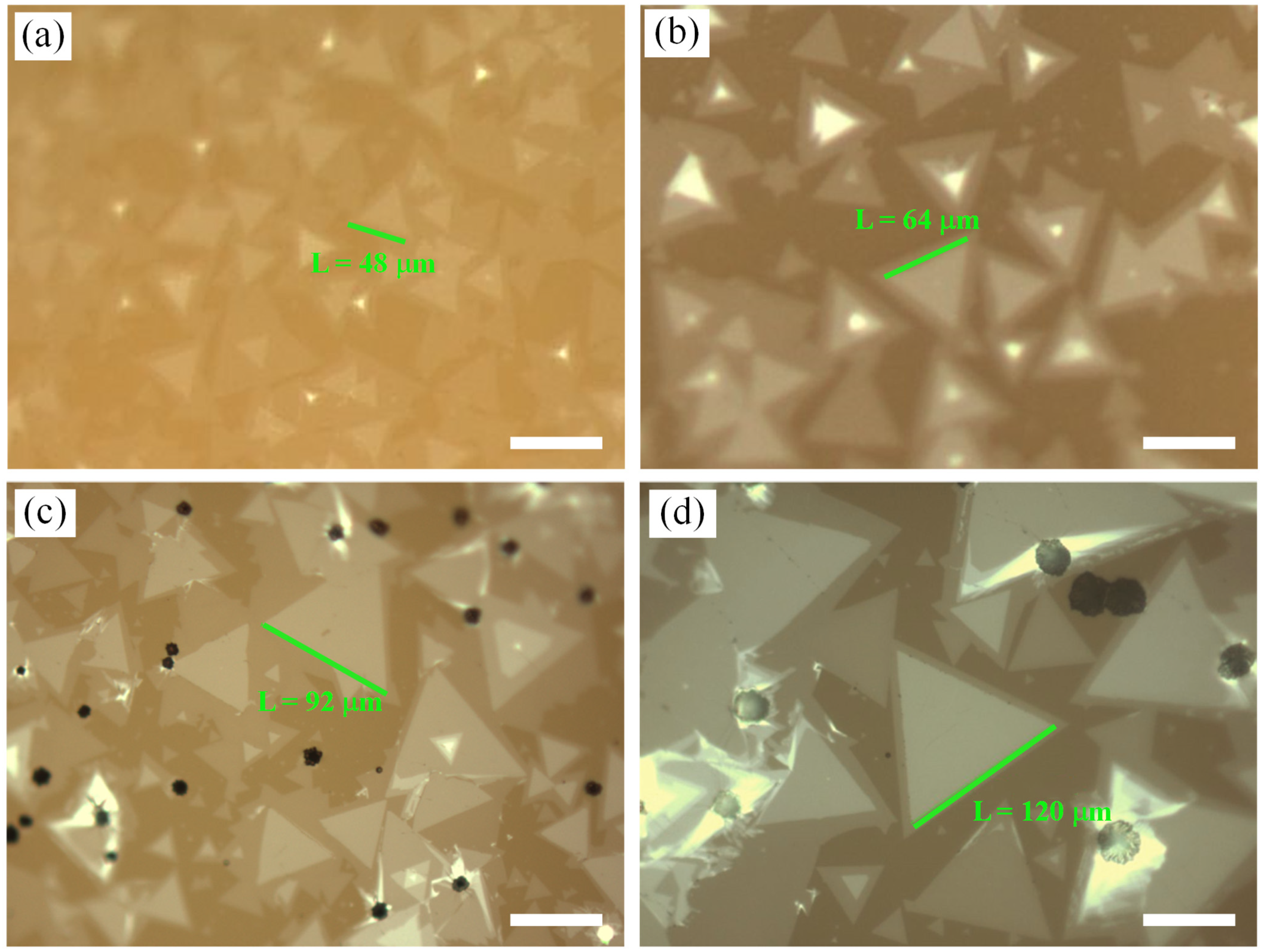
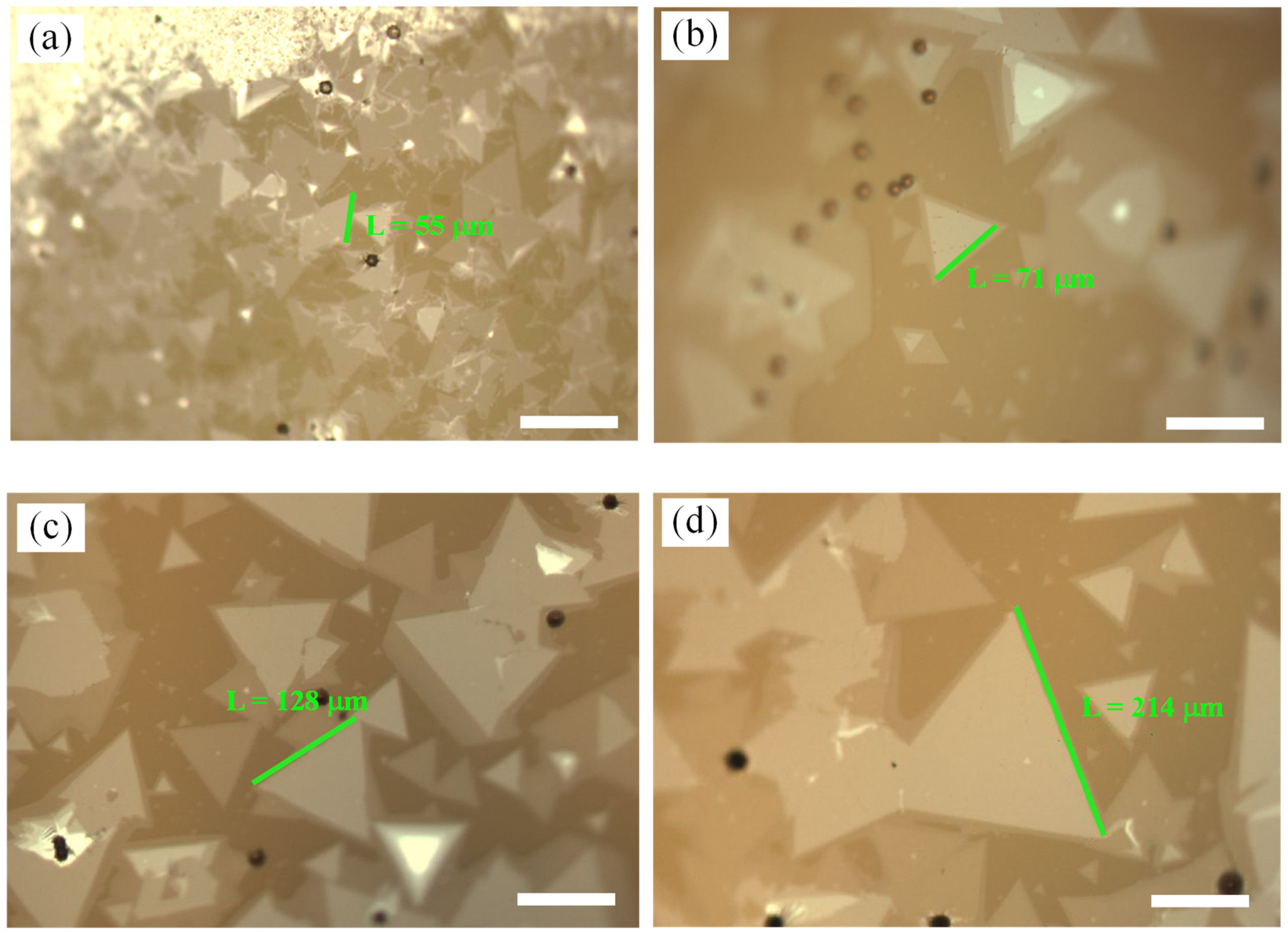
3.2. Effect of Mo Mass under NaCl Assistance
3.3. Effect of Growth Pressure under NaCl Assistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Hersam, M.C. Electronic transport in two-dimensional materials. Annu. Rev. Phys. Chem. 2018, 69, 299–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinwande, D.; Huyghebaert, C.; Wang, C.-H.; Serna, M.I.; Goossens, S.; Li, L.-J.; Wong, H.S.P.; Koppens, F.H.L. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef]
- Seravalli, L.; Bosi, M. A review on chemical vapour deposition of two-dimensional MoS2 flakes. Materials 2021, 14, 7590. [Google Scholar] [CrossRef]
- Zhang, X.; Grajal, J.; Vazquez-Roy, J.L.; Radhakrishna, U.; Wang, X.; Chern, W.; Zhou, L.; Lin, Y.; Shen, P.-C.; Ji, X.; et al. Two-dimensional MoS2-enabled flexible rectenna for Wi-Fi-band wireless energy harvesting. Nature 2019, 566, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tan, J.; Nong, H.; Liu, B.; Cheng, H.-M. Chemical vapor deposition growth of two-dimensional compound materials: Controllability, material quality, and growth mechanism. Acc. Mater. Res. 2021, 2, 36–47. [Google Scholar] [CrossRef]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.-C.; Wang, D.-Y.; Kuo, T.-R. Phase-dependent MoS2 nanoflowers for light-driven antibacterial application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Gao, Q.; Lu, J.; Chen, S.; Chen, L.; Xu, Z.; Lin, D.; Xu, S.; Liu, P.; Zhang, X.; Cai, W.; et al. Chemical vapor deposition of uniform and large-domain molybdenum disulfide crystals on glass/Al2O3 substrates. Nanomaterials 2022, 12, 2719. [Google Scholar] [CrossRef]
- Wu, F.; Tian, H.; Shen, Y.; Hou, Z.; Ren, J.; Gou, G.; Sun, Y.; Yang, Y.; Ren, T.-L. Vertical MoS2 transistors with sub-1-nm gate lengths. Nature 2022, 603, 259–264. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Ma, L.; Li, W.; Gao, S.; Sun, W.; Dong, R.; Zou, X.; Fan, D.; Shao, L.; et al. Uniform nucleation and epitaxy of bilayer molybdenum disulfide on sapphire. Nature 2022, 605, 69–75. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.; Yi, Z.; Pan, X.; Chi, F.; Liu, L.; Li, X.; Wu, Y. Improved low-frequency noise in CVD bilayer MoS2 field-effect transistors. Appl. Phys. Lett. 2021, 118, 153103. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Si, M.; Li, S.; Huang, M.; Ye, P.; Wu, Y. Performance potential and limit of MoS2 transistors. Adv. Mater. 2015, 27, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, Z.; Xu, X.; Song, J.; Li, X.; Wu, Y. Scalable high performance radio frequency electronics based on large domain bilayer MoS2. Nat. Commun. 2018, 9, 4778. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.B.; Madhvapathy, S.R.; Sachid, A.B.; Llinas, J.P.; Wang, Q.; Ahn, G.H.; Pitner, G.; Kim, M.J.; Bokor, J.; Hu, C.; et al. MoS2 transistors with 1-nanometer gate lengths. Science 2016, 354, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zhu, J.; Ma, Q.; Ma, J.; Bai, H.; Chen, L.; Mu, S. Two-dimensional MoS2: Structural properties, synthesis methods, and regulation strategies toward oxygen reduction. Micromachines 2021, 12, 240. [Google Scholar] [CrossRef]
- Xia, B.; An, L.; Gao, D.; Shi, S.; Xi, P.; Xue, D. Hierarchical ultrathin Mo(SxSe1−x)2 nanosheets with tunable ferromagnetism and efficient hydrogen evolution reaction activity: Towards defect site effect. CrystEngComm 2015, 17, 6420–6425. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Correction to photoluminescence from chemically exfoliated MoS2. Nano Lett. 2012, 12, 526. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 2012, 8, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Li, H.; Li, L.-J. Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques. Chem. Soc. Rev. 2015, 44, 2744–2756. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Wu, Y.; Jiao, L. Controlled synthesis of highly crystalline MoS2 flakes by chemical vapor deposition. J. Am. Chem. Soc. 2013, 135, 5304–5307. [Google Scholar] [CrossRef]
- Amani, M.; Chin, M.L.; Birdwell, A.G.; O’Regan, T.P.; Najmaei, S.; Liu, Z.; Ajayan, P.M.; Lou, J.; Dubey, M. Electrical performance of monolayer MoS2 field-effect transistors prepared by chemical vapor deposition. Appl. Phys. Lett. 2013, 102, 193107. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Si, M.; Najmaei, S.; Neal, A.T.; Du, Y.; Ajayan, P.M.; Lou, J.; Ye, P.D. Statistical study of deep submicron dual-gated field-effect transistors on monolayer chemical vapor deposition molybdenum disulfide films. Nano Lett. 2013, 13, 2640–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.-H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.S.; Sangwan, V.K.; Jariwala, D.; Wood, J.D.; Park, S.; Chen, K.-S.; Shi, F.; Ruiz-Zepeda, F.; Ponce, A.; Jose-Yacaman, M.; et al. Influence of stoichiometry on the optical and electrical properties of chemical vapor deposition derived MoS2. ACS Nano 2014, 8, 10551–10558. [Google Scholar] [CrossRef] [Green Version]
- Ji, Q.; Su, C.; Mao, N.; Tian, X.; Idrobo, J.-C.; Miao, J.; Tisdale William, A.; Zettl, A.; Li, J.; Kong, J. Revealing the Brønsted-Evans-Polanyi relation in halide-activated fast MoS2 growth toward millimeter-sized 2D crystals. Sci. Adv. 2021, 7, eabj3274. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Wang, D.; Tang, B.; Yang, P.; Shi, Y.; Zhou, F.; Fu, J.; Huan, Y.; Cui, F.; et al. Ultrafast growth of large-area uniform, millimeter-size MoSe2 single crystals on low-cost soda-lime glass. Adv. Mater. Interfaces 2021, 8, 2100415. [Google Scholar] [CrossRef]
- Qin, B.; Ma, H.; Hossain, M.; Zhong, M.; Xia, Q.; Li, B.; Duan, X. Substrates in the synthesis of two-dimensional materials via chemical vapor deposition. Chem. Mater. 2020, 32, 10321–10347. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Tang, J.; Zhu, J.; Zhang, Q.; Jia, Q.; Lu, Y.; Wei, Z.; Yu, H.; Zhao, Y.; et al. Wafer-scale highly oriented monolayer MoS2 with large domain sizes. Nano Lett. 2020, 20, 7193–7199. [Google Scholar] [CrossRef]
- Li, T.; Guo, W.; Ma, L.; Li, W.; Yu, Z.; Han, Z.; Gao, S.; Liu, L.; Fan, D.; Wang, Z.; et al. Epitaxial growth of wafer-scale molybdenum disulfide semiconductor single crystals on sapphire. Nat. Nanotechnol. 2021, 16, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Song, J.; Gao, Q.; Li, S.; Hu, Q.; Li, X.; Wu, Y. High-performance transistors based on monolayer CVD MoS2 grown on molten glass. Appl. Phys. Lett. 2018, 113, 202103. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Pan, S.; Tang, B.; Liang, Y.; Zhao, X.; Zhang, Z.; Shi, J.; Huan, Y.; Shi, Y.; et al. Epitaxial growth of centimeter-scale single-crystal MoS2 monolayer on Au(111). ACS Nano 2020, 14, 5036–5045. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shi, J.; Zheng, X.; Liu, J.; Dou, W.; Gao, Y.; Yuan, X.; Ouyang, F.; Huang, H. CVD grown MoS2 nanoribbons on MoS2 covered sapphire(0001) without catalysts. Phys. Status Solidi-Rapid Res. Lett. 2019, 13, 1900063. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Gao, J.; Du, F.; Han, Q.; Nie, Y.; Chen, Z.; Bachmatiuk, A.; Priydarshi, M.K.; Ma, D.; et al. Growing uniform graphene disks and films on molten glass for heating devices and cell culture. Adv. Mater. 2015, 27, 7839–7846. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, L. Controllable growth of graphene on liquid surfaces. Adv. Mater. 2019, 31, 1800690. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, J.; Zhou, L.; Mendes, R.G.; Dong, Y.; Zhang, M.-Y.; Cui, Z.-H.; Cai, Z.; Zhang, Z.; Zhu, D.; et al. Bandgap tuning of two-dimensional materials by sphere diameter engineering. Nat. Mater. 2020, 19, 528–533. [Google Scholar] [CrossRef]
- Cai, Q.; Ju, Q.; Hong, W.; Jian, C.; Wang, T.; Liu, W. Water assisted growth of two-dimensional MoS2/MoSe2 vertical heterostructures on molten glass. Nanoscale 2022, 14, 1990–1996. [Google Scholar] [CrossRef]
- Yang, P.; Zou, X.; Zhang, Z.; Hong, M.; Shi, J.; Chen, S.; Shu, J.; Zhao, L.; Jiang, S.; Zhou, X.; et al. Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 2018, 9, 979. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Tan, S.J.R.; Xu, H.; Wu, B.; Liu, B.; Fu, D.; Fu, W.; Geng, D.; Liu, Y.; et al. Chemical vapor deposition of large-size monolayer MoSe2 crystals on molten glass. J. Am. Chem. Soc. 2017, 139, 1073–1076. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Lee, Y.-H.; Shi, Y.; Hsu, A.; Chin, M.L.; Li, L.-J.; Dubey, M.; Kong, J.; Palacios, T. Integrated circuits based on bilayer MoS2 transistors. Nano Lett. 2012, 12, 4674–4680. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Zhang, C.; Yang, K.; Pan, X.; Zhang, Z.; Yang, J.; Yi, Z.; Chi, F.; Liu, L. High-performance CVD bilayer MoS2 radio frequency transistors and gigahertz mixers for flexible nanoelectronics. Micromachines 2021, 12, 451. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhao, D.; Li, J.; Kang, H.; Zhao, S.; Jin, T.; Zhang, J.; Xue, Z.; Wang, Y.; et al. Fast and controllable synthesis of AB-stacked bilayer MoS2 for photoelectric detection. 2D Mater. 2021, 9, 15016. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Tang, D.-M.; Zhao, W.; Xu, H.; Chu, L.; Bando, Y.; Golberg, D.; Eda, G. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 2015, 1, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lin, Y.-C.; Zhao, W.; Wu, J.; Wang, Z.; Hu, Z.; Shen, Y.; Tang, D.-M.; Wang, J.; Zhang, Q.; et al. Vapour–liquid–solid growth of monolayer MoS2 nanoribbons. Nat. Mater. 2018, 17, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Lin, J.; Huang, X.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.; Lei, J.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef]
- Chen, L.; Zang, L.; Chen, L.; Wu, J.; Jiang, C.; Song, J. Study on the catalyst effect of NaCl on MoS2 growth in a chemical vapor deposition process. CrystEngComm 2021, 23, 5337–5344. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Z.; Sun, M.; Lin, F.; Cheng, T.; Shi, J.; Xie, C.; Shi, Y.; Jiang, S.; Huan, Y.; et al. Thickness tunable wedding-cake-like MoS2 flakes for high-performance optoelectronics. ACS Nano 2019, 13, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Beinik, I.; Lauritsen, J.V. Single-layer MoS2 formation by sulfidation of molybdenum oxides in different oxidation states on Au(111). Phys. Chem. Chem. Phys. 2017, 19, 14020–14029. [Google Scholar] [CrossRef]
- Kojima, K.; Lim, H.E.; Liu, Z.; Zhang, W.; Saito, T.; Nakanishi, Y.; Endo, T.; Kobayashi, Y.; Watanabe, K.; Taniguchi, T.; et al. Restoring the intrinsic optical properties of CVD-grown MoS2 monolayers and their heterostructures. Nanoscale 2019, 11, 12798–12803. [Google Scholar] [CrossRef] [Green Version]
- Bhaviripudi, S.; Jia, X.; Dresselhaus, M.S.; Kong, J. Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett. 2010, 10, 4128–4133. [Google Scholar] [CrossRef]
- Zhou, S.; Gan, L.; Wang, D.; Li, H.; Zhai, T. Space-confined vapor deposition synthesis of two dimensional materials. Nano Res. 2018, 11, 2909–2931. [Google Scholar] [CrossRef]
- Verble, J.L.; Wieting, T.J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 1970, 25, 362–365. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Yan, X.; Lu, Z.; Qiu, H.; Xu, G.; Zhou, X.; Wang, P.; Pan, X.; Liu, K.; Jiao, L. High-mobility multilayered MoS2 flakes with low contact resistance grown by chemical vapor deposition. Adv. Mater. 2017, 29, 1604540. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Chen, L.; Chen, S.; Zhang, Z.; Yang, J.; Pan, X.; Yi, Z.; Liu, L.; Chi, F.; Liu, P.; et al. NaCl-Assisted Chemical Vapor Deposition of Large-Domain Bilayer MoS2 on Soda-Lime Glass. Nanomaterials 2022, 12, 2913. https://doi.org/10.3390/nano12172913
Gao Q, Chen L, Chen S, Zhang Z, Yang J, Pan X, Yi Z, Liu L, Chi F, Liu P, et al. NaCl-Assisted Chemical Vapor Deposition of Large-Domain Bilayer MoS2 on Soda-Lime Glass. Nanomaterials. 2022; 12(17):2913. https://doi.org/10.3390/nano12172913
Chicago/Turabian StyleGao, Qingguo, Lvcheng Chen, Simin Chen, Zhi Zhang, Jianjun Yang, Xinjian Pan, Zichuan Yi, Liming Liu, Feng Chi, Ping Liu, and et al. 2022. "NaCl-Assisted Chemical Vapor Deposition of Large-Domain Bilayer MoS2 on Soda-Lime Glass" Nanomaterials 12, no. 17: 2913. https://doi.org/10.3390/nano12172913
APA StyleGao, Q., Chen, L., Chen, S., Zhang, Z., Yang, J., Pan, X., Yi, Z., Liu, L., Chi, F., Liu, P., & Zhang, C. (2022). NaCl-Assisted Chemical Vapor Deposition of Large-Domain Bilayer MoS2 on Soda-Lime Glass. Nanomaterials, 12(17), 2913. https://doi.org/10.3390/nano12172913






