The Effect of Liquid-Phase Exfoliated Graphene Film on Neurodifferentiation of Stem Cells from Apical Papilla
Abstract
:1. Introduction
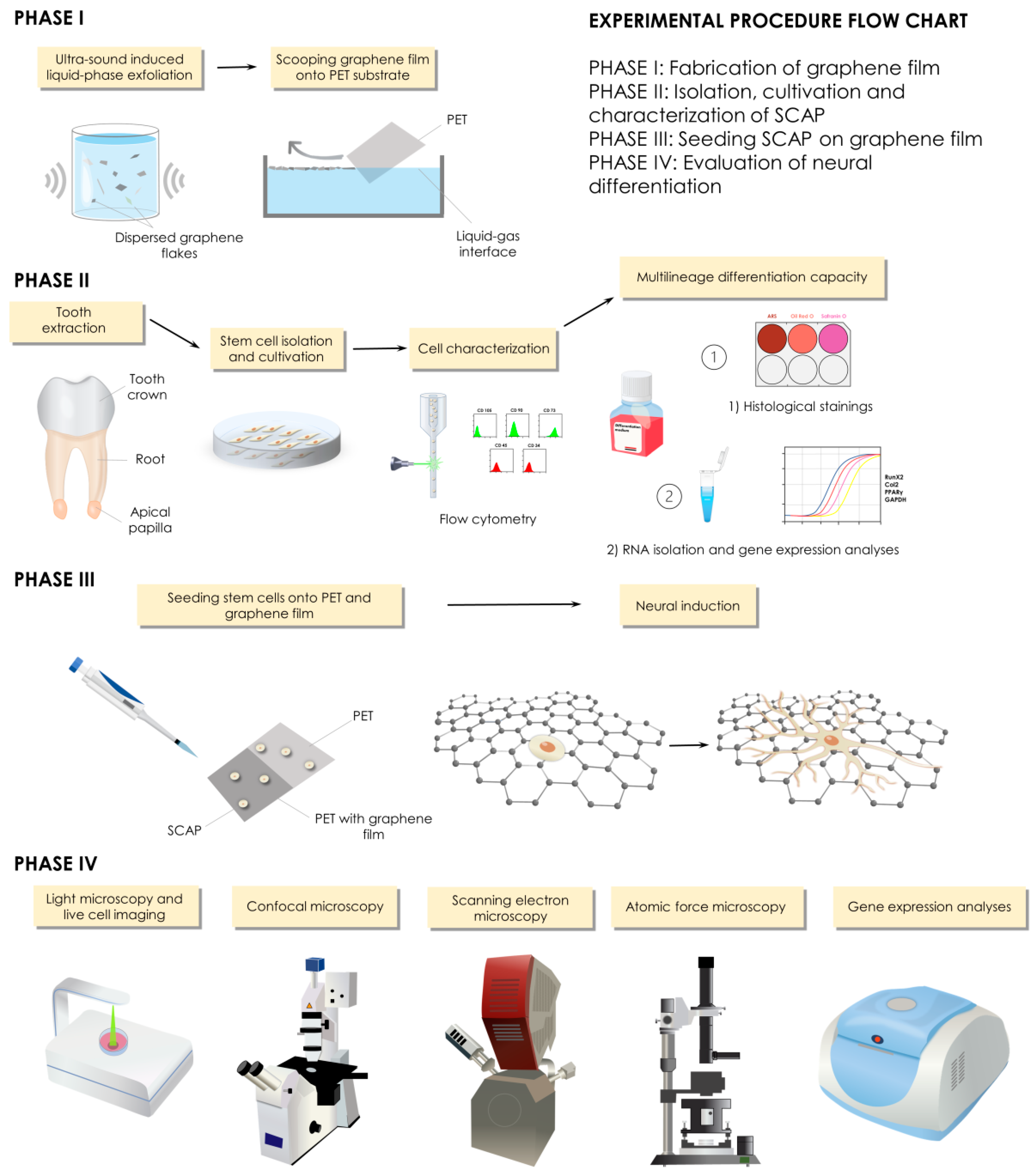
2. Materials and Methods
2.1. Fabrication of Graphene Film
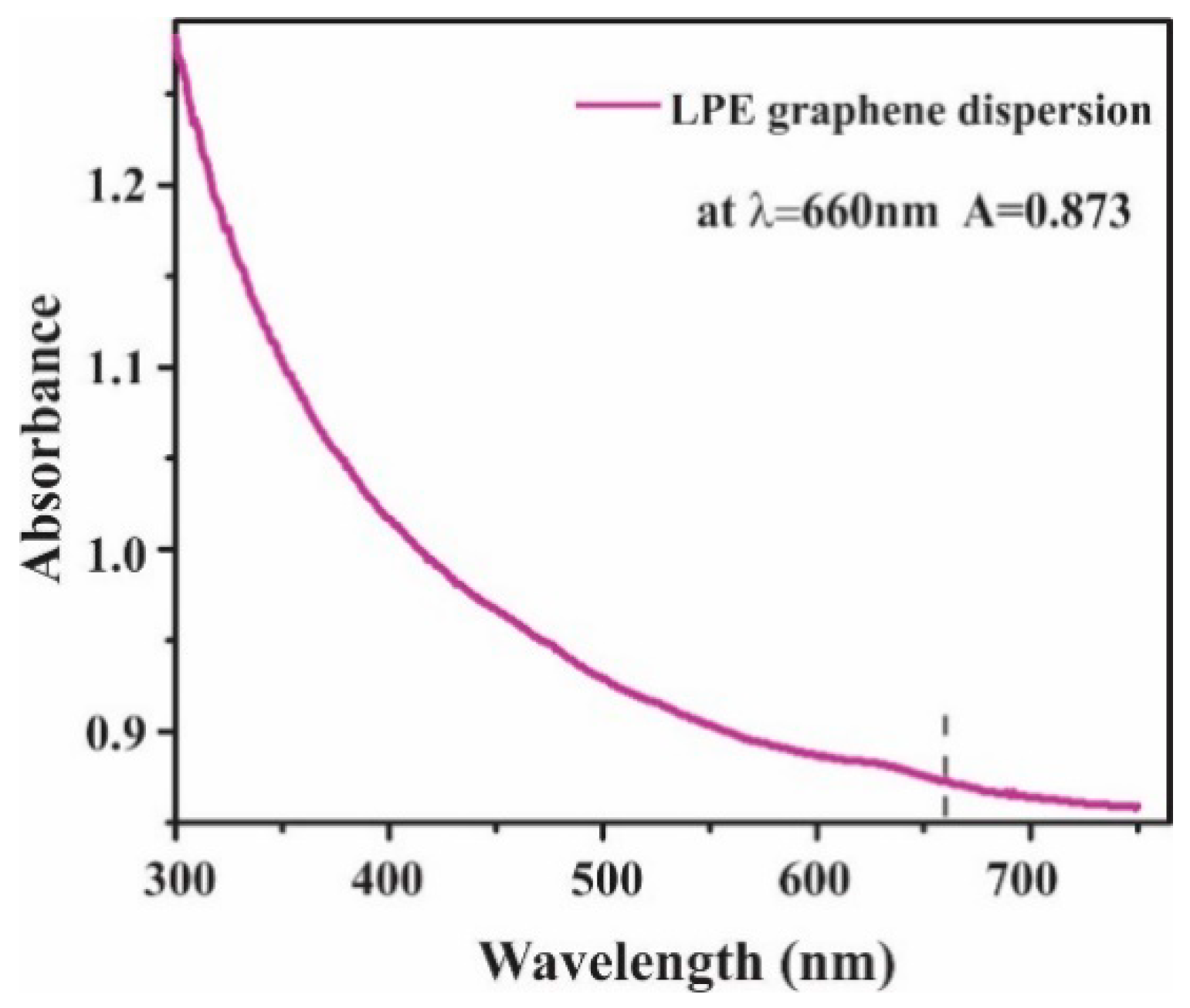
2.1.1. Preparation of Graphene Dispersion
2.1.2. Liquid-Phase Exfoliated Graphene Film Fabrication
2.2. Graphene Film Characterization
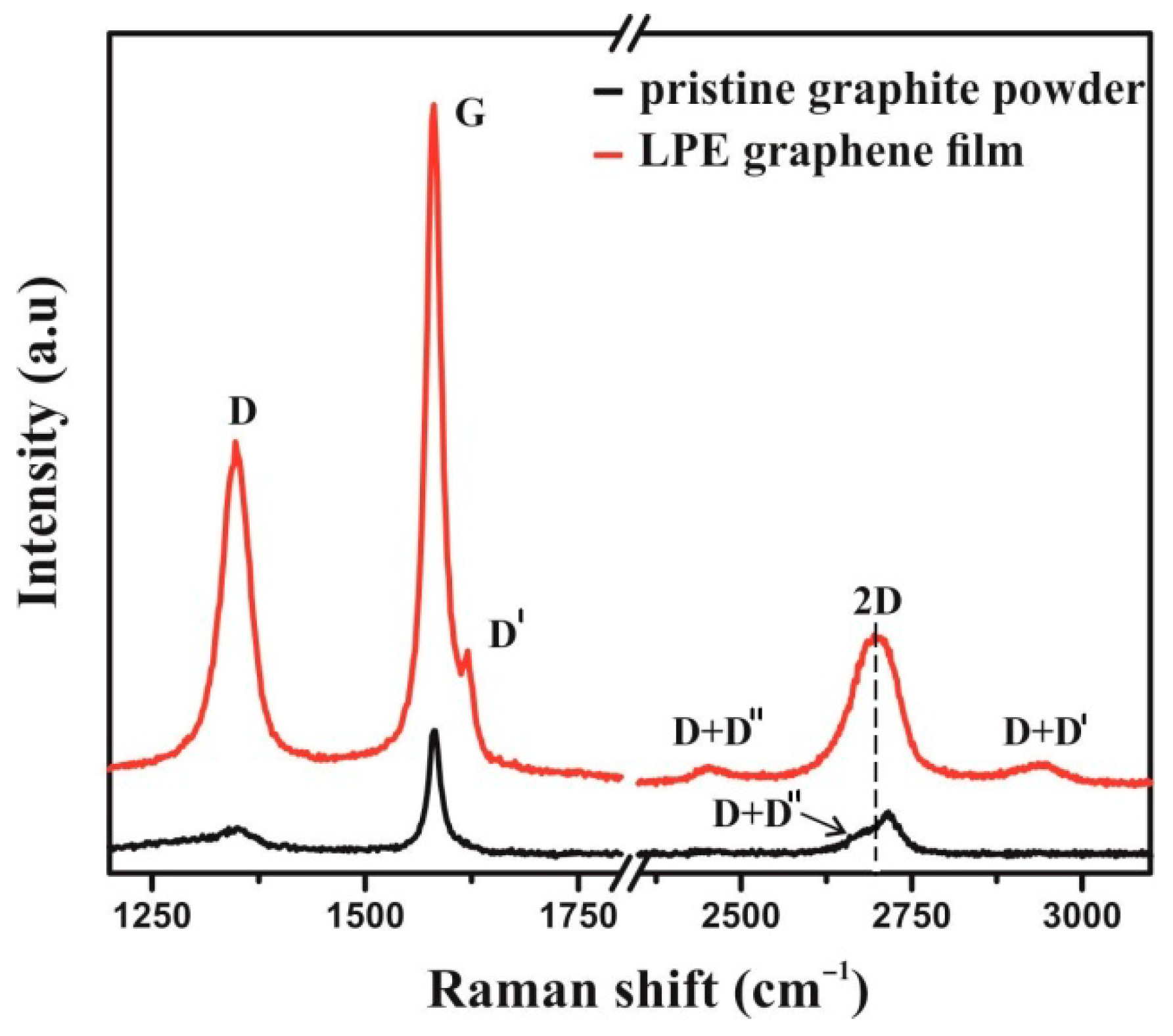
2.2.1. Raman Spectroscopy of Graphene Film
2.2.2. Scanning Electron Microscopy (SEM) of Graphene Film
2.2.3. Atomic Force Microscopy (AFM) of Graphene Film
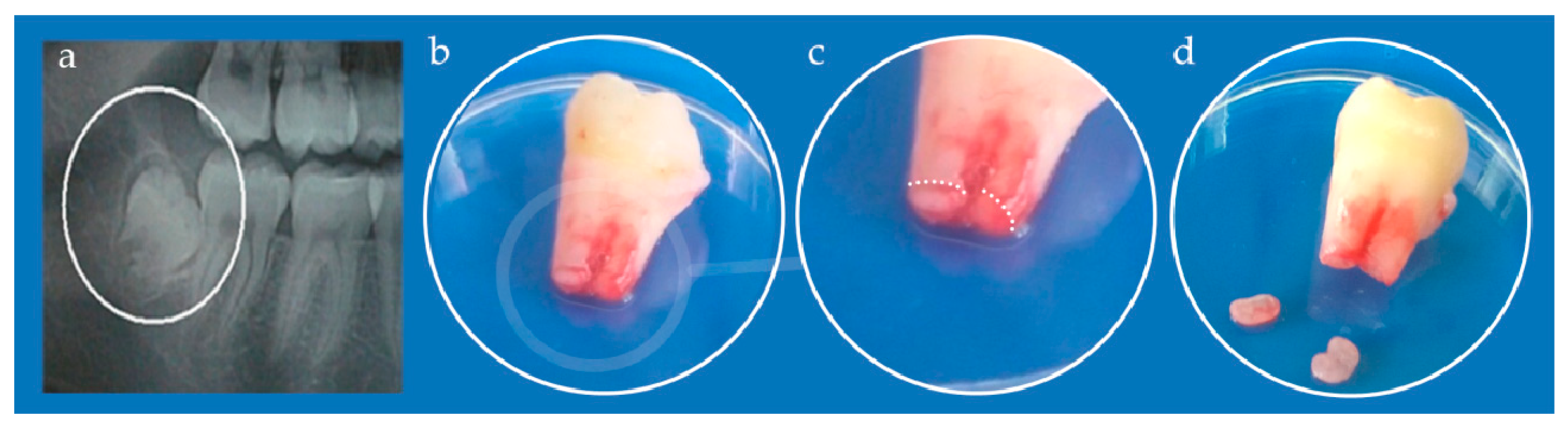
2.3. Cell Cultures
2.4. SCAP Characterization
2.4.1. Flow Cytometry
2.4.2. Multilineage Differentiation Capacity
Osteo-Differentiation
Chondro-Differentiation
Adipo-Differentiation
2.5. LPEG Neuro-Induction
2.6. Cell Morphology Analysis Following LPEG Neuro-Induction
2.6.1. Light Microscopy
2.6.2. Confocal Microscopy
2.6.3. AFM of Neuron-like Cells
2.6.4. SEM of Neuron-like Cells
2.7. RNA Isolation and Gene Expression
2.8. Statistical Analysis
3. Results
3.1. Graphene Film Characterization
3.1.1. Raman Spectroscopy of Graphene Film
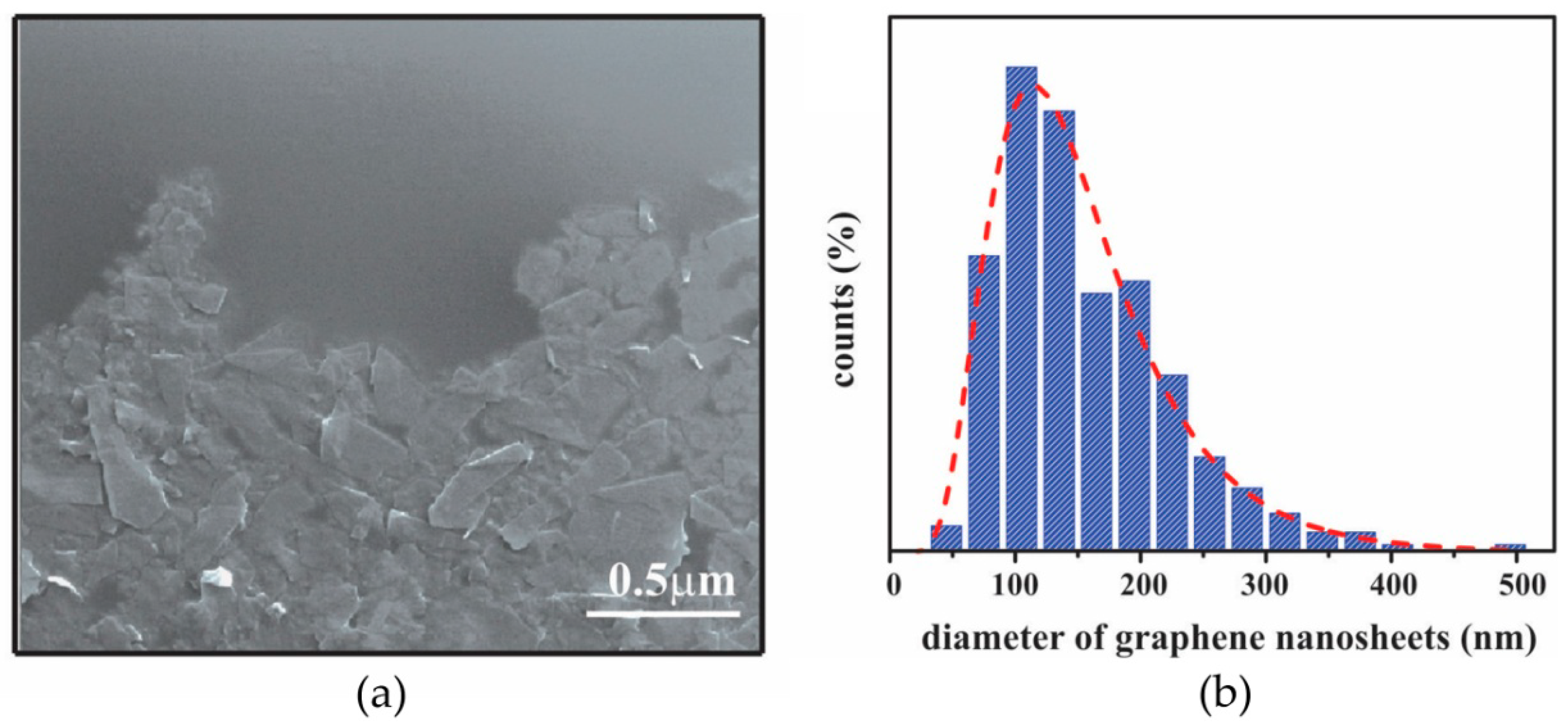
3.1.2. SEM Characterization of Graphene Film
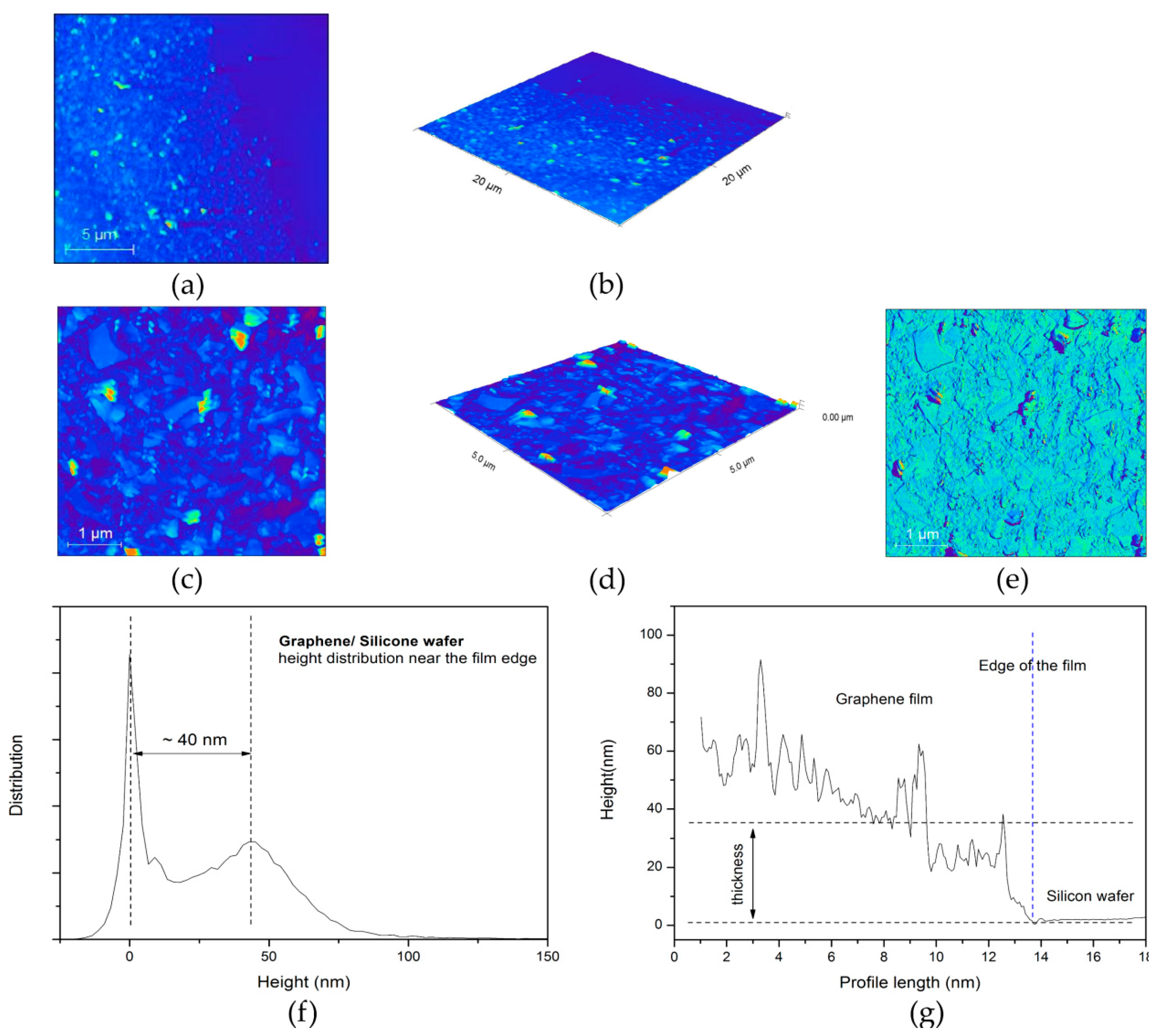
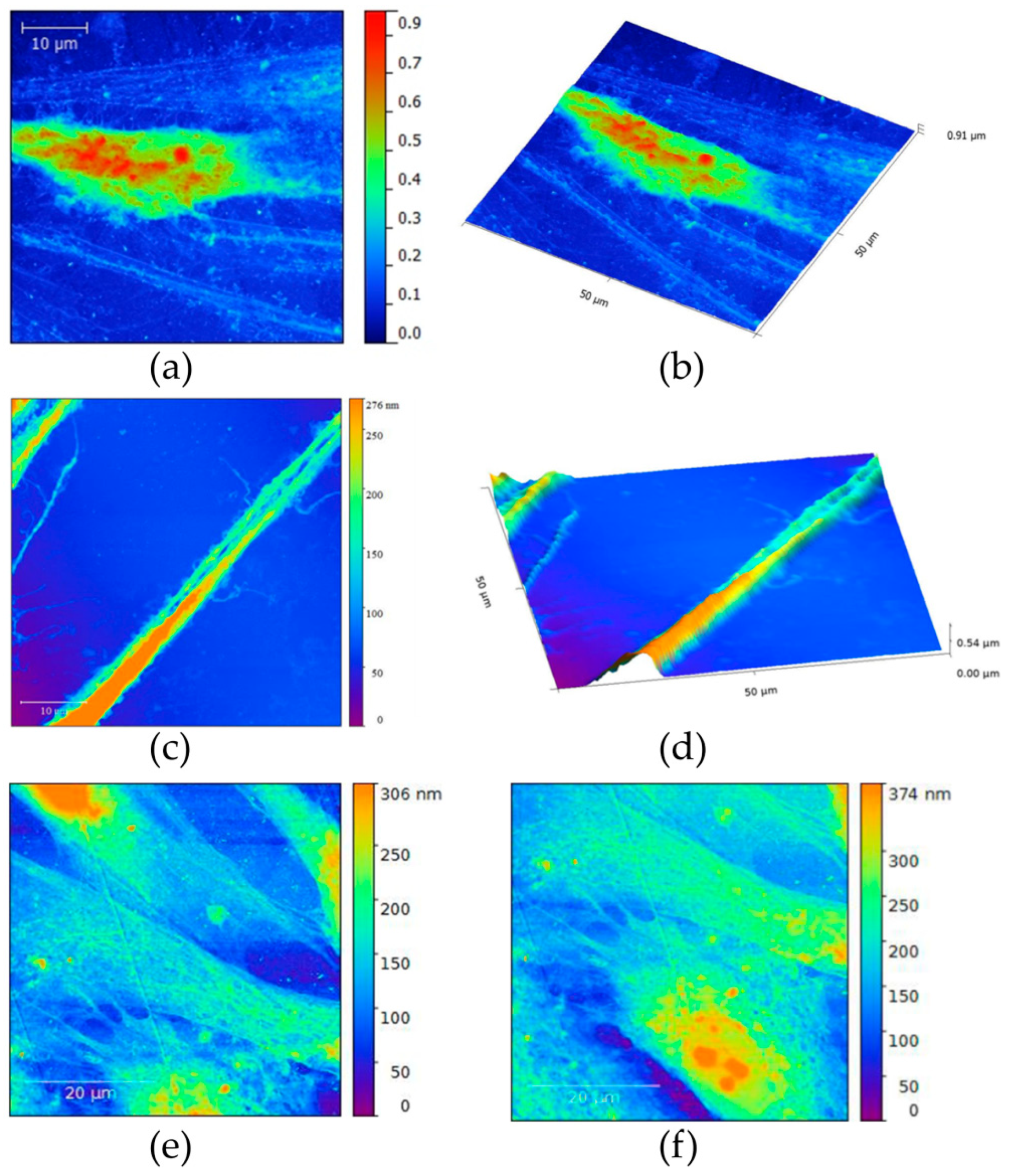
3.1.3. AFM Characterization of Graphene Film
3.2. SCAP Characterization
3.2.1. Flow Cytometry Analysis
3.2.2. Multilineage Differentiation Capacity
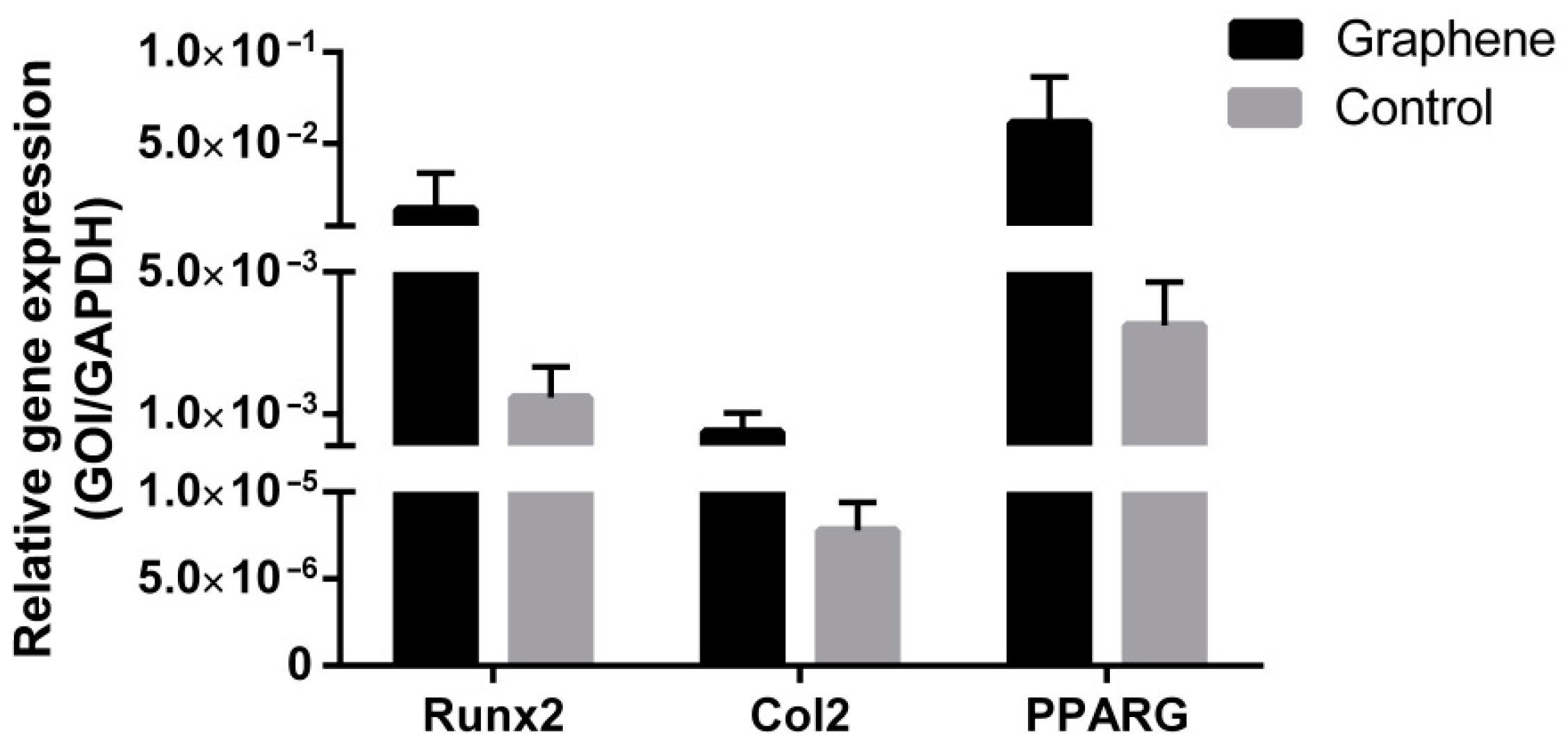
3.2.3. Gene Expression Analysis of Multilineage Differentiation
3.3. LPEG Neuro-Induction of SCAP
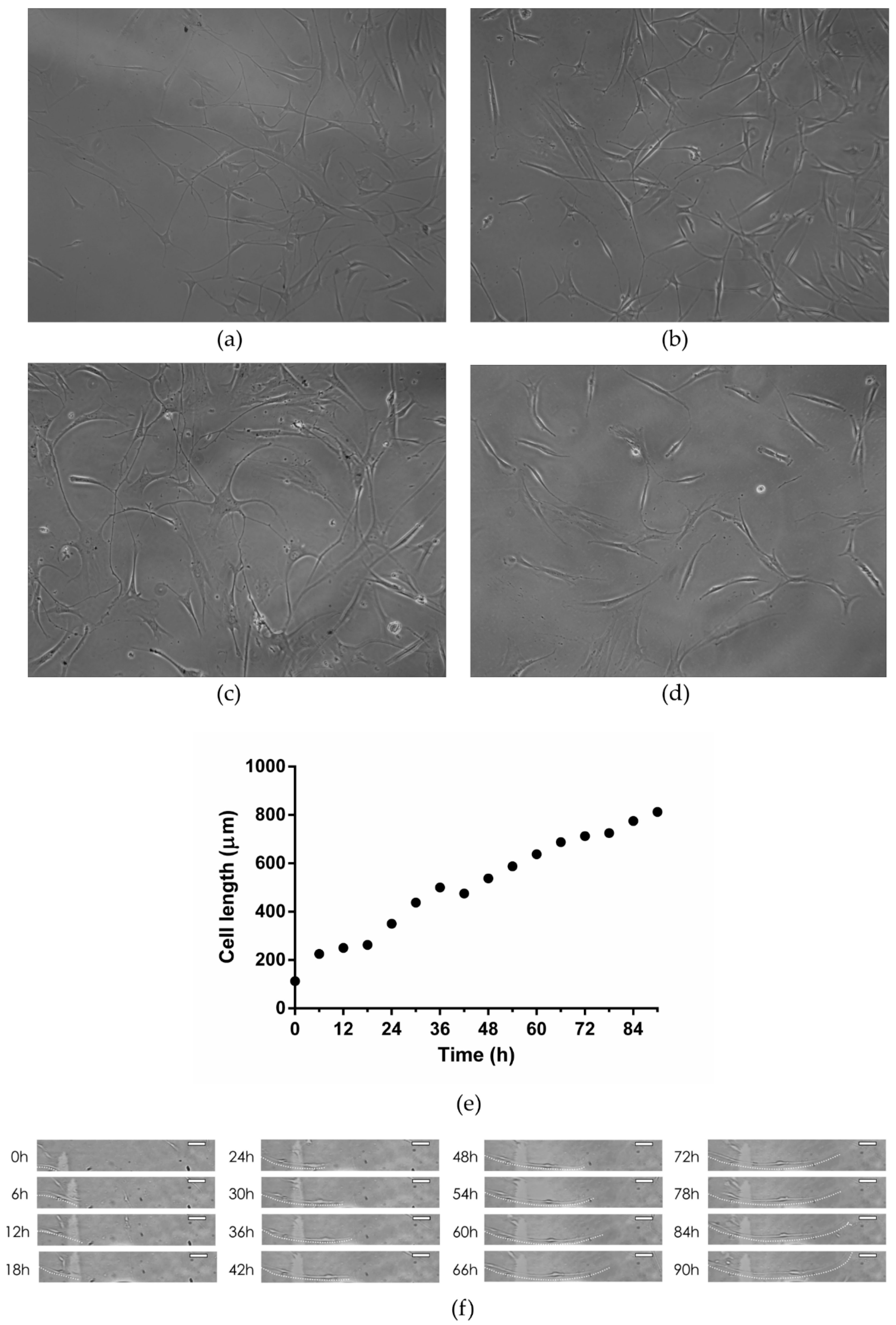
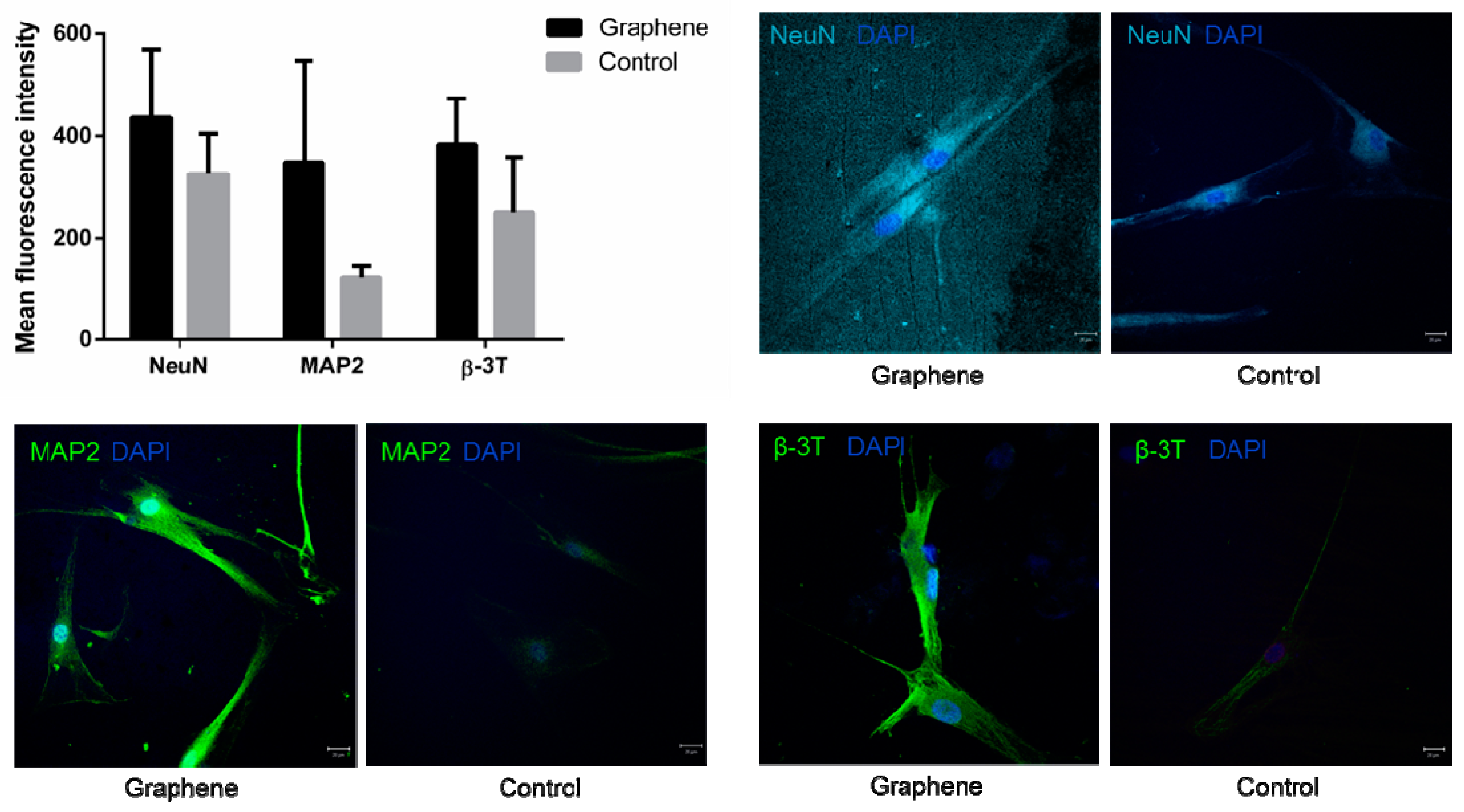
3.3.1. Light Microscopy
3.3.2. Confocal Microscopy
3.3.3. AFM of Neuron-like Cells
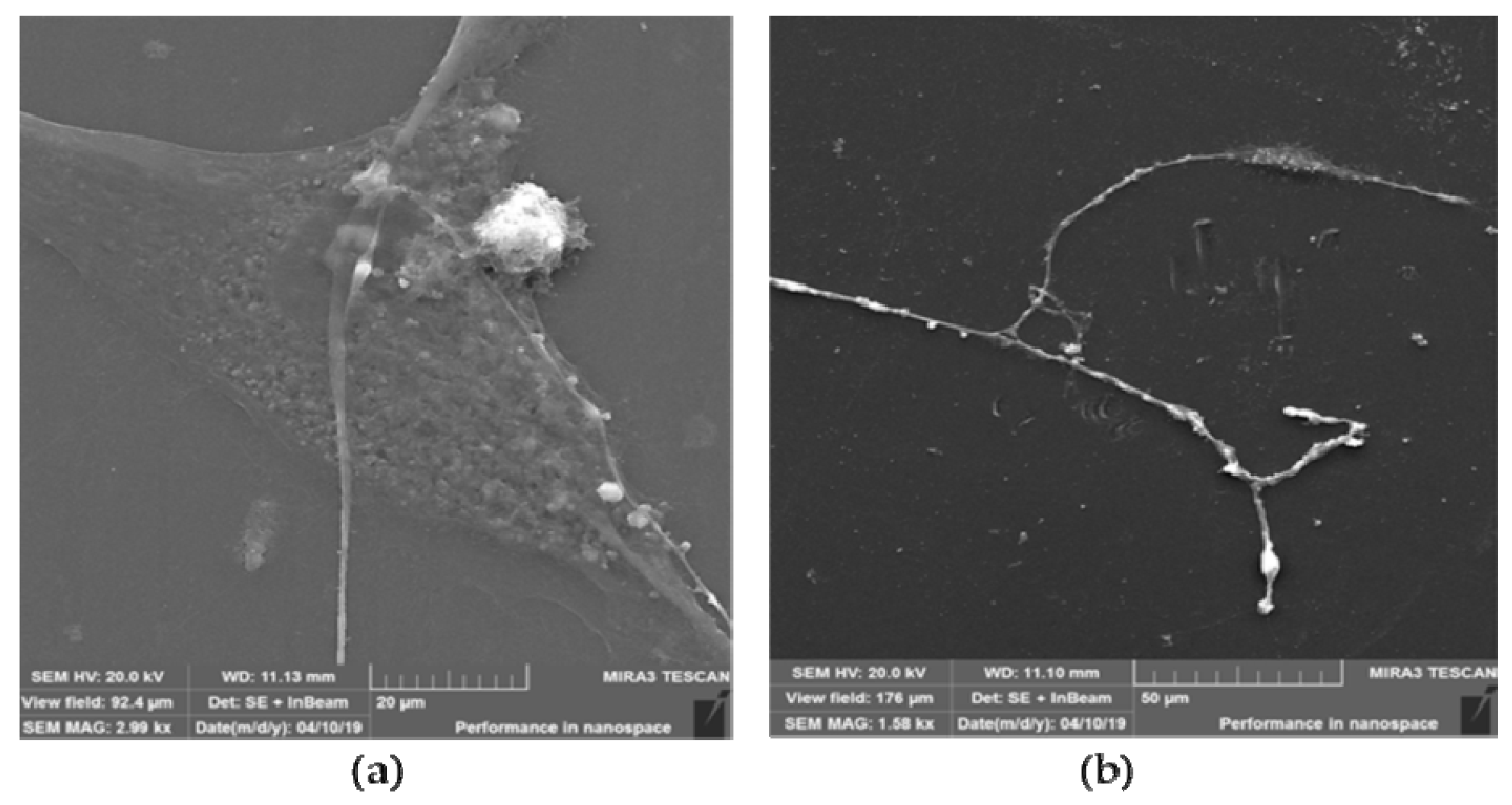
3.3.4. SEM of Neuron-like Cells
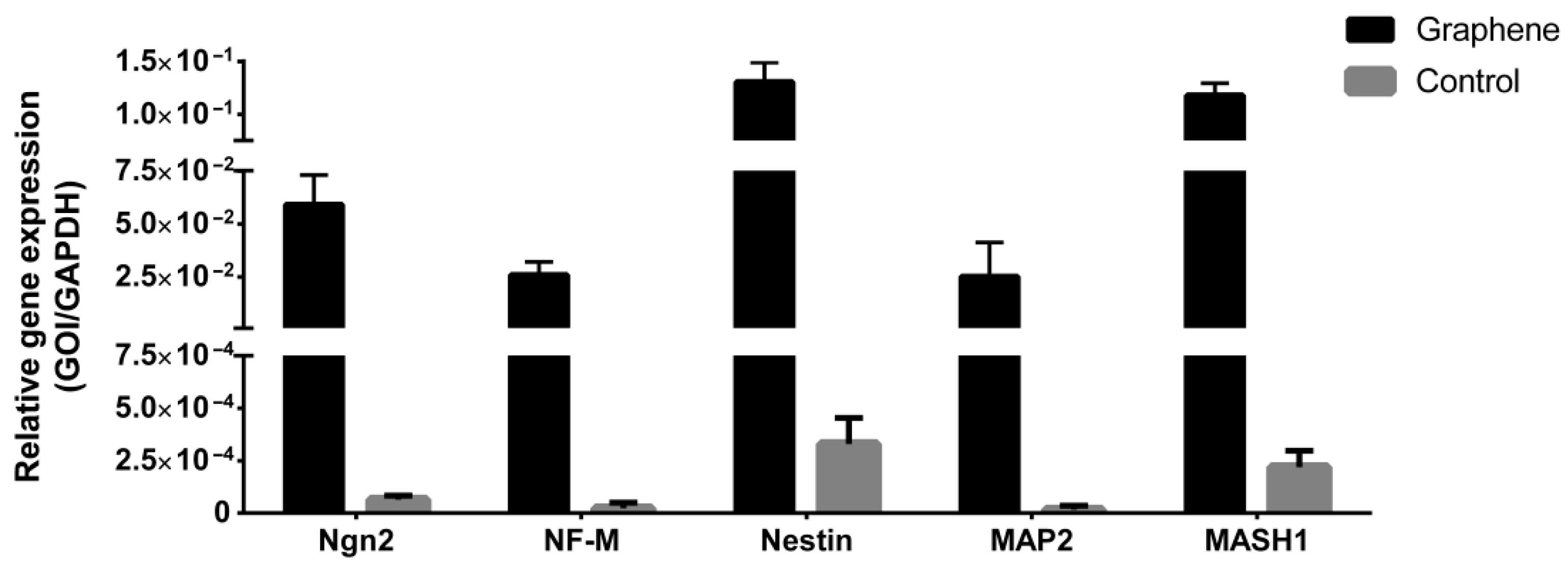
3.3.5. Gene Expression Analysis after LPEG Neuro-Induction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitrečić, D.; Hribljan, V.; Jagečić, D.; Isaković, J.; Lamberto, F.; Horánszky, A.; Zana, M.; Foldes, G.; Zavan, B.; Pivoriūnas, A.; et al. Regenerative Neurology and Regenerative Cardiology: Shared Hurdles and Achievements. Int. J. Mol. Sci. 2022, 23, 855. [Google Scholar] [CrossRef]
- Liu, A.; Long, Y.; Li, J.; Gu, L.; Karim, A.; Wang, X.; Gibson, A.L.F. Accelerated complete human skin architecture restoration after wounding by nanogenerator-driven electrostimulation. J. Nanobiotechnol. 2021, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wei, H.; Li, J.; Yao, G.; Yu, B.; Ni, D.; Gibson, A.L.; Lan, X.; Jiang, Y.; Cai, W.; et al. Effective Wound Healing Enabled by Discrete Alternative Electric Fields from Wearable Nanogenerators. ACS Nano 2018, 12, 12533–12540. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, A.; Sharpe, P.; Shi, S.; Ramalingam, M. (Eds.) Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Egusa, H.; Iida, K.; Kobayashi, M.; Lin, T.Y.; Zhu, M.; Zuk, P.A.; Wang, C.J.; Thakor, D.K.; Hedrick, M.H.; Nishimura, I. Downregulation of extracellular matrix-related gene clusters during osteogenic differentiation of human bone marrow- and adipose tissue-derived stromal cells. Tissue Eng. 2007, 13, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Fernandes, K.J.; McKenzie, I.A.; Mill, P.; Smith, K.M.; Akhavan, M.; Barnabé-Heider, F.; Biernaskie, J.; Junek, A.; Kobayashi, N.R.; Toma, J.G.; et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004, 6, 1082–1093. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Huang, G.T.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef]
- Abe, S.; Yamaguchi, S.; Amagasa, T. Multilineage Cells from Apical Pulp of Human Tooth with Immature Apex. Oral. Sci. Int. 2007, 4, 45–48. [Google Scholar] [CrossRef]
- Germain, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Jacobs, D.; Vandermeulen, G.; Bouzin, C.; Préat, V.; Dupont-Gillain, C.; et al. Fibrin hydrogels to deliver dental stem cells of the apical papilla for regenerative medicine. Regen. Med. 2015, 10, 153–167. [Google Scholar] [CrossRef]
- Vanacker, J.; Viswanath, A.; De Berdt, P.; Everard, A.; Cani, P.D.; Bouzin, C.; Feron, O.; Diogenes, A.; Leprince, J.G.; des Rieux, A. Hypoxia modulates the differentiation potential of stem cells of the apical papilla. J. Endod. 2014, 40, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.H.; Shi, H.; Tang, L.A.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- La, W.G.; Park, S.; Yoon, H.H.; Jeong, G.J.; Lee, T.J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.S. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Seo, T.H.; Lee, S.; Jang, W.; Kim, M.J.; Sung, J.S. Neuronal differentiation of human mesenchymal stem cells in response to the domain size of graphene substrates. J. Biomed. Mater. Res. A 2018, 106, 43–51. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Yousefi, K.; Hashemi, S.A.; Afsa, M.; Bahrani, S.; Gholami, A.; Ghahramani, Y.; Alizadeh, A.; Chiang, W.H. Renewable Carbon Nanomaterials: Novel Resources for Dental Tissue Engineering. Nanomaterials 2021, 11, 2800. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Gobbato, L.; Ludovichetti, F.S.; Tocco, I.; Carraro, A.; Piattelli, A.; Zavan, B. Graphene based scaffolds effects on stem cells commitment. J. Transl. Med. 2014, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, 263–267. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, W.C.; Manga, K.K.; Ang, P.K.; Lu, J.; Liu, Y.P.; Lim, C.T.; Loh, K.P. Fluorinated graphene for promoting neuro-induction of stem cells. Adv. Mater. 2012, 24, 4285–4290. [Google Scholar] [CrossRef]
- Tang, M.; Song, Q.; Li, N.; Jiang, Z.; Huang, R.; Cheng, G. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 2013, 34, 6402–6411. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, J.H.; Kang, S.H.; Hwang, E.Y.; Hwang, Y.S.; Lee, M.H.; Han, D.W.; Park, J.C. Enhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetic substrates. Biomed. Res. Int. 2014, 2014, 212149. [Google Scholar] [CrossRef] [PubMed]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, J.M.; Hollister, S.J. Tailoring the mechanical properties of 3D-designed poly (glycerol sebacate) scaffolds for cartilage applications. J. Biomed. Mater. Res. A 2010, 94, 9–18. [Google Scholar] [CrossRef]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001. [Google Scholar] [CrossRef]
- Lee, T.J.; Park, S.; Bhang, S.H.; Yoon, J.K.; Jo, I.; Jeong, G.J.; Hong, B.H.; Kim, B.S. Graphene enhances the cardiomyogenic differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2014, 452, 174–180. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Bhadra, D.; Moroni, L.; Pramanik, K. Myoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide-polymer composite fibrous meshes: Importance of graphene oxide conductivity and dielectric constant on their biocompatibility. Biofabrication 2015, 7, 015009. [Google Scholar] [CrossRef]
- Ayán-Varela, M.; Villar-Rodil, S.; Paredes, J.I.; Munuera, J.M.; Pagán, A.; Lozano-Pérez, A.A.; Cenis, J.L.; Martínez-Alonso, A.; Tascón, J.M. Investigating the Dispersion Behavior in Solvents, Biocompatibility, and Use as Support for Highly Efficient Metal Catalysts of Exfoliated Graphitic Carbon Nitride. ACS Appl. Mater. Interfaces 2015, 7, 24032–24045. [Google Scholar] [CrossRef]
- Gopinathan, J.; Quigley, A.F.; Bhattacharyya, A.; Padhye, R.; Kapsa, R.M.; Nayak, R.; Shanks, R.A.; Houshyar, S. Preparation, characterisation, and in vitro evaluation of electrically conducting poly(ɛ-caprolactone)-based nanocomposite scaffolds using PC12 cells. J. Biomed. Mater. Res. A 2016, 104, 853–865. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- Matković, A.; Milošević, I.; Milićević, M.; Tomašević-Ilić, T.; Pešić, J.; Musić, M.; Spasenović, M.; Jovanović, Đ.; Vasić, B.; Deeks, C.; et al. Enhanced sheet conductivity of Langmuir–Blodgett assembled graphene thin films by chemical doping. 2D Mater. 2016, 3, 015002. [Google Scholar] [CrossRef]
- Simonovic, J.; Toljic, B.; Nikolic, N.; Peric, M.; Vujin, J.; Panajotovic, R.; Gajic, R.; Bekyarova, E.; Cataldi, A.; Parpura, V.; et al. Differentiation of stem cells from apical papilla into neural lineage using graphene dispersion and single walled carbon nanotubes. J. Biomed. Mater. Res. A 2018, 106, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Milošević, I.R.; Vasić, B.; Matković, A.; Vujin, J.; Aškrabić, S.; Kratzer, M.; Griesser, T.; Teichert, C.; Gajić, R. Single-step fabrication and work function engineering of Langmuir-Blodgett assembled few-layer graphene films with Li and Au salts. Sci. Rep. 2020, 10, 8476. [Google Scholar] [CrossRef] [PubMed]
- Shehadat, S.A.; Gorduysus, M.O.; Hamid, S.S.A.; Abdullah, N.A.; Samsudin, A.R.; Ahmad, A. Optimization of scanning electron microscope technique for amniotic membrane investigation: A preliminary study. Eur. J. Dent. 2018, 12, 574–578. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Han, F.; Ma, X.; Zhai, Y.; Cui, L.; Yang, L.; Zhu, Z.; Hao, Y.; Cheng, G. Strategy for Designing a Cell Scaffold to Enable Wireless Electrical Stimulation for Enhanced Neuronal Differentiation of Stem Cells. Adv. Healthc. Mater. 2021, 10, 2100027. [Google Scholar] [CrossRef]
- Madanagopal, T.T.; Tai, Y.K.; Lim, S.H.; Fong, C.H.; Cao, T.; Rosa, V.; Franco-Obregón, A. Pulsed electromagnetic fields synergize with graphene to enhance dental pulp stem cell-derived neurogenesis by selectively targeting TRPC1 channels. Eur. Cells Mater. 2021, 41, 216–232. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Yin, J. Free-Standing Buckle-Delaminated 2D Organic Nanosheets with Enhanced Mechanical Properties and Multifunctionality. Adv. Mater. Interfaces 2019, 6, 1900561. [Google Scholar] [CrossRef]
- Hung, H.S.; Kung, M.L.; Chen, F.C.; Ke, Y.C.; Shen, C.C.; Yang, Y.C.; Tang, C.M.; Yeh, C.A.; Hsieh, H.H.; Hsu, S.H. Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells. Nanomaterials 2021, 11, 2046. [Google Scholar] [CrossRef]
- Lee, J.S.; Lipatov, A.; Ha, L.; Shekhirev, M.; Andalib, M.N.; Sinitskii, A.; Lim, J.Y. Graphene substrate for inducing neurite outgrowth. Biochem. Biophys. Res. Commun. 2015, 460, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Losada, N.; Wendelbob, R.; Ocaña, M.C.; Casares, A.D.; Guzman de Villoría, R.; Aguirre Gomez, J.A.; Arraez, M.A.; Gonzalez-Alegre, P.; Medina, M.A.; Arenas, E.; et al. Graphene Oxide and Reduced Derivatives, as Powder or Film Scaffolds, Differentially Promote Dopaminergic Neuron Differentiation and Survival. Front. Neurosci. 2020, 14, 570409. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Lin, Z.; Lu, Y.; Chen, H.; Li, B.; Li, Z.; Xia, H.; Li, L.; Zhang, T. Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 2022, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte, M.V.; Lacconi, G.I.; Urreta, S.E.; Torres, L.E.F.F. On the Nature of Defects in Liquid-phase Exfoliated Graphene. J. Phys. Chem. C 2014, 118, 15455–15459. [Google Scholar] [CrossRef]
- Amaro-Gahete, J.; Benítez, A.; Otero, R.; Esquivel, D.; Jiménez-Sanchidrián, C.; Morales, J.; Caballero, Á.; Romero-Salguero, F.J. A Comparative Study of Particle Size Distribution of Graphene Nanosheets Synthesized by an Ultrasound-Assisted Method. Nanomaterials 2019, 9, 152. [Google Scholar] [CrossRef]
- Qu, G.; Li, Y.; Chen, L.; Chen, Q.; Zou, D.; Yang, C.; Zhou, Q. Comparison of Osteogenic Differentiation Potential of Human Dental-Derived Stem Cells Isolated from Dental Pulp, Periodontal Ligament, Dental Follicle, and Alveolar Bone. Stem. Cells Int. 2021, 2021, 6631905. [Google Scholar] [CrossRef]
- Son, Y.B.; Kang, Y.H.; Lee, H.J.; Jang, S.J.; Bharti, D.; Lee, S.L.; Jeon, B.G.; Park, B.W.; Rho, G.J. Evaluation of odonto/osteogenic differentiation potential from different regions derived dental tissue stem cells and effect of 17β-estradiol on efficiency. BMC Oral. Health. 2021, 21, 15. [Google Scholar] [CrossRef]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S.; et al. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, Y.; Kim, D.H.; Choung, P.H.; Cho, C.S.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. A 2013, 101, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, R.; Zara, S.; Ventrella, A.; Siani, G.; Da Ros, T.; Iezzi, G.; Cataldi, A.; Fontana, A. Covalent Decoration of Cortical Membranes with Graphene Oxide as a Substrate for Dental Pulp Stem Cells. Nanomaterials 2019, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Jiang, S.; Yi, B.; Gong, T.; Lim, L.W.; Zhang, C. Small molecules enhance neurogenic differentiation of dental-derived adult stem cells. Arch. Oral. Biol. 2019, 102, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Xiang, A.P.; Mao, F.F.; Zhang, L.; Di, C.G.; Liu, X.M.; Shao, Y.; Ma, B.F.; Lee, J.H.; Ha, K.S.; et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 2010, 28, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Hochuli, A.H.D.; Senegaglia, A.C.; Selenko, A.H.; Fracaro, L.; Brofman, P.R.S. Dental Pulp from Human Exfoliated Deciduous Teeth-derived Stromal Cells Demonstrated Neuronal Potential: In Vivo and In Vitro Studies. Curr. Stem. Cell Res. Ther. 2021, 16, 495–506. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Reddy, S.; He, L.; Ramakrishana, S.; Luo, H. Graphene nanomaterials for regulating stem cell fate in neurogenesis and their biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 69–78. [Google Scholar] [CrossRef]

| Primer Name | Sequences (5′→3′) | |
|---|---|---|
| Runx2 | Forward Reverse | ACAAACAACCACAGAACCACAAGT GTCTCGGTGGCTGGTAGTGA |
| Col2 | Forward Reverse | TTCAGCTATGGAGATGACAATC AGAGTCCTAGAGTGACTGAG |
| PPARG | Forward Reverse | GCTGTGCAGGAGATCACAGA GGCTCCATAAAGTCACCAA |
| Ngn2 | Forward Reverse | CCTGGAAACCATCTCACTTCA TACCCAAAGCCAAGAAATGC |
| NF-M | Forward Reverse | TGGGAAATGGCTCGTCATTT CTTCATGGAAACGGCCAA |
| Nestin | Forward Reverse | AACAGCGACGGAGGTCTCTA TTCTCTTGTCCCGCAGACTT |
| MAP2 | Forward Reverse | AACCCTTTGAGAACACGACA TCTTTCCGTTCATCTGCCA |
| MASH1 | Forward Reverse | CCAGTTGTACTTCAGCACC TGCCACTTTGAGTTTGGAC |
| GAPDH | Forward Reverse | TCATGACCACAGTCCATGCCATCA CCCTGTTGCTGTAGCCAAATTCGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonovic, J.; Toljic, B.; Lazarevic, M.; Markovic, M.M.; Peric, M.; Vujin, J.; Panajotovic, R.; Milasin, J. The Effect of Liquid-Phase Exfoliated Graphene Film on Neurodifferentiation of Stem Cells from Apical Papilla. Nanomaterials 2022, 12, 3116. https://doi.org/10.3390/nano12183116
Simonovic J, Toljic B, Lazarevic M, Markovic MM, Peric M, Vujin J, Panajotovic R, Milasin J. The Effect of Liquid-Phase Exfoliated Graphene Film on Neurodifferentiation of Stem Cells from Apical Papilla. Nanomaterials. 2022; 12(18):3116. https://doi.org/10.3390/nano12183116
Chicago/Turabian StyleSimonovic, Jelena, Bosko Toljic, Milos Lazarevic, Maja Milosevic Markovic, Mina Peric, Jasna Vujin, Radmila Panajotovic, and Jelena Milasin. 2022. "The Effect of Liquid-Phase Exfoliated Graphene Film on Neurodifferentiation of Stem Cells from Apical Papilla" Nanomaterials 12, no. 18: 3116. https://doi.org/10.3390/nano12183116







