Impedance Analysis of Chitin Nanofibers Integrated Bulk Acoustic Wave Humidity Sensor with Asymmetric Electrode Configuration
Abstract
:1. Introduction
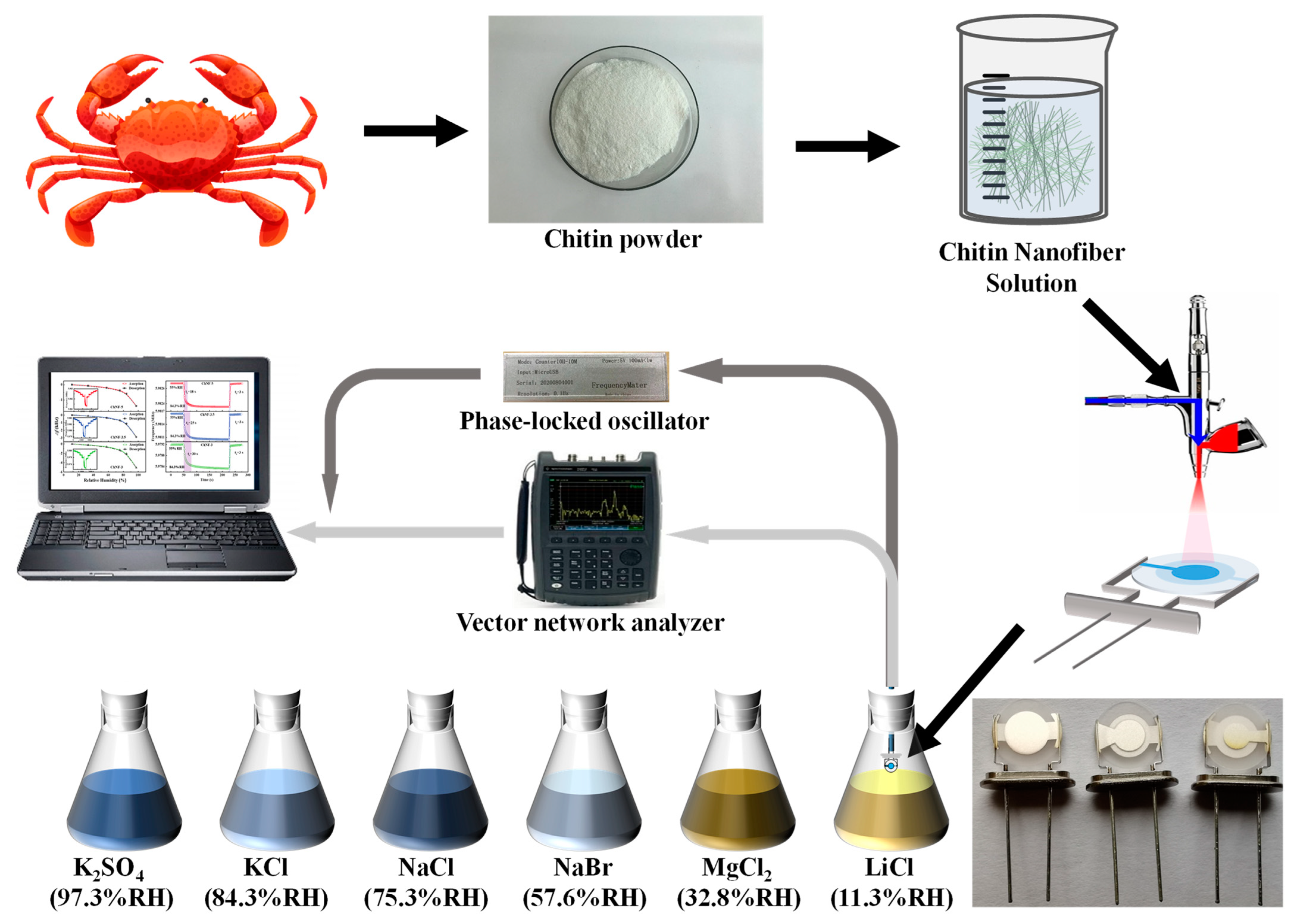
2. Materials and Methods
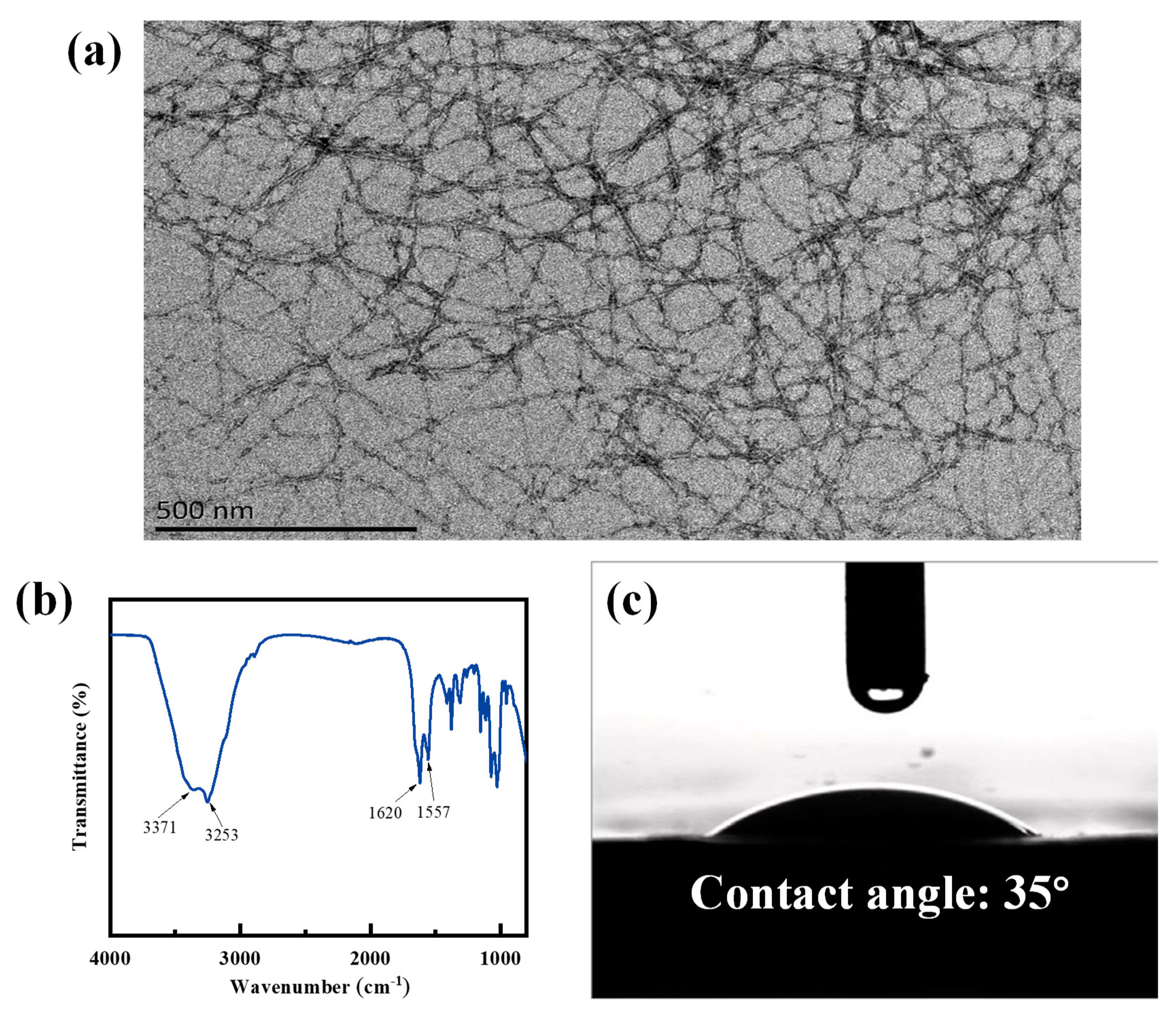
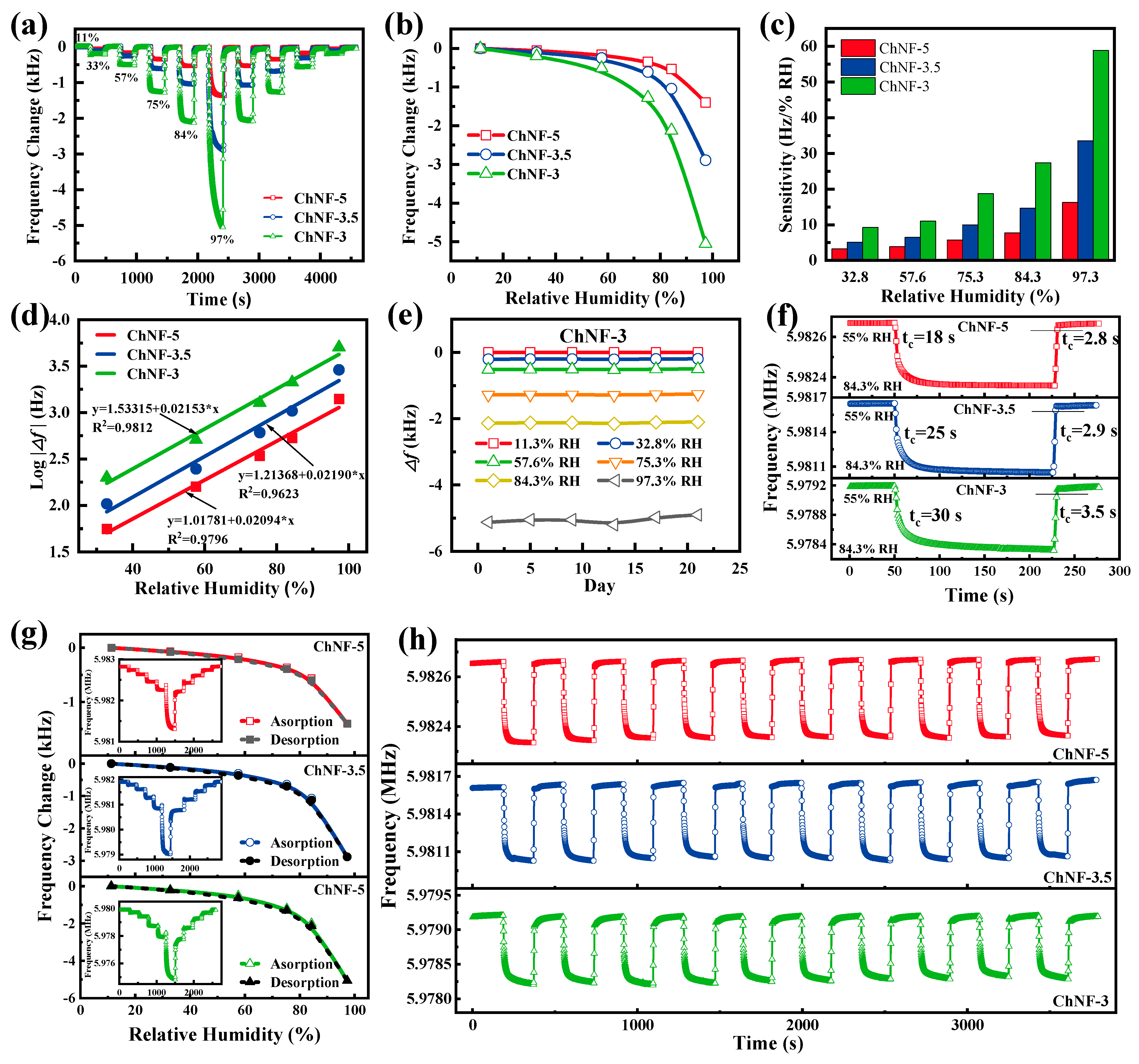
3. Results and Discussion
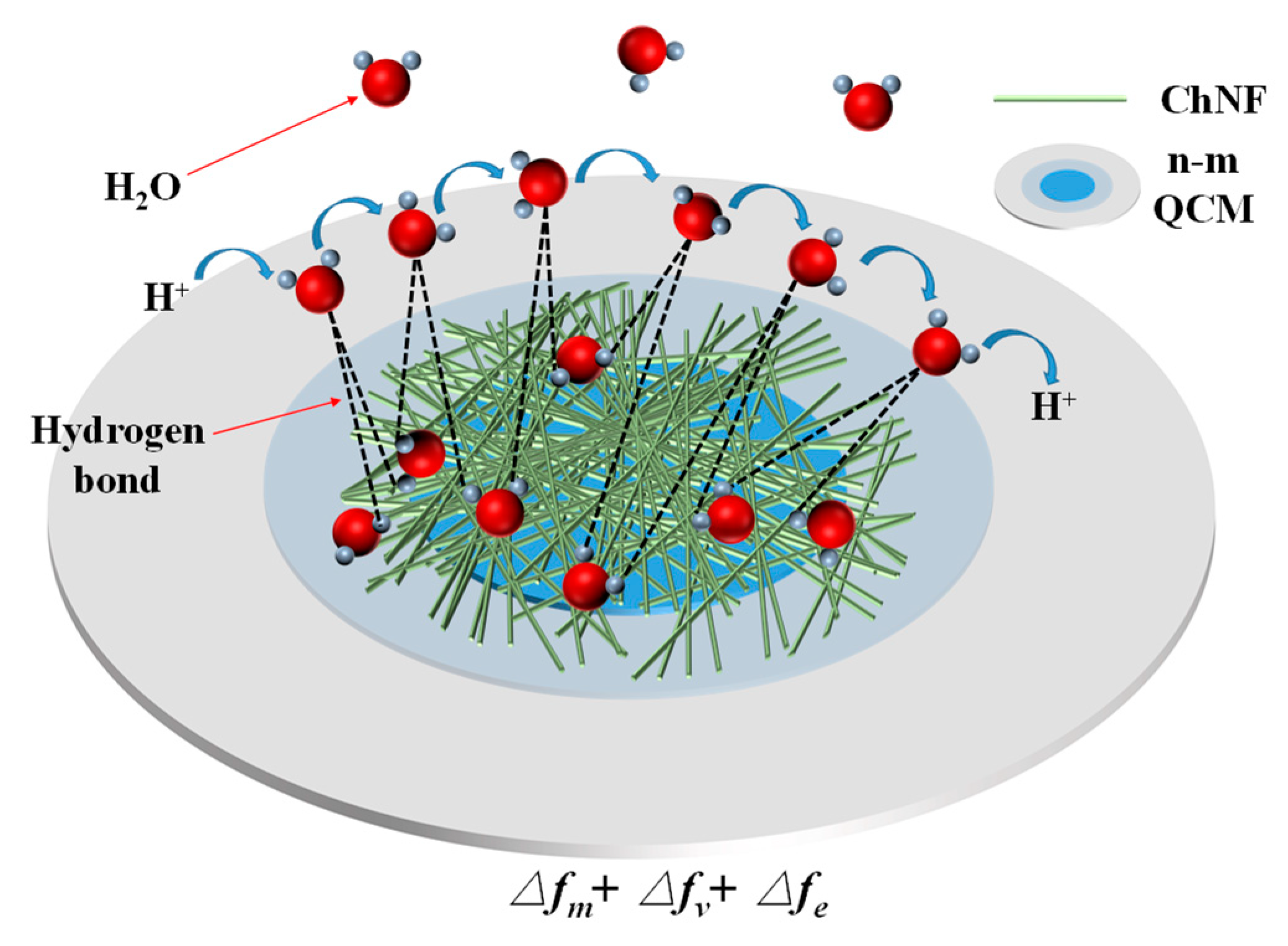
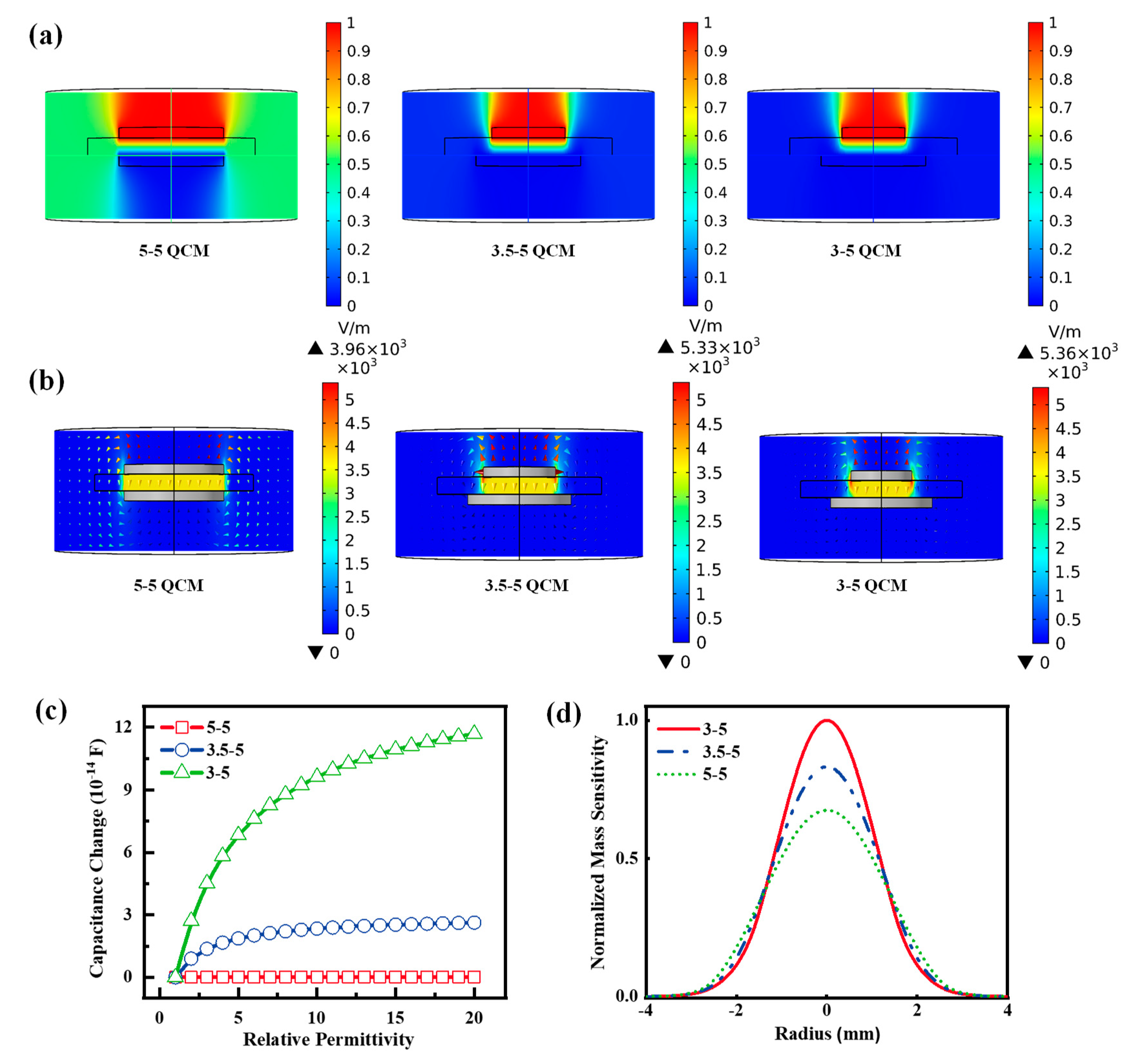
4. Analysis of Sensitivity Enhancement Mechanisms
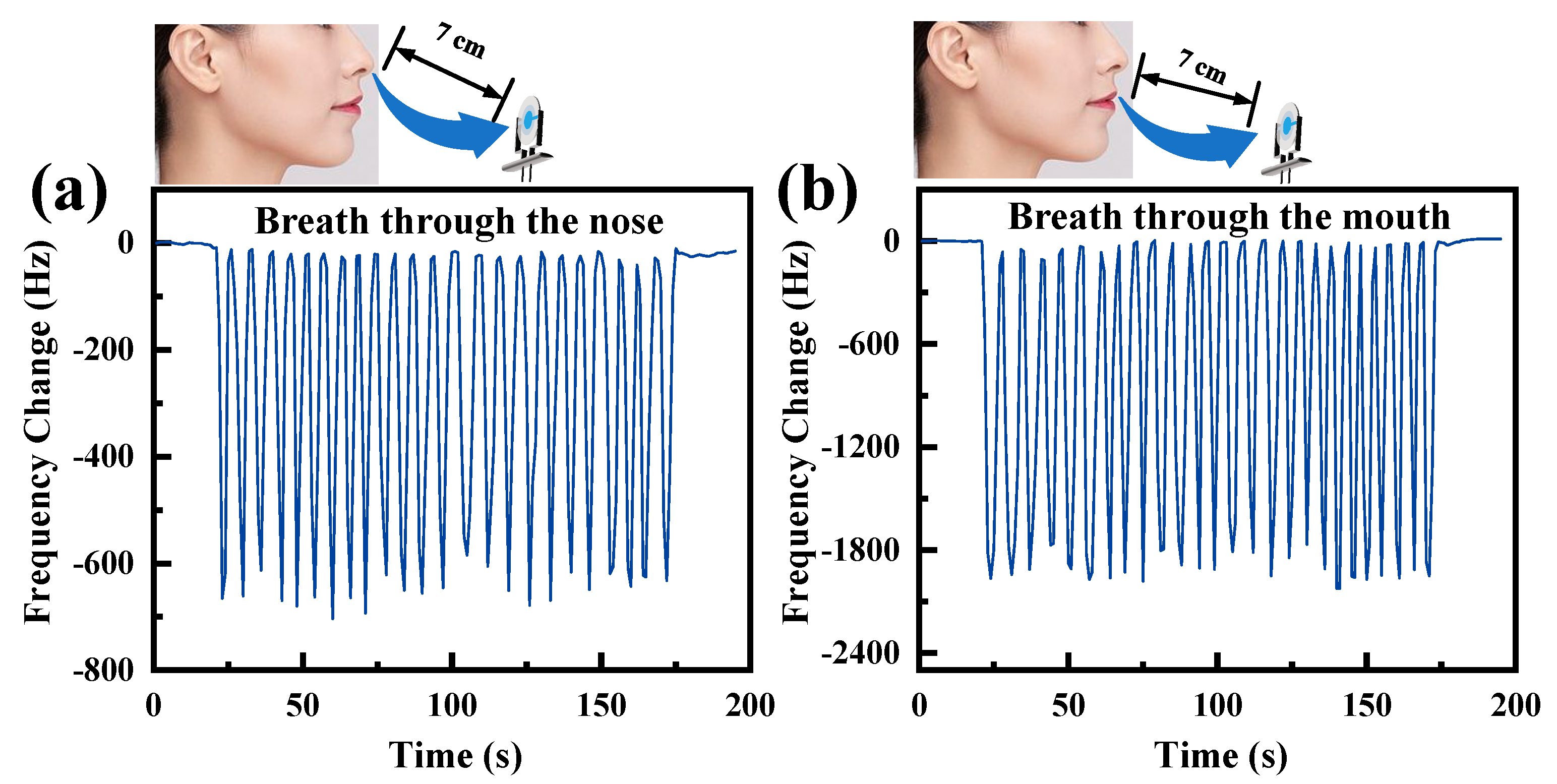
5. Sensor Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of Breath Analysis Based on Humidity and Gas Sensors: Potential and Challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Xu, L.; Zhai, H.; Chen, X.; Liu, Y.; Wang, M.; Liu, Z.; Umar, M.; Ji, C.; Chen, Z.; Jin, L.; et al. Coolmax/Graphene-Oxide Functionalized Textile Humidity Sensor with Ultrafast Response for Human Activities Monitoring. Chem. Eng. J. 2021, 412, 128639. [Google Scholar] [CrossRef]
- Tong, X.; Wang, H.; Ding, H.; Li, J.; Zhao, H.; Lin, Z.; Xi, H.; Zhang, X. Flexible Humidity Sensors Based on Multidimensional Titanium Dioxide/Cellulose Nanocrystals Composite Film. Nanomaterials 2022, 12, 1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Yang, Q.; Atashbar, M.Z. Rapid, Highly Sensitive, and Highly Repeatable Printed Porous Paper Humidity Sensor. Chem. Eng. J. 2021, 433, 133751. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent Trends of Ceramic Humidity Sensors Development: A Review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.H.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.F.; Shi, Y.; Cai, J.G.; Watanabe, A. Recent Advances in Graphene-Based Humidity Sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, L.F. An Overview of High Frequency Acoustic Sensors—Qcms, Saws and Fbars—Chemical and Biochemical Applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Kim, N.; Wong, W.S.; Yeow, J.T.W. Inkjet-Printed Cmut Humidity Sensors with High Sensitivity and Low Hysteresis. Sens. Actuators B Chem. 2021, 327, 128920. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, N.; Huang, X.; Yao, Y.; Jin, Y.; Pan, W.; Liu, D. Humidity-Sensing Properties of a Biocl-Coated Quartz Crystal Microbalance. ACS Omega 2020, 5, 18818–18825. [Google Scholar] [CrossRef]
- Li, Z.; Teng, M.; Yang, R.; Lin, F.; Fu, Y.; Lin, W.; Zheng, J.; Zhong, X.; Chen, X.; Yang, B.; et al. Sb-Doped Wo3 Based Qcm Humidity Sensor with Self-Recovery Ability for Real-Time Monitoring of Respiration and Wound. Sens. Actuators B Chem. 2022, 361, 131691. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Ma, W.; Ling, W. Quartz Crystal Microbalance Humidity Sensors Based on Nanodiamond Sensing Films. IEEE Trans. Nanotechnol. 2014, 13, 386–393. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, X.; Zhang, B.; Zhang, Z.; Hou, D.; Zhou, Z. Facile Fabrication of High Sensitivity Cellulose Nanocrystals Based Qcm Humidity Sensors with Asymmetric Electrode Structure. Sens. Actuators B Chem. 2019, 302, 127192. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, G. Effects of Surface Physical Sorption on Characteristic of Coated Quartz-Crystal Humidity Sensor. Sens. Actuators B Chem. 1995, 24, 62–64. [Google Scholar] [CrossRef]
- Zhang, C.; Vetelino, J.F. Bulk Acoustic Wave Sensors for Sensing Measurand-Induced Electrical Property Changes in Solutions. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 773–778. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Huang, X. Enhanced Sensitivity of Quartz Crystal Proximity Sensors Using an Asymmetrical Electrodes Configuration. Sens. Actuators A Phys. 2017, 258, 95–100. [Google Scholar] [CrossRef]
- Dickert, F.; Halikias, K.; Hayden, O.; Piu, L.; Sikorski, R. Sensors Based on Fingerprints of Neutral and Ionic Analytes in Polymeric Materials. Sens. Actuators B Chem. 2001, 76, 295–298. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.; Pan, W.; Xu, Y.; Fan, Z. Investigation on Mass Sensitivity of N-M Type Electrode Quartz Crystal Microbalance. Sensors 2019, 19, 2125. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.-H.; Yao, Y.; Luo, K.-B.; Pan, H.-Z.; Wang, Q. Ringed Electrode Configuration Enhances the Sensitivity of Qcm Humidity Sensor Based on Lignin through Fringing Field Effect. IEEE Sens. J. 2021, 21, 22450–22458. [Google Scholar] [CrossRef]
- Ramaprasad, A.T.; Rao, V. Chitin–Polyaniline Blend as Humidity Sensor. Sens. Actuators B Chem. 2010, 148, 117–125. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultrafine Polysaccharide Nanofibrous Membranes for Water Purification. Biomacromolecules 2011, 12, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Afnas, V.M.; Unnikrishnan, G.; Budhe, S.; Manaf, O.; Ameen, J. Pva/Gelatin/Chitin Ternary Blend as a Humidity Sensing Material. J. Mater. Sci. Mater. Electron. 2021, 33, 2031–2043. [Google Scholar] [CrossRef]
- Li, H.; Yoshida, S.; Mitani, N.; Egusa, M.; Takagi, M.; Izawa, H.; Matsumoto, T.; Kaminaka, H.; Ifuku, S. Disease Resistance and Growth Promotion Activities of Chitin/Cellulose Nanofiber from Spent Mushroom Substrate to Plant. Carbohydr. Polym. 2022, 284, 119233. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, P.; Gharaie, S.; Yousefi, H.; Paulino, M.; Kaynak, A.; Varley, R.; Kouzani, A.Z. A 3d Printable Dynamic Nanocellulose/Nanochitin Self-Healing Hydrogel and Soft Strain Sensor. Carbohydr. Polym. 2022, 291, 119545. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Wang, S.-T.; Chang, K.-L.B. Enhancing Saltiness Perception Using Chitin Nanomaterials. Polymers 2019, 11, 719. [Google Scholar] [CrossRef]
- Jlassi, K.; Mallick, S.; Eribi, A.; Chehimi, M.M.; Ahmad, Z.; Touati, F.; Krupa, I. Facile Preparation of N-S Co-Doped Graphene Quantum Dots (Gqds) from Graphite Waste for Efficient Humidity Sensing. Sens. Actuators B Chem. 2020, 328, 129058. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, X.; Tang, Z.; Chen, Q.; Li, H.; Wang, X.; Yan, Y. Method for Qcm Resonator Device Equivalent Circuit Parameter Extraction and Electrode Quality Assessment. Micromachines 2021, 12, 1086. [Google Scholar] [CrossRef]
- Butterworth, S. On a Null Method of Testing Vibration Galvanometers. Proc. Phys. Soc. Lond. 1913, 26, 264–273. [Google Scholar] [CrossRef]
- Van Dyke, K.S. The Piezo-Electric Resonator and Its Equivalent Network. Proc. Inst. Radio Eng. 1928, 16, 742–764. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Nakamori, T.; Mori, H. Measuring of Isothermal Water Vapor Adsorption/Desorption Rate Using Qcm Method and Its Mass Transfer Resistance of a Layer Coated with Silica-Gel Micro Particles in a Moist Air. Int. J. Refrig. 2019, 105, 11–18. [Google Scholar] [CrossRef]
- Lee, S.-W.; Choi, B.I.; Kim, J.C.; Woo, S.-B.; Kim, Y.-G. Reducing Individual Difference and Temperature Dependency of Qcm Humidity Sensors Based on Graphene Oxides through Normalization of Frequency Shifts. Sens. Actuators B Chem. 2020, 313, 128043. [Google Scholar] [CrossRef]
- Rianjanu, A.; Julian, T.; Hidayat, S.N.; Yulianto, N.; Majid, N.; Syamsu, I.; Wasisto, H.S.; Triyana, K. Quartz Crystal Microbalance Humidity Sensors Integrated with Hydrophilic Polyethyleneimine-Grafted Polyacrylonitrile Nanofibers. Sens. Actuators B Chem. 2020, 319, 128286. [Google Scholar] [CrossRef]
- Choi, J.; Baek, S.; Jeon, S.; Yim, C. Laser-Induced Graphene on a Quartz Crystal Microbalance for Humidity Sensing. Crystals 2021, 11, 289. [Google Scholar] [CrossRef]
- Chang, Q.; Wu, D.; Huang, Y.; Liang, C.; Liu, L.; Liu, H.; He, Y.; Huang, Q.; Qiu, J.; Tang, X. A Lead-Free K2cubr3 Microwires-Based Humidity Sensor Realized Via Qcm for Real-Time Breath Monitoring. Sens. Actuators B Chem. 2022, 367, 132112. [Google Scholar] [CrossRef]
- Li, R.; Fan, Y.; Ma, Z.; Zhang, D.; Liu, Y.; Xu, J. Controllable Preparation of Ultrathin Mxene Nanosheets and Their Excellent Qcm Humidity Sensing Properties Enhanced by Fluoride Doping. Microchim. Acta Acta 2021, 188, 81. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z. Graphene Oxide Thin Film Coated Quartz Crystal Microbalance for Humidity Detection. Appl. Surf. Sci. 2011, 257, 7778–7782. [Google Scholar] [CrossRef]
- Gao, N.; Li, H.-Y.; Zhang, W.; Zhang, Y.; Zeng, Y.; Zhixiang, H.; Liu, J.; Jiang, J.; Miao, L.; Yi, F.; et al. Qcm-Based Humidity Sensor and Sensing Properties Employing Colloidal Sno2 Nanowires. Sens. Actuators B Chem. 2019, 293, 129–135. [Google Scholar] [CrossRef]
- Tang, L.; Chen, W.; Chen, B.; Lv, R.; Zheng, X.; Rong, C.; Lu, B.; Huang, B. Sensitive and Renewable Quartz Crystal Microbalance Humidity Sensor Based on Nitrocellulose Nanocrystals. Sens. Actuators B Chem. 2021, 327, 128944. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Chen, Y.; Feng, L.; Lu, J.; Du, L.; Guo, J.; Cheng, Z.; Shi, Z.; Zhao, L. High-Sensitivity and Environmentally Friendly Humidity Sensors Deposited with Recyclable Green Microspheres for Wireless Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 15608–15622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, F.; Qi, P.; Gao, B.; Zhang, T. Study on a Humidity Sensor of Quartz Crystal Microbalance Modified with Multi-Pore Polydopamine. IEEE Electron Device Lett. 2022, 43, 611–614. [Google Scholar] [CrossRef]
- Zhang, D.; Song, X.; Wang, Z.; Chen, H. Ultra-Highly Sensitive Humidity Sensing by Polydopamine/Graphene Oxide Nanostructure on Quartz Crystal Microbalance. Appl. Surf. Sci. 2021, 538, 147816. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, X.; Chen, Q.; Zhang, Z.; Ling, W. High Sensitivity and High Stability Qcm Humidity Sensors Based on Polydopamine Coated Cellulose Nanocrystals/Graphene Oxide Nanocomposite. Nanomaterials 2020, 10, 2210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, K.; Xu, R.; Jiang, D.; Luo, L.; Zhu, Z. Quartz Crystal Microbalance Coated with Carbon Nanotube Films Used as Humidity Sensor. Sens. Actuators A Phys. 2005, 120, 142–146. [Google Scholar] [CrossRef]
- Qi, P.; Xu, Z.; Zhang, T.; Fei, T.; Wang, R. Chitosan Wrapped Multiwalled Carbon Nanotubes as Quartz Crystal Microbalance Sensing Material for Humidity Detection. J. Colloid Interface Sci. 2020, 560, 284–292. [Google Scholar] [CrossRef]
- Rahman, S.A.; Khan, S.A.; Rehman, M.M.; Kim, W.-Y. Highly Sensitive and Stable Humidity Sensor Based on the Bi-Layered Pva/Graphene Flower Composite Film. Nanomaterials 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.M.M.U.; Khan, M.; Rehman, M.M.; Khan, S.A.; Kim, W.Y. High-Performance Humidity Sensor for Multipurpose Applications by Recycling of Potato Peel Bio-Waste. Sens. Actuators A Phys. 2022, 343, 113662. [Google Scholar] [CrossRef]
- Saqib, M.; Khan, S.A.; Mutee Ur Rehman, H.M.; Yang, Y.; Kim, S.; Rehman, M.M.; Young Kim, W. High-Performance Humidity Sensor Based on the Graphene Flower/Zinc Oxide Composite. Nanomaterials 2021, 11, 242. [Google Scholar] [CrossRef]
- Rehman, M.U.; Hafiz, M.; Rehman, M.M.; Saqib, M.; Khan, S.A.; Khan, M.; Yang, Y.; Kim, S.; Rahman, S.A.; Kim, W. Highly Efficient and Wide Range Humidity Response of Biocompatible Egg White Thin Film. Nanomaterials 2021, 11, 1815. [Google Scholar] [CrossRef]
- Khan, S.A.; Saqib, M.; Rehman, M.M.; Rehman, H.M.M.U.; Rahman, S.A.; Yang, Y.; Kim, S.; Kim, W. A Full-Range Flexible and Printed Humidity Sensor Based on a Solution-Processed P(Vdf-Trfe)/Graphene-Flower Composite. Nanomaterials 2021, 11, 1915. [Google Scholar] [CrossRef]
- Zheng, Z.; Yao, Y.; Liu, J.A.; Sun, Y.; Yeow, J.T. Highly Sensitive Cmut-Based Humidity Sensors Built with Nitride-to-Oxide Wafer Bonding Technology. Sens. Actuators B Chem. 2019, 294, 123–131. [Google Scholar] [CrossRef]
- Alam, S.; Islam, T.; Mittal, U. A Sensitive Inexpensive Saw Sensor for Wide Range Humidity Measurement. IEEE Sens. J. 2019, 20, 546–551. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Fang, Z.; Liu, Z.; Zhu, Y.; Du, L. High-Performance Fbar Humidity Sensor Based on the Pi Film as the Multifunctional Layer. Sens. Actuators B Chem. 2020, 308, 127694. [Google Scholar] [CrossRef]
- Le, X.; Peng, L.; Pang, J.; Xu, Z.; Gao, C.; Xie, J. Humidity Sensors Based on Aln Microcantilevers Excited at High-Order Resonant Modes and Sensing Layers of Uniform Graphene Oxide. Sens. Actuators B Chem. 2018, 283, 198–206. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Meng, F.-Y.; Liang, J.-G.; Kashan, H.S.; Adhikari, K.K.; Wang, L.; Kim, E.-S.; Kim, N.-Y. Design and Analysis of Ultrafast and High-Sensitivity Microwave Transduction Humidity Sensor Based on Belt-Shaped Moo3 Nanomaterial. Sens. Actuators B Chem. 2019, 304, 127138. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Ding, X.; Yu, X.; Zhao, X.; Chen, X. Facile Fabrication of Flower-Like Mos2/Nanodiamond Nanocomposite toward High-Performance Humidity Detection. Sens. Actuators B Chem. 2020, 317, 128168. [Google Scholar] [CrossRef]
- Josse, F.; Lee, Y.; Martin, S.J.; Cernosek, R.W. Analysis of the Radial Dependence of Mass Sensitivity for Modified-Electrode Quartz Crystal Resonators. Anal. Chem. 1998, 70, 237–247. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung Von Schwingquarzen Zur Wägung Dünner Schichten Und Zur Mikrowägung. Z. Fur Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.; Yao, Y.; Mao, K. Analysis of the Effect of Electrode Materials on the Sensitivity of Quartz Crystal Microbalance. Nanomaterials 2022, 12, 975. [Google Scholar] [CrossRef]

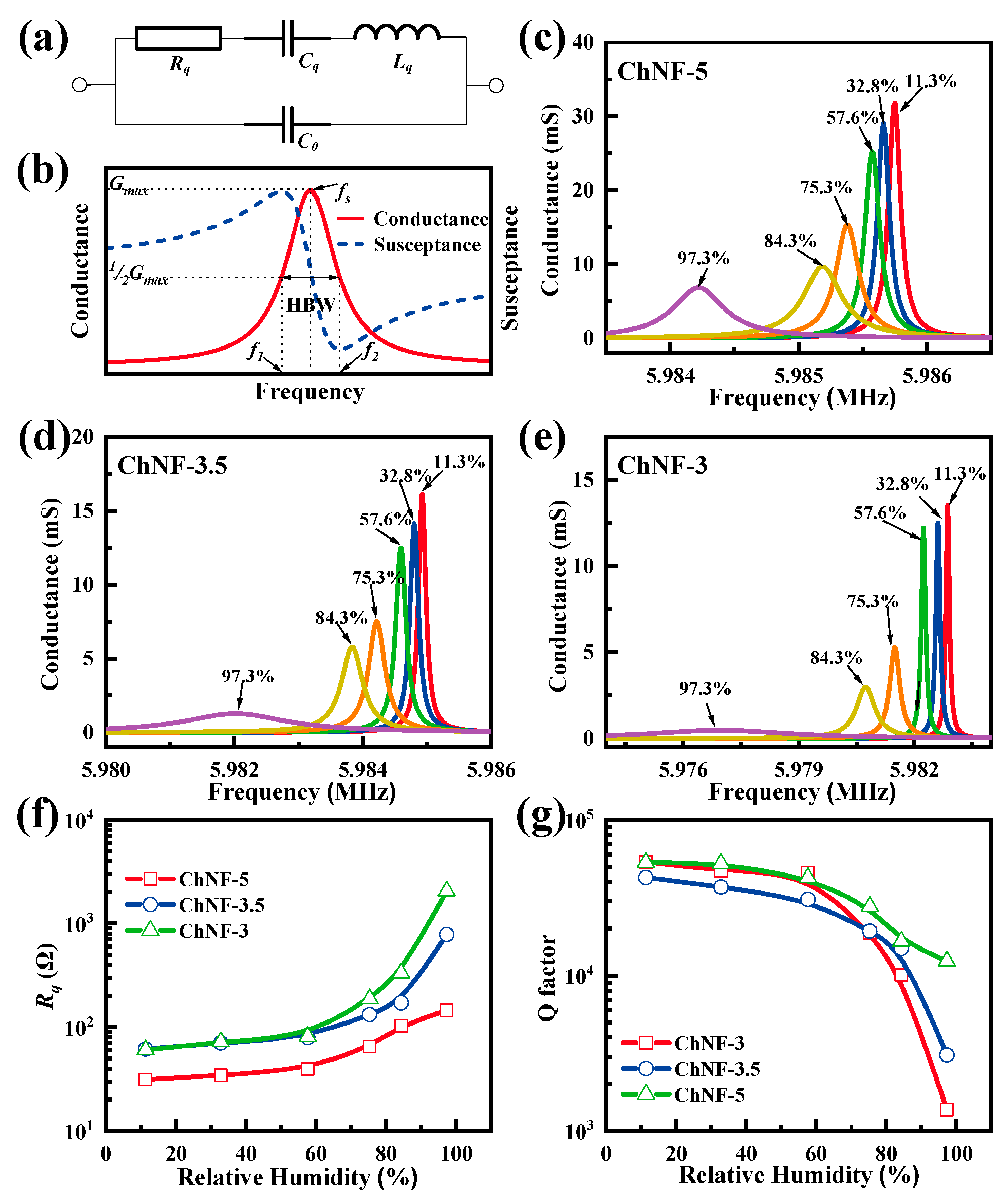
| (Ω) | (mH) | (fF) | (pF) | Q | |
|---|---|---|---|---|---|
| ChNF-5 | 29.1729 | 4.7402 | 14.9185 | 4.2481 | 47,084 |
| ChNF-3.5 | 54.1703 | 7.1384 | 9.9066 | 3.2460 | 49,529 |
| ChNF-3 | 61.9854 | 8.5007 | 8.3190 | 2.9921 | 51,544 |
| (Ω) | (mH) | (fF) | (pF) | Q | ||
|---|---|---|---|---|---|---|
| ChNF-5 | 11.30% | 31.29 | 44.38 | 15.95 | 4.34 | 53,283 |
| 32.80% | 34.42 | 48.15 | 14.7 | 4.01 | 52,553 | |
| 57.60% | 39.5 | 44.79 | 15.8 | 4.32 | 42,603 | |
| 75.30% | 65.15 | 48.07 | 14.72 | 4.03 | 27,720 | |
| 84.30% | 103.46 | 45.65 | 15.5 | 4.26 | 16,576 | |
| 97.30% | 146.32 | 48.21 | 14.69 | 4.04 | 12,378 | |
| ChNF-3.5 | 11.30% | 61.81 | 70.21 | 10.08 | 3 | 42,669 |
| 32.80% | 70.76 | 69.8 | 10.14 | 3.02 | 37,058 | |
| 57.60% | 80 | 65.67 | 10.78 | 3.2 | 30,835 | |
| 75.30% | 133.08 | 68.25 | 10.37 | 3.08 | 19,263 | |
| 84.30% | 172.94 | 68.63 | 10.32 | 3.06 | 14,905 | |
| 97.30% | 782.86 | 64.26 | 11.03 | 3.21 | 3082 | |
| ChNF-3 | 11.30% | 60.34 | 86.2 | 8.22 | 2.88 | 53,649 |
| 32.80% | 72.45 | 90.9 | 7.79 | 2.74 | 47,117 | |
| 57.60% | 81.26 | 98.56 | 7.19 | 2.52 | 45,544 | |
| 75.30% | 189.27 | 94.42 | 7.51 | 2.6 | 18,731 | |
| 84.30% | 334.81 | 89.92 | 7.88 | 2.68 | 10,082 | |
| 97.30% | 2061.53 | 75 | 9.46 | 2.98 | 1365 |
| Materials | Sensing Range (RH) | Sensitivity (ppm/%RH) | Response/ Recovery Time (s) | Humidity Hysteresis (%RH) | Reference |
|---|---|---|---|---|---|
| Lignin | 11.3–97.3% | 6.1 | 29/5 | 6.2 | [19] |
| CNCs | 11.3–97.3% | 12.0 | 60/15 | 7.3 | [13] |
| S-Ti3C2 | 11.3−97.3% | 1.28 | 6/2 | 1.16 | [35] |
| GO | 11.3−97.3% | 2.21 | 45/21 | Not given | [36] |
| SnO2 | 11.3−97.3% | 2.9 | 10/3 | Not given | [37] |
| NCNCs | 11.3−84.3 % | 1.3 | 18/10 | 1.6 | [38] |
| green microspheres | 11.3−97.3% | 3.0 | 48/65 | 0.08 | [39] |
| Sb/WO3 | 0–85%RH | 3.6 | 10/1.6 | Not given | [11] |
| multi-pore PDA | 11−97.3% | 0.5 | 12/39 | 3.66 | [40] |
| PDA/GO | 0–97.3% | 12.5 | 18/2 | 2.1 | [41] |
| PDA@CNCs/GO | 11.3–97.3% | 5.5 | 37/5 | 4.3 | [42] |
| CNT | 5–97% | 0.5 | 60/70 | Not given | [43] |
| MWCNTs-CS | 11–95% | 4.7 | 75/34 | 0.8 | [44] |
| Chitin nanofiber | 11.3–97.3% | 9.8 | 30/3.5 | 2.5 | This work |
| Materials | Sensing Principle | Sensing Range (RH) | Sensitivity (Operating Frequency) | Response/ Recovery Time (s) | Humidity Hysteresis (%RH) | Reference |
|---|---|---|---|---|---|---|
| PVA/GF | Capacitance | 40–90% | 29 nF/%RH (10 kHz) | 2/3.2 | Not given | [45] |
| Potato peel | Impedance | 10–90% | 70 kΩ/% RH (1 kHz) | 8/12 | 2.1 | [46] |
| ZnO/GrF | Resistance | 15–86% | 7.7 µA/%RH (Not give) | 0.4/4 | Not given | [47] |
| egg white | Impedance | 10–85% | 50 kΩ/%RH (1 kHz) | 1.2/1.7 | Not given | [48] |
| P(VDF-TrFE)/GF | Capacitance | 8–98% | 0.056 pF/%RH (10 kHz) | 0.8/2.5 | >20 | [49] |
| GO | CMUT | 22.5–43.2% | 241.67 ppm/%RH (10 MHz) | 10/4 | Not given | [50] |
| PVA | SAW | 0–98.8% | 7.35 kHz/%RH (433 MHz) | 35/46 | 0.004 | [51] |
| PI | FBAR | 15–85% | 67.3 KHz/%RH (1055 MHz) | 17/26 | 1.77 | [52] |
| GO | Cantilever | 10–90 | 84 Hz/%RH (2 MHz) | 17/12 | <3 | [53] |
| MoO3 | SIW | 10–90 | 2.062 MHz/%RH (9.1 GHz) | 3/2 | 0.25 | [54] |
| Chitin nanofiber | QCM | 11.3–97.3% | 58.84 Hz/%RH (6 MHz) | 30/3.5 | 2.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Liu, D.; Huang, X.-H.; Yao, Y.; Mao, K.-L. Impedance Analysis of Chitin Nanofibers Integrated Bulk Acoustic Wave Humidity Sensor with Asymmetric Electrode Configuration. Nanomaterials 2022, 12, 3035. https://doi.org/10.3390/nano12173035
Chen Q, Liu D, Huang X-H, Yao Y, Mao K-L. Impedance Analysis of Chitin Nanofibers Integrated Bulk Acoustic Wave Humidity Sensor with Asymmetric Electrode Configuration. Nanomaterials. 2022; 12(17):3035. https://doi.org/10.3390/nano12173035
Chicago/Turabian StyleChen, Qiao, Dong Liu, Xian-He Huang, Yao Yao, and Kun-Lei Mao. 2022. "Impedance Analysis of Chitin Nanofibers Integrated Bulk Acoustic Wave Humidity Sensor with Asymmetric Electrode Configuration" Nanomaterials 12, no. 17: 3035. https://doi.org/10.3390/nano12173035
APA StyleChen, Q., Liu, D., Huang, X. -H., Yao, Y., & Mao, K. -L. (2022). Impedance Analysis of Chitin Nanofibers Integrated Bulk Acoustic Wave Humidity Sensor with Asymmetric Electrode Configuration. Nanomaterials, 12(17), 3035. https://doi.org/10.3390/nano12173035







