Facile Synthesis of NixCo3−xS4 Microspheres for High-Performance Supercapacitors and Alkaline Aqueous Rechargeable NiCo-Zn Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
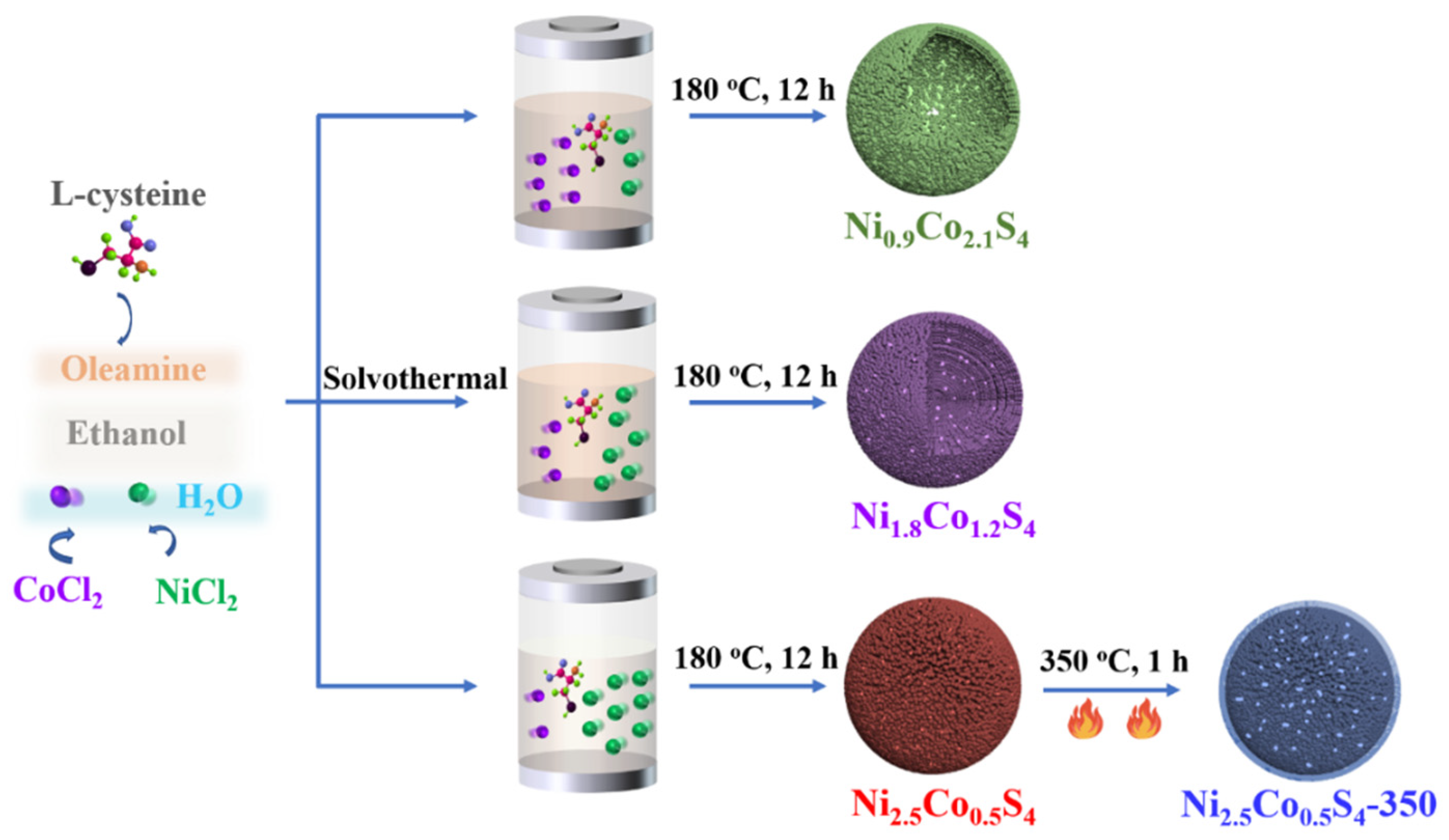
2.2. Preparation of NixCo3−xS4 Microspheres
2.3. Material Characterization
2.4. Electrode Preparation and Electrochemical Measurements
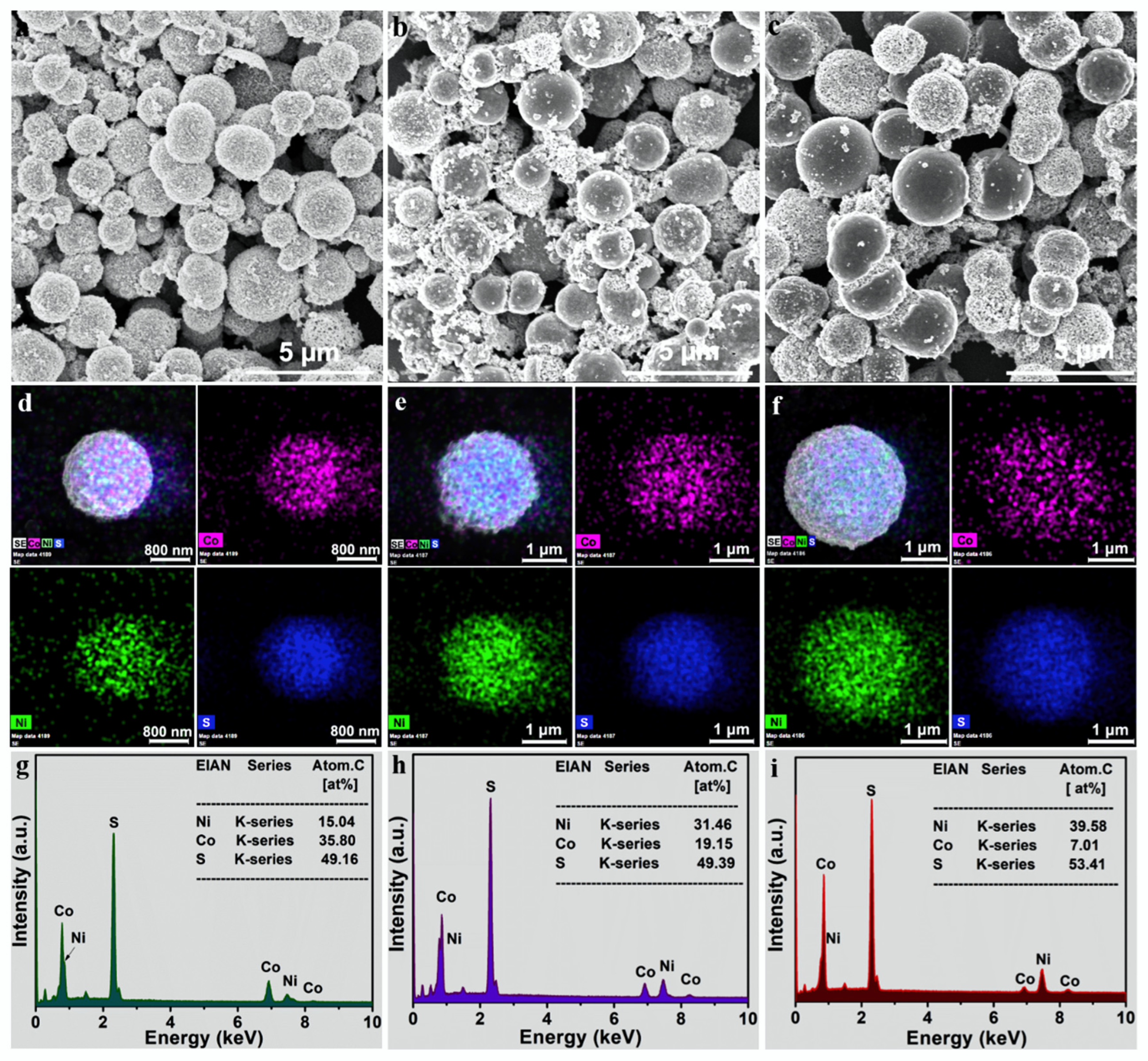
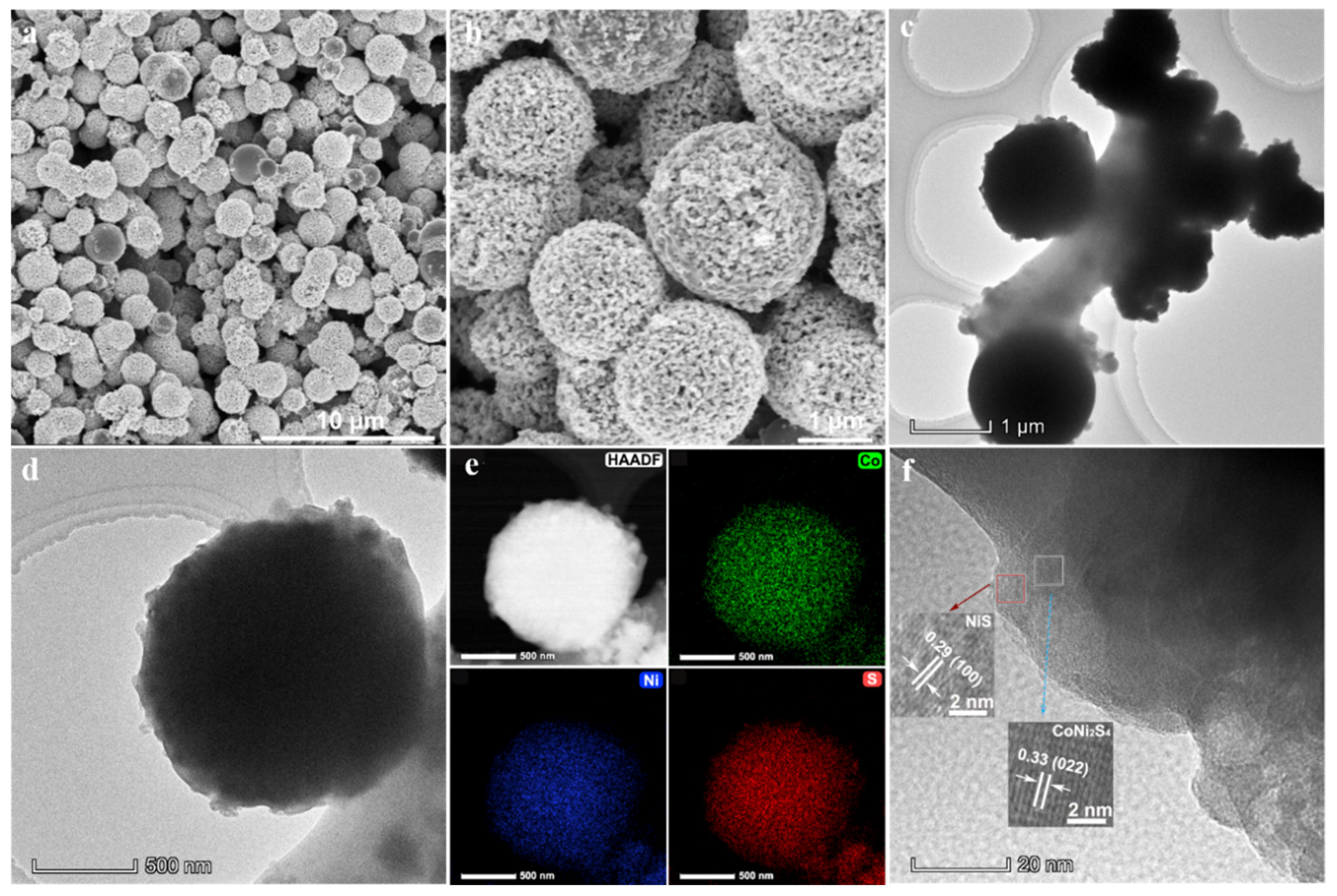
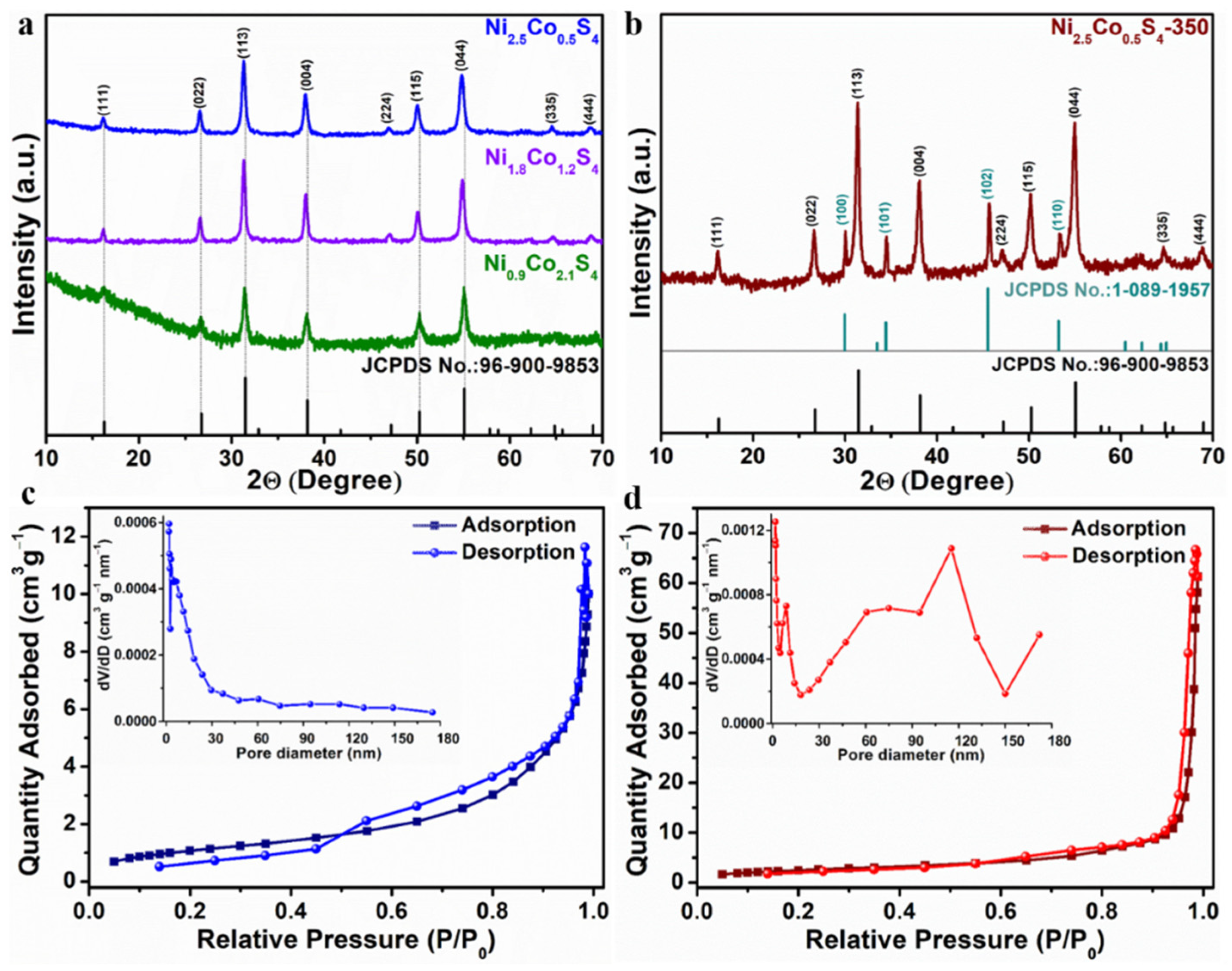
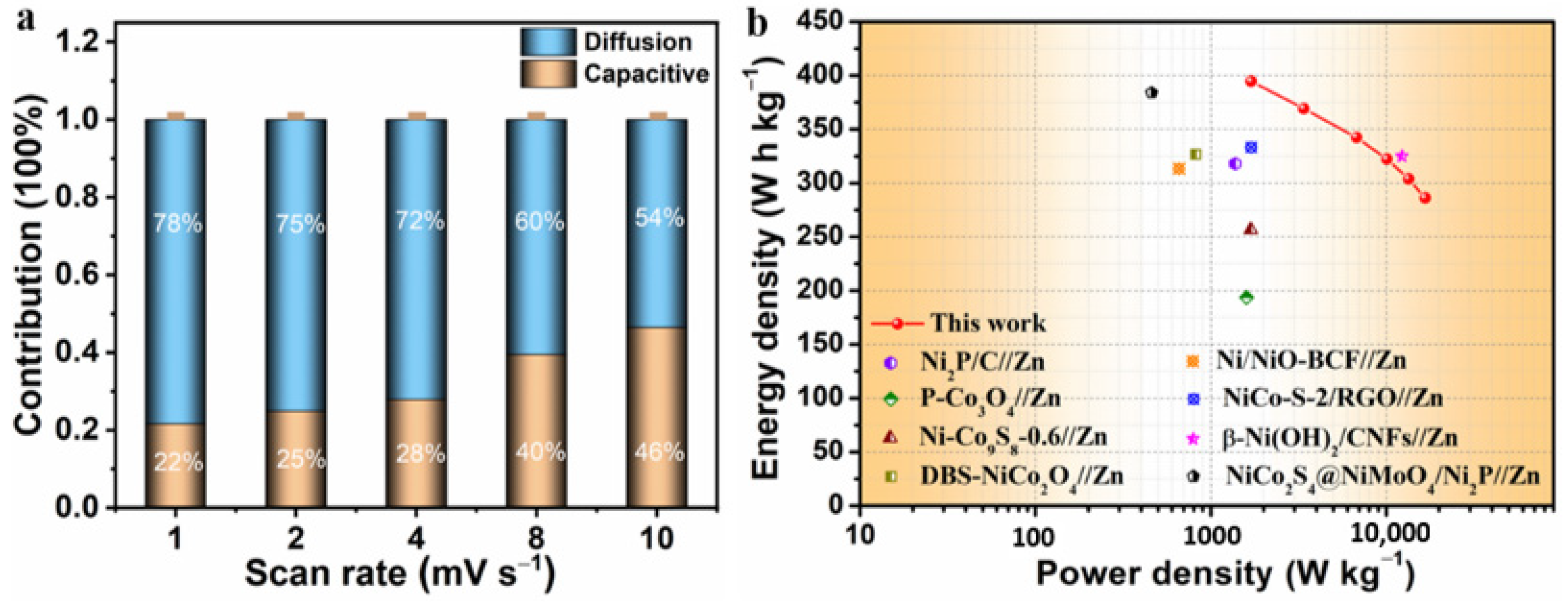
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleischmann, S.; Mitchell, J.B.; Wang, R.C.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Lou, X.W. Mixed Metal Sulfides for Electrochemical Energy Storage and Conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.P.; Hu, Z.; Zhang, X.L.; Tao, Z.L.; Chen, J. Nanostructured Mn-Based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Q.; Zhu, J.X.; Yan, Q.Y.; Dou, S.X.; Sun, W.P. Nanostructured Metal Chalcogenides for Energy Storage and Electrocatalysis. Adv. Funct. Mater. 2017, 27, 1702317. [Google Scholar] [CrossRef]
- Zhou, G.M.; Xu, L.; Hu, G.W.; Mai, L.Q.; Cui, Y. Nanowires for Electrochemical Energy Storage. Chem. Rev. 2019, 119, 11042–11109. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Chao, Y.G.; Wang, F.; Dai, J.Y.; Qin, Y.F.; Bao, X.B.; Yang, Y.; Guo, S.J. Intimately coupled WS2 nanosheets in hierarchical hollow carbon nanospheres as the high-Performance anode material for lithium-Ion storage. Rare Met. 2022, 41, 1245–1254. [Google Scholar] [CrossRef]
- Guo, Y.N.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.L.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Kulkarni, P.; Nataraj, S.K.; Balakrishna, R.G.; Nagarajua, D.H.; Reddy, M.V. Nanostructured binary and ternary metal sulfides: Synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040–22094. [Google Scholar] [CrossRef]
- Zhu, W.D.; Cheng, Y.; Wang, C.; Ni, P.N.; Lu, X.F. Transition metal sulfides meet electrospinning: Versatile synthesis, distinct properties and prospective applications. Nanoscale 2021, 13, 9112–9146. [Google Scholar] [CrossRef]
- Dai, M.; Wang, R. Synthesis and Applications of Nanostructured Hollow Transition Metal Chalcogenides. Small 2021, 17, 2006813. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.G.; He, F.L.; Ye, M.D. Nickel and cobalt sulfide-based nanostructured materials for electrochemical energy storage devices. Chem. Eng. J. 2021, 409, 127237. [Google Scholar]
- Maurya, O.; Khaladkar, S.; Horn, M.R.; Sinha, B.; Deshmukh, R.; Wang, H.X.; Kim, T.Y.; Dubal, D.P.; Kalekar, A. Emergence of Ni-Based Chalcogenides (S and Se) for Clean Energy Conversion and Storage. Small 2021, 17, 2100361. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.Q.; Cao, Z.Y.; Yin, Z.H.; Ma, T.L.; Chen, S.R. Current progress of metal sulfides derived from metal–Organic frameworks for advanced electrocatalysis: Potential electrocatalysts with diverse applications. J. Mater. Chem. A 2022, 10, 1617–1641. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, Y.H.; Zhang, Z.Y.; Dong, S.T.; Wang, H.Y. Facile synthesis of NixCo3−xS4 hollow nanoprism with broader electromagnetic absorption properties: Effect of Ni/Co atomic ratios. J. Alloy. Compd. 2018, 767, 323–329. [Google Scholar] [CrossRef]
- Jia, Z.R.; Wang, B.B.; Feng, A.L.; Liu, J.J.; Zhang, C.H.; Zhang, M.; Wu, G.L. Fabrication of NixCo3−xS4 hollow nanosphere as wideband electromagnetic absorber at thin matched thickness. Ceram. Int. 2019, 45, 15854–15859. [Google Scholar] [CrossRef]
- Yu, Y.W.; Hu, X.L.; Wang, S.; Qiao, H.D.; Liu, Z.Y.; Song, K.F.; Shen, X.D. High mass loading Ni4Co1-OH@CuO core-Shell nanowire arrays obtained by electrochemical reconstruction for alkaline energy storage. Nano Res. 2022, 15, 685–693. [Google Scholar] [CrossRef]
- Chhetri, K.; Kim, T.; Acharya, D.; Muthurasu, A.; Dahal, B.; Bhattarai, R.M.; Lohani, P.C.; Pathak, I.; Ji, S.; Ko, T.H.; et al. Hollow Carbon Nanofibers with Inside-Outside Decoration of Bi-Metallic MOF Derived Ni-Fe Phosphides as Electrode Materials for Asymmetric Supercapacitors. Chem. Eng. J. 2022, 450, 138363. [Google Scholar] [CrossRef]
- Dahiya, Y.; Hariram, M.; Kumar, M.; Jain, A.; Sarkar, D. Modified transition metal chalcogenides for high performance supercapacitors: Current trends and emerging opportunities. Coord. Chem. Rev. 2022, 451, 214265. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Wang, R.; Lin, D.; Zeng, Y.X.; Lu, X.H. Ni-Based Nanostructures as High-Performance Cathodes for Rechargeable Ni-Zn Battery. ChemNanoMat 2018, 4, 525–536. [Google Scholar] [CrossRef]
- He, Y.P.; Zhang, P.P.; Huang, H.; Li, X.B.; Zhai, X.H.; Chen, B.M.; Guo, Z.C. Engineering Sulfur Vacancies of Ni3S2 Nanosheets as a Binder-Free Cathode for an Aqueous Rechargeable Ni-Zn Battery. ACS Appl. Energy Mater. 2020, 3, 3863–3875. [Google Scholar]
- Tong, X.; Li, Y.; Pang, N.; Zhou, Y.; Wu, D.J.; Xiong, D.Y.; Xu, S.H.; Wang, L.W.; Chu, P.K. Highly active cobalt-Doped nickel sulfide porous nanocones for highperformance quasi-Solid-State zinc-Ion batteries. J. Energy Chem. 2022, 66, 237–249. [Google Scholar] [CrossRef]
- Zhou, Y.; Tong, X.; Pang, N.; Deng, Y.P.; Yan, C.H.; Wu, D.J.; Xu, S.H.; Xiong, D.Y.; Wang, L.W.; Chu, P.K. Ni3S2 Nanocomposite Structures Doped with Zn and Co as Long-Lifetime, High-Energy-Density, and Binder-Free Cathodes in Flexible Aqueous Nickel-Zinc Batteries. ACS Appl. Mater. Interfaces 2021, 13, 34292–34300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Deng, Y.; Wu, Y.Q.; Xiao, Z.Y.; Liu, X.B.; Li, Z.J.; Bu, R.R.; Zhang, Q.; Sun, W.; Wang, L. Chemically coupled 0D-3D hetero-Structure of Co9S8-Ni3S4 hollow spheres for Zn-Based supercapacitors. Chem. Eng. J. 2022, 430, 132836. [Google Scholar]
- Shi, W.H.; Mao, J.; Xu, X.L.; Liu, W.X.; Zhang, L.; Cao, X.H.; Lu, X.H. An ultra-Dense NiS2/reduced graphene oxide composite cathode for high-Volumetric/gravimetric energy density nickel–Zinc batteries. J. Mater. Chem. A 2019, 7, 15654–15661. [Google Scholar] [CrossRef]
- Cui, Z.X.; Shen, S.; Yu, J.Q.; Si, J.H.; Cai, D.P.; Wang, Q.T. Electrospun carbon nanofibers functionalized with NiCo2S4 nanoparticles as lightweight, flexible and binder-Free cathode for aqueous Ni-Zn batteries. Chem. Eng. J. 2021, 426, 130068. [Google Scholar] [CrossRef]
- Zhou, J.C.; Wang, Y.C.; Zhou, J.J.; Chen, K.; Han, L. Well-Defined hollow tube@sheets NiCo2S4 core–Shell nanoarrays for ultrahigh capacitance supercapacitor. Dalton Trans. 2021, 50, 15129–15139. [Google Scholar] [CrossRef]
- Qu, G.M.; Li, C.L.; Hou, P.Y.; Zhao, G.; Wang, X.; Zhang, X.L.; Xu, X.J. Hierarchically hollow structured NiCo2S4@NiS for high-Performance flexible hybrid supercapacitors. Nanoscale 2020, 12, 4686–4694. [Google Scholar]
- Chen, L.N.; Wan, J.F.; Fan, L.; Wei, Y.H.; Zou, J.L. Construction of CoNi2S4 hollow cube structures for excellent performance asymmetric supercapacitors. Appl. Surf. Sci. 2021, 570, 151174. [Google Scholar] [CrossRef]
- Cao, X.; He, J.; Li, H.; Kang, L.P.; He, X.X.; Sun, J.; Jiang, R.B.; Xu, H.; Lei, Z.B.; Liu, Z.H. CoNi2S4 Nanoparticle/Carbon Nanotube Sponge Cathode with Ultrahigh Capacitance for Highly Compressible Asymmetric Supercapacitor. Small 2018, 14, 1800998. [Google Scholar] [CrossRef]
- Liao, X.B.; Li, Z.H.; He, Q.; Xia, L.X.; Li, Y.; Zhu, S.H.; Wang, M.M.; Wang, H.; Xu, X.; Mai, L.Q.; et al. Three-Dimensional Porous Nitrogen-Doped Carbon Nanosheet with Embedded NixCo3−xS4 Nanocrystals for Advanced Lithium−Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 9181–9189. [Google Scholar]
- Yu, J.Q.; Cai, D.P.; Si, J.H.; Zhan, H.B.; Wang, Q.T. MOF-Derived NiCo2S4 and carbon hybrid hollow spheres compactly concatenated by electrospun carbon nanofibers as self-Standing electrodes for aqueous alkaline Zn batteries. J. Mater. Chem. A 2022, 10, 4100–4109. [Google Scholar]
- Peng, T.; Qian, Z.Y.; Wang, J.; Song, D.L.; Liu, J.Y.; Liu, Q.; Wang, P. Construction of mass-Controllable mesoporous NiCo2S4 electrodes for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 19376–19382. [Google Scholar]
- Lu, W.; Yang, M.; Jiang, X.; Yu, Y.; Liu, X.C.; Xing, Y. Template-Assisted synthesis of hierarchically hollow C/NiCo2S4 nanospheres electrode for high performance supercapacitors. Chem. Eng. J. 2020, 382, 122943. [Google Scholar]
- Yu, L.; Zhang, L.; Wu, H.B.; Lou, X.W. Formation of NixCo3−xS4 Hollow Nanoprisms with Enhanced Pseudocapacitive Properties. Angew. Chemie 2014, 126, 3785–3788. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Chen, S.M.; Lu, Q.C.; Song, L.; Wei, Y.; Wang, X. Atomic-Level molybdenum oxide nanorings with full-Spectrum absorption and photoresponsive properties. Nat. Commun. 2017, 8, 1559. [Google Scholar]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-Encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar]
- Yang, Y.; Yao, H.Q.; Yu, Z.H.; Islam, S.M.; He, H.Y.; Yuan, M.W.; Yue, Y.H.; Xu, K.; Hao, W.C.; Sun, G.B.; et al. Hierarchical Nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a Highly Efficient Electro-Catalyst for Overall Water Splitting in a Wide pH Range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar]
- Chhetri, K.; Dahal, B.; Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, T.; Chae, S.-H.; Kim, H.Y. Controlled Selenium Infiltration of Cobalt Phosphide Nanostructure Arrays from a Two-Dimensional Cobalt Metal−Organic Framework: A Self-Supported Electrode for Flexible Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.-H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-Derived nanoporous carbon nanocomposite wrapped with Co3O4-Polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar]
- Mohamed, S.G.; Hussain, I.; Shim, J.-J. One-Step synthesis of hollow C-NiCo2S4 nanostructures for high-Performance supercapacitor electrodes. Nanoscale 2018, 10, 6620–6628. [Google Scholar]
- Guan, B.Y.; Yu, L.; Wang, X.; Song, S.Y.; Lou, X.W. Formation of Onion-Like NiCo2S4 Particles via Sequential Ion-Exchange for Hybrid Supercapacitors. Adv. Mater. 2017, 29, 1605051. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.Q.; He, W.D.; Deng, X.L.; Ma, H.; Xu, X.J. Controlled synthesis of NiCo2S4 hollow spheres as high-Performance electrode materials for supercapacitors. J. Alloy. Compd. 2018, 735, 1395–1401. [Google Scholar]
- Ning, X.L.; Li, F.; Zhou, Y.; Miao, Y.E.; Wei, C.; Liu, T.X. Confined growth of uniformly dispersed NiCo2S4 nanoparticles on nitrogen-Doped carbon nanofibers for high-Performance asymmetric supercapacitors. Chem. Eng. J. 2017, 328, 599–608. [Google Scholar]
- Liu, Y.P.; Li, Z.L.; Yao, L.; Chen, S.M.; Zhang, P.X.; Deng, L.B. Confined growth of NiCo2S4 nanosheets on carbon flakes derived from eggplant with enhanced performance for asymmetric supercapacitors. Chem. Eng. J. 2019, 366, 550–559. [Google Scholar]
- Wang, Q.H.; Gao, F.; Xu, B.Y.; Cai, F.X.; Zhan, F.P.; Gao, F.; Wang, Q.X. ZIF-67 derived amorphous CoNi2S4 nanocages with nanosheet arrays on the shell for a high-Performance asymmetric supercapacitor. Chem. Eng. J. 2017, 327, 387–396. [Google Scholar]
- Zhang, P.; Guan, B.Y.; Yu, L.; Lou, X.W. Formation of Double-Shelled Zinc–Cobalt Sulfide Dodecahedral Cages from Bimetallic Zeolitic Imidazolate Frameworks for Hybrid Supercapacitors. Angew. Chem. Int. Ed. 2017, 56, 7141–7145. [Google Scholar]
- Shang, W.X.; Yu, W.T.; Tan, P.; Chen, B.; Xu, H.R.; Ni, M. A high-Performance Zn battery based on self-Assembled nanostructured NiCo2O4 electrode. J. Power Sources 2019, 421, 6–13. [Google Scholar]
- Zhang, H.; Zhang, X.; Li, H.; Zhang, Y.; Zeng, Y.; Tong, Y.; Zhang, P.; Lu, X. Flexible rechargeable Ni//Zn battery based on self-Supported NiCo2O4 nanosheets with high power density and good cycling stability. Green Energy Environ. 2018, 3, 56–62. [Google Scholar]
- Wen, J.; Feng, Z.; Liu, H.R.; Chen, T.; Yang, Y.Q.; Li, S.Z.; Sheng, S.; Fang, G.J. In-Situ synthesized Ni2P nanosheet arrays as the cathode for novel alkaline Ni//Zn rechargeable battery. Appl. Surf. Sci. 2019, 485, 462–467. [Google Scholar]
- Wang, X.W.; Wang, F.X.; Wang, L.Y.; Li, M.X.; Wang, Y.F.; Chen, B.W.; Zhu, Y.S.; Fu, L.J.; Zha, L.S.; Zhang, L.X.; et al. An aqueous rechargeable Zn//Co3O4 battery with high energy density and good cycling behavior. Adv. Mater. 2016, 28, 4904–4911. [Google Scholar] [PubMed]
- Wang, X.W.; Li, M.X.; Wang, Y.F.; Chen, B.W.; Zhu, Y.S.; Wu, Y.P. A Zn–NiO rechargeable battery with long lifespan and high energy density. J. Mater. Chem. A 2015, 3, 8280–8283. [Google Scholar]
- Hu, P.; Wang, T.S.; Zhao, J.W.; Zhang, C.J.; Ma, J.; Du, H.P.; Wang, X.G.; Cui, G.L. Ultrafast Alkaline Ni/Zn Battery Based on Ni-Foam-Supported Ni3S2 Nanosheets. ACS Appl. Mater. Interfaces 2015, 7, 26396–26399. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Wang, D.M.; Huang, M.Z.; Jia, H.L.; Sun, J.H.; Song, X.K.; Guan, M.Y. Facile Synthesis of Ni(OH)2/Carbon Nanofiber Composites for Improving NiZn Battery Cycling Life. ACS Sustain. Chem. Eng. 2017, 5, 6827–6834. [Google Scholar]
- Zhang, D.J.; Zhang, J.C.; Li, J.Q.; Li, C.X.; Li, Y.T.; Liu, Y.Y.; Zhang, R.C. Facile synthesis of mesoporous NixCo9-xS8 hollow spheres for high-Performance supercapacitors and aqueous Ni/Co–Zn batteries. RSC Adv. 2022, 12, 20447–20453. [Google Scholar]
- Lai, C.W.; Wang, Y.X.; Fu, L.; Song, H.X.; Liu, B.; Pan, D.; Guo, Z.H.; Seok, I.; Li, K.W.; Zhang, H.R.; et al. Aqueous flexible all-Solid-State NiCo-Zn batteries with high capacity based on advanced ion-Buffering reservoirs of NiCo2O4. Adv. Compos. Hybrid Mater. 2022, 5, 536–546. [Google Scholar]
- Yang, F.; Zhang, K.; Cen, Z.; Xu, K.B. Rational construction of multidimensional oxygen-Deficient Co3O4 nanosheet/nanowire arrays as high-Performance electrodes for aqueous Zn-Ion batteries and asymmetric supercapacitors. J. Alloy. Compd. 2021, 879, 160439. [Google Scholar]
- Cen, Z.; Yang, F.; Wan, J.; Xu, K.B. The in situ construction of oxygen-Vacancy-Rich NiCo2S4@NiMoO4/Ni2P multilevel nanoarrays for high-Performance aqueous Zn-Ion batteries. New J. Chem. 2022, 46, 6587–6595. [Google Scholar]
- Jiang, L.L.; Li, L.; Luo, S.; Xu, H.; Xia, L.Y.; Wang, H.K.; Liu, X.G.; Wu, Y.Q.; Qing, Y. Configuring hierarchical Ni/NiO 3D-Network assisted with bamboo cellulose nanofibers for high-Performance Ni–Zn aqueous batteries. Nanoscale 2020, 12, 14651. [Google Scholar]
- Peng, Z.W.; Yang, C.; Zhao, Q.Y.; Liang, F.L.; Yun, S.L.; Liu, R.; Zhang, Z.Q.; Liao, Y.X.; Chen, H.C. Ultra-Dispersed nickel–cobalt sulfides on reduced graphene oxide with improved power and cycling performances for nickel-Zinc batteries. J. Colloid Interface Sci. 2022, 607, 61–67. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Jiang, B.; Li, C.; Bian, H.; Liu, Y.; Bu, Y.; Zhang, R.; Zhang, J. Facile Synthesis of NixCo3−xS4 Microspheres for High-Performance Supercapacitors and Alkaline Aqueous Rechargeable NiCo-Zn Batteries. Nanomaterials 2022, 12, 2994. https://doi.org/10.3390/nano12172994
Zhang D, Jiang B, Li C, Bian H, Liu Y, Bu Y, Zhang R, Zhang J. Facile Synthesis of NixCo3−xS4 Microspheres for High-Performance Supercapacitors and Alkaline Aqueous Rechargeable NiCo-Zn Batteries. Nanomaterials. 2022; 12(17):2994. https://doi.org/10.3390/nano12172994
Chicago/Turabian StyleZhang, Daojun, Bei Jiang, Chengxiang Li, Hao Bian, Yang Liu, Yingping Bu, Renchun Zhang, and Jingchao Zhang. 2022. "Facile Synthesis of NixCo3−xS4 Microspheres for High-Performance Supercapacitors and Alkaline Aqueous Rechargeable NiCo-Zn Batteries" Nanomaterials 12, no. 17: 2994. https://doi.org/10.3390/nano12172994



