Nanoencapsulation of Acetamiprid by Sodium Alginate and Polyethylene Glycol Enhanced Its Insecticidal Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Chemicals
2.3. Preparation of AL–Acetamiprid Nanoparticles
2.4. Preparation of PEG–Acetamiprid Nanoparticles
2.5. Description of Nanoparticles
2.6. Oral Toxicity Assay
2.7. Preparation of the Whole-Body Homogenates for Biochemical Analysis
2.8. Energy Reserves
2.9. Detoxifying Enzymes
2.10. Data Analysis
3. Results
3.1. Characterization of Synthesized Nanoparticles
3.2. Larvicidal Activity
3.3. Energy Reserves
3.4. Detoxifying Enzymes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefoe, G.; Dominiak, B.; Worsley, P.; Davies, J. Elm Leaf Beetle “Xanthogaleruca luteola” (Muller) Dispersal across South Eastern Australia (1989–2011). Plant Prot. Q. 2014, 29, 61–65. Available online: https://search.informit.org/doi/10.3316/informit.592873696099273 (accessed on 29 July 2022).
- Chiffelle, Í.; Huerta, A.; Bobadilla, V.; Macuada, G.; Araya, J.E.; Curkovic, T.; Ceballos, R. Antifeedant and insecticidal effects of extracts from Melia azedarach fruits and Peumus boldus leaves on Xanthogaleruca luteola larvae. Chil. J. Agri. Res. 2019, 79, 609–615. [Google Scholar] [CrossRef]
- Singh, N.S.; Sharma, R.; Parween, T.; Patanjali, P.K. Pesticide contamination and human health risk factor. In Modern Age Environmental Problems and their Remediation, 1st ed.; Oves, M., Zain Khan, M., Ismail, I., Eds.; Springer: Cham, The Netherlands, 2018; pp. 46–68. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosane alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Lu, Y. Colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 90–94. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Sansukcharearnpon, A.; Wanichwecharungruang, S.; Leepipatpaiboon, N.; Kerdcharoen, T.; Arayachukeat, S. High loading fragrance encapsulation based on a polymerblend: Preparation and release behavior. Int. J. Pharm. 2010, 391, 267–273. [Google Scholar] [CrossRef]
- Liu, S.Q.; Yuan, L.; Yue, X.L.; Zhang, Z.Z.; Tang, Z.Y. Recent Advances in nanosensors for organophosphate pesticide detection. Adv. Powder Technol. 2008, 19, 419–441. [Google Scholar] [CrossRef]
- Wu, W.Y.; Bian, Z.P.; Wang, W.; Zhu, J. Gold Nanoparticle composite film-based silver enhanced colorimetric detection of cardiac troponin. Sens Actuators B Chem. 2010, 147, 298–303. [Google Scholar] [CrossRef]
- Horwat, D.I.; Zakharov, J.; Endrino, L. Chemistry, Phase formation, and catalytic activity of thin palladium-containing oxide films synthesized by plasma-assisted physical vapor deposition. Surf. Coat. 2011, 205, 171–177. [Google Scholar] [CrossRef]
- Valizadeh, B.; Samarfard, S.; Sendi, J.J.; Karbanowicz, T.P. Developing an Ephestia kuehniella hemocyte cell line to assess the bio-insecticidal potential of microencapsulated Helicoverpa armigera Nucleopolyhedrovirus against cotton bollworm (Lepidoptera: Noctuidae) Larva. J. Eco. Entomol. 2020, 113, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira, J., Jr.; Alves, T. Nanopesticides in agriculture: Benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A.; Setzer, W.N.; Krutmuang, P. Mulberry protection through flowering-stage essential oil of Artemisia annua against the lesser mulberry pyralid Glyphodes Pyloalis Walker. Foods 2021, 10, 210. [Google Scholar] [CrossRef]
- Chandraboss, V.L.; Senthilvelan, S.; Natanapatham, L.M.; Murugavelu, B.; Loganathan, B.; Karthikeyan, J. Photocatalytic effect of ag and ag/pt doped silicate non crystalline material on methyl violet—experimental and theoretical studies. J. Non. Cryst. Solids 2013, 368, 23–28. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Dey, S.; Sreenivasan, K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohydr. Polym. 2014, 99, 499–507. [Google Scholar] [CrossRef]
- Ravichandran, V.; Jayakrishnan, A. Synthesis and evaluation of anti-fungal activities of sodium alginate-amphotericin B conjugates. Int. J. Biol. Macromol. 2018, 108, 1101–1109. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Yihua, Y.; Mengdan, Y.; Jing, X.; Weiquan, C.; Ying, Y.; Guanghua, H.; Yue, D.; Ting, Z.; Mengqing, J. A sodium alginate-based nano-pesticide delivery system for enhanced in vitro photostability and insecticidal efficacy of phloxine B. Carbohydr. Polym. 2020, 247, 0144–8617. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Nazarzadeh Zare, E.; Makvandi, P.; Černík, M. Nanoparticles and nanofibres based on tree gums: Biosynthesis and applications. Compr. Anal. Chem. 2021, 94, 223–265. [Google Scholar] [CrossRef]
- Yang, F.-L.; Li, X.-G.; Zhu, F.; Lei, C.-L. Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, K.; Tuguntaev, R.G.; Mozhi, A.; Zhang, J.; Wang, P.C.; Liang, X.J. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine 2016, 12, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Lewicka, K.; Dobrzynski, P.; Rychter, P. PLAGA-PEG-PLAGA terpolymer-based carriers of herbicides for potential application in environment-friendly, controlled release systems of agrochemicals. Materials 2020, 13, 2778. [Google Scholar] [CrossRef]
- Shi, X.B.; Jiang, L.L.; Wang, H.Y.; Qiao, K.; Wang, D.; Wang, K.Y. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manage. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.; Zhou, J.; Song, W.; Xiao, Y.; Cui, C.; Gao, W.; Ke, F.; Zhu, J.; Gu, Z.; et al. Delivery of acetamiprid to tea leaves enabled by porous silica nanoparticles: Efficiency, distribution and metabolism of acetamiprid in tea plants. BMC Plant Biol. 2021, 21, 337. [Google Scholar] [CrossRef]
- Fogel, M.N.; Schneider, M.I.; Desneux, N.; González, B.; Ronco, A.E. Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef]
- Padmavathi, P.; Vasundhara, N.; Kovvuri, S.; Venugopal, N. Synthesis and characterization of nano-acetamiprid—New plant safeguard nanomaterial. Am. J. Anal. Chem. 2021, 11, 197–204. [Google Scholar] [CrossRef]
- Su, Y.; Ren, X.; Ma, X.; Wang, D.; Hu, H.; Song, X.; Cui, J.; Ma, Y.; Yao, Y. Evaluation of the toxicity and sublethal effects of acetamiprid and dinotefuran on the predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Toxics 2022, 10, 309. [Google Scholar] [CrossRef]
- Slattery, M.; Harper, B.; Harper, S. Pesticide encapsulation at the nanoscale drives changes to the hydrophobic partitioning and toxicity of an active ingredient. Nanomaterials 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Khdair, A.; Patil, Y.; Handa, H.; Mao, G.; Panyam, J. Polymer-surfactant nanoparticles for sustained release of water-soluble drugs. J. Pharm. Sci. 2007, 96, 3379–3389. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, P.; Amini, B.; Bagherzadeh, R.; Kashfi, M.; Latifi, M.; Yavari, N.; Asadi Kani, S.; Kong, L. Flexible hybrid structure piezoelectric nanogenerator based on ZnO nanorod/PVDF nanofibers with improved output. RSC Adv. 2019, 9, 10117–10123. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Siegert, K.J. Carbohydrate metabolism in Manduca sexta during late larval development. J. Insect Physiol. 1987, 33, 421–427. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Oppenorth, F.J.; van der Pas, L.J.T.; Houx, N.W.H. Glutathione S-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of house fly and their influence on resistance. Pestic. Bioch. Physiol. 1979, 11, 176–178. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Sendi, J.J.; Setzer, W.N.; Changbunjong, T. Encapsulation of Eucalyptus largiflorens essential oil by mesoporous silicates for effective control of the cowpea weevil, Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae). Molecules 2022, 27, 3531. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R.; Dilbaghi, N. Development and evaluation of alginate–chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Ng’ambi, J.W. Effect of polyethylene glycol 4000 supplementation on the performance of yearling male Pedi goats fed dietary mixture levels of Acacia karroo leaf meal and Setaria verticillata grass hay. Trop. Anim. Health Prod. 2017, 49, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. Applications of absorbent polymers for sustainable plant protection and crop yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Adak, T.; Kumar, J.; Shakil, N.A.; Walia, S. Development of Controlled release formulations of imidacloprid employing novel nano-ranged amphiphilic polymers. J. Environ. Sci. Health Part B 2012, 47, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Bajpai, J.; Saini, R.; Bajpai, A.K.; Acharya, S. Sustained release of pesticide (Cypermethrin) from nanocarriers: An effective technique for environmental and crop protection. Process Saf. Environ. Prot. 2018, 117, 315–325. [Google Scholar] [CrossRef]
- Bhan, S.; Mohan, L.; Srivastava, C.N. Relative larvicidal potentiality of nano-encapsulated temephos and imidacloprid against Culex quinquefasciatus. J. Asia Pac. Entomol. 2014, 17, 787–791. [Google Scholar] [CrossRef]
- Hemalatha, N.; Kumar, K.R. Synthesis and characterization of PEG-cinnamon essential oil nanoparticles and their application as an insecticidal agent. J. Adv. Sci. Res. 2021, 12, 142–148. [Google Scholar] [CrossRef]
- Keawchaoonm, L.; Yoksanm, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B. 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Takei, T.; Yoshida, M.; Hatate, Y.; Shiomori, K.; Kiyoyama, S. Preparation of polylactide/poly(ε-caprolactone) microspheres enclosing acetamiprid and evaluation of release behavior. Polym. Bull. 2008, 61, 391–397. [Google Scholar] [CrossRef]
- Bursell, E. The Role of Proline in Energy Metabolism. In Energy Metabolism in Insects, 1st ed.; Downer, R.G.H., Ed.; Springer: Boston, MA, USA, 1981; pp. 135–154. [Google Scholar] [CrossRef]
- Gall, M.L.; Behmer, S.T. Effects of protein and carbohydrate on an insect herbivore: The vista from a fitness landscape. Integr. Comp. Biol. 2014, 54, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Physiological Systems in Insects, 3rd ed.; Academic Press: London, UK, 2013; p. 696. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Ugrankar, R.; Theodoropoulos, P.; Akdemir, F.; Henne, W.M.; Graff, J.M. Circulating glucose levels inversely correlate with Drosophila larval feeding through insulin signaling and SLC5A11. Commun. Boil. 2018, 1, 110. [Google Scholar] [CrossRef]
- Wu, M.Y.; Ying, Y.Y.; Zhang, S.S.; Li, X.G.; Yan, W.H.; Yao, Y.C.; Shah, S.; Wu, G.; Yang, F.L. Effects of diallyl trisulfide, an active substance from garlic essential oil, on energy metabolism in male moth Sitotroga cerealella (Olivier). Insects 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Toxicity and deleterious effects of Artemisia annua essential oil extracts on mulberry pyralid (Glyphodes pyloalis). Pestic. Biochem. Physiol. 2020, 170, 104702. [Google Scholar] [CrossRef] [PubMed]
- Nardini, L.; Christian, R.N.; Coetzer, N.; Ranson, H.; Coetzee, M.; Koekemoer, L.L. Detoxification enzymes associated with insecticide resistance in laboratory strains of Anopheles arabiensis of different geographic origin. Parasit. Vectors 2012, 5, 113. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
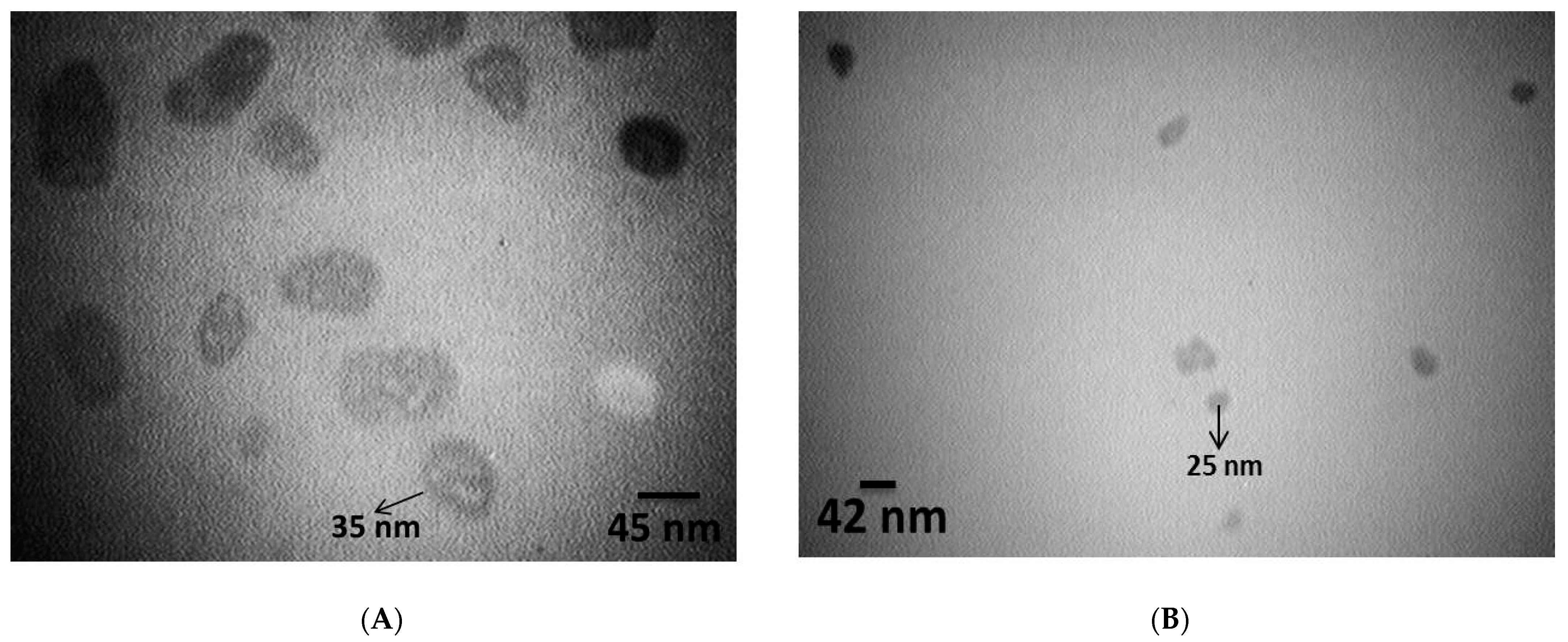
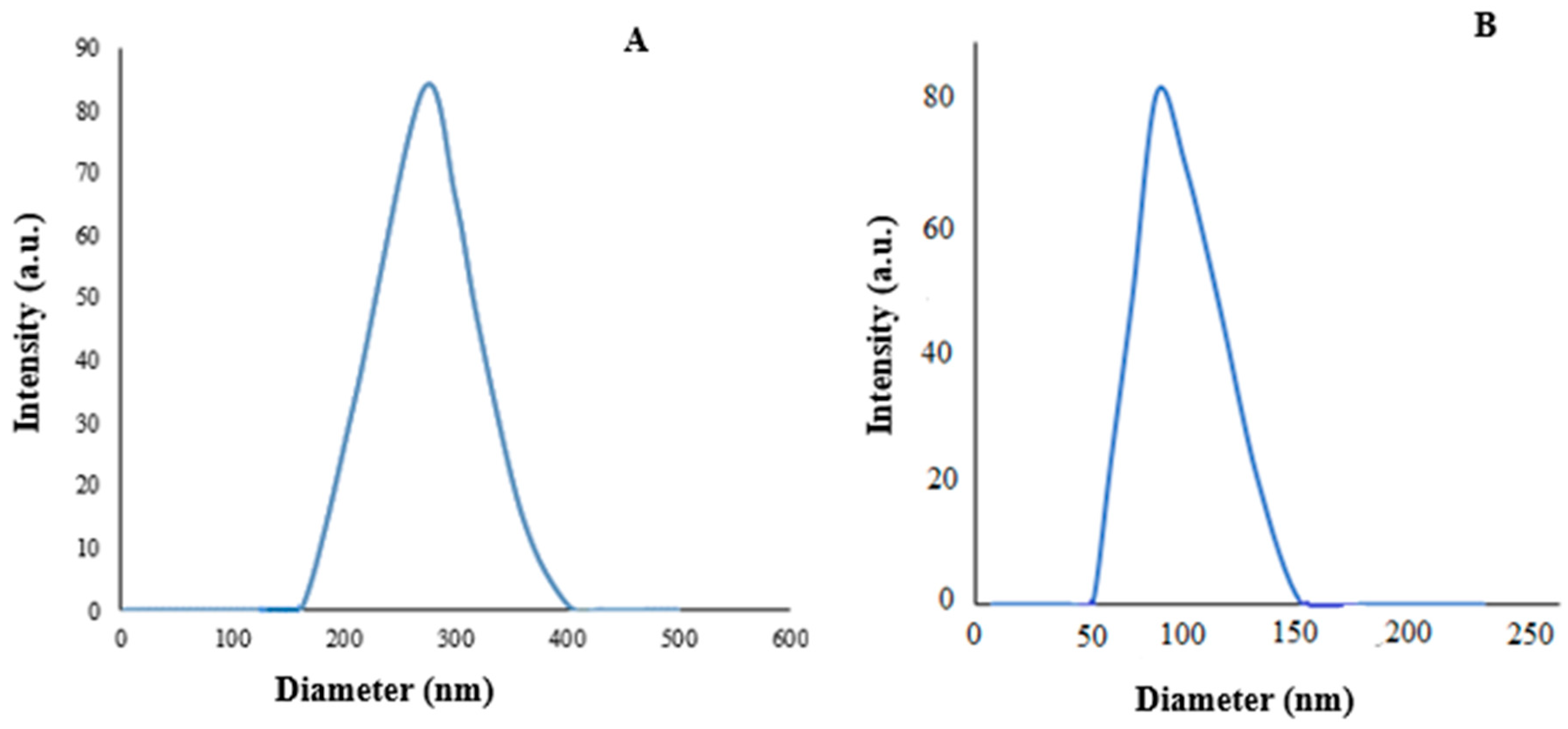
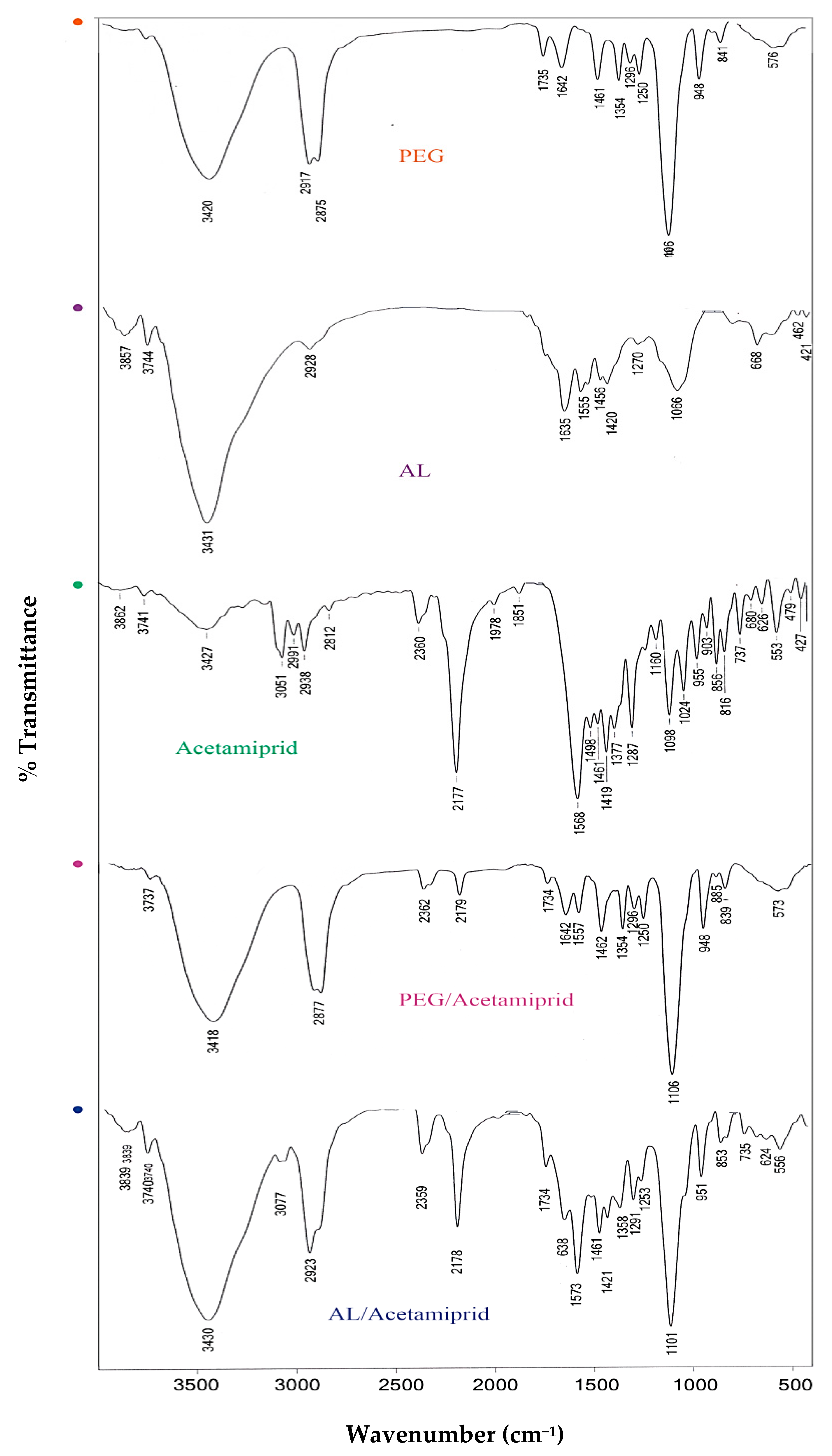
| Bioassay | LC30 (95% Confidence Limits) (ppm) | LC50 (95% Confidence Limits) (ppm) | Slope ± SE | Intercept ± SE | χ² (df = 3) | Relative Potency |
|---|---|---|---|---|---|---|
| AL–acetamiprid | 0.015 (0.005–0.028) | 0.048 (0.025–0.076) | 1.071 ± 0.198 | −0.731 ± 0.211 | 2.734 * | 14.16 |
| PEG–acetamiprid | 0.024 (0.008–0.048) | 0.081 (0.046–0.135) | 1.002 ± 0.196 | −0.913 ± 0.219 | 0.321 * | 8.39 |
| Acetamiprid | 0.338 (0.191–0.475) | 0.680 (0.486–0.915) | 1.729 ± 0.304 | −3.170 ± 0.588 | 0.838 * | 1.00 |
| Bio-Assay | Concentrations | Protein (mg/dL) | Glucose (mg/dL) | Triglyceride (mg/dL) |
|---|---|---|---|---|
| Acetamiprid | Control | 1.233 ± 0.0360 a | 0.0933 ± 0.0047 a | 1.8900 ± 0.0145 a |
| LC30 | 1.0900 ± 0.0030 b | 0.0790 ± 0.0208 a | 1.633 ± 0.2185 ab | |
| LC50 | 1.0566 ± 0.0098 b | 0.0733 ± 0.0317 b | 1.5100 ± 0.0965 b | |
| F-Value | 10.94 | 49.80 | 5.04 | |
| Pr | 0.0018 | 0.0001 | 0.0300 | |
| AL–acetamiprid | LC30 | 0.9700 ± 0.0057 b | 0.0623 ± 0.0090 a | 1.5557 ± 0.0431 a |
| LC50 | 0.9433 ± 0.0088 b | 0.0433 ± 0.0083 b | 1.1600 ± 0.0677 b | |
| F-Value | 2.19 | 29.51 | 17.65 | |
| Pr | 0.0170 | 0.0001 | 0.0005 | |
| PEG–acetamiprid | LC30 | 0.8500 ± 0.0012 c | 0.0690 ± 0.0008 a | 1.433 ± 0.1185 ab |
| LC50 | 0.8366 ± 0.0098 c | 0.0513 ± 0.0017 b | 1.3000 ± 0.0765 b | |
| F-Value | 12.84 | 46.10 | 5.10 | |
| Pr | 0.0019 | 0.0001 | 0.0300 |
| Bio-Assay | Concentrations | Esterase (U/mg Protein) | GST (U/mg Protein) |
|---|---|---|---|
| Acetamiprid | Control | 0.02300 ± 0.001 a | 0.0853 ± 0.004 a |
| LC30 | 0.0065 ± 0.0015 b | 0.06366 ± 0.002 ab | |
| LC50 | 0.0001 ± 0.00001 b | 0.05700 ± 0.001 b | |
| F-Value | 21.76 | 11.13 | |
| Pr | 0.0003 | 0.0483 | |
| AL–acetamiprid | LC30 | 0.0010 ± 0.0001 b | 0.0506 ± 0.0063 b |
| LC50 | 0.0001 ± 0.0000 c | 0.0490 ± 0.0024 b | |
| F-Value | 28.13 | 22.87 | |
| Pr | 0.0001 | 0.0005 | |
| PEG–acetamiprid | LC30 | 0.0018 ± 0.0001 b | 0.0696 ± 0.0053 ab |
| LC50 | 0.0003 ± 0.0000 c | 0.0580 ± 0.0021 b | |
| F-Value | 30.23 | 21.27 | |
| Pr | 0.0001 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebadollahi, A.; Valizadeh, B.; Panahandeh, S.; Mirhosseini, H.; Zolfaghari, M.; Changbunjong, T. Nanoencapsulation of Acetamiprid by Sodium Alginate and Polyethylene Glycol Enhanced Its Insecticidal Efficiency. Nanomaterials 2022, 12, 2971. https://doi.org/10.3390/nano12172971
Ebadollahi A, Valizadeh B, Panahandeh S, Mirhosseini H, Zolfaghari M, Changbunjong T. Nanoencapsulation of Acetamiprid by Sodium Alginate and Polyethylene Glycol Enhanced Its Insecticidal Efficiency. Nanomaterials. 2022; 12(17):2971. https://doi.org/10.3390/nano12172971
Chicago/Turabian StyleEbadollahi, Asgar, Bita Valizadeh, Saleh Panahandeh, Hadiseh Mirhosseini, Maryam Zolfaghari, and Tanasak Changbunjong. 2022. "Nanoencapsulation of Acetamiprid by Sodium Alginate and Polyethylene Glycol Enhanced Its Insecticidal Efficiency" Nanomaterials 12, no. 17: 2971. https://doi.org/10.3390/nano12172971
APA StyleEbadollahi, A., Valizadeh, B., Panahandeh, S., Mirhosseini, H., Zolfaghari, M., & Changbunjong, T. (2022). Nanoencapsulation of Acetamiprid by Sodium Alginate and Polyethylene Glycol Enhanced Its Insecticidal Efficiency. Nanomaterials, 12(17), 2971. https://doi.org/10.3390/nano12172971








