Molecular Dynamics and Experimental Investigation on the Interfacial Binding Mechanism in the Fe/Cu1−x-Nix Bimetallic Interface
Abstract
:1. Introduction
2. Computational and Experimental Methodology
2.1. Computational Method
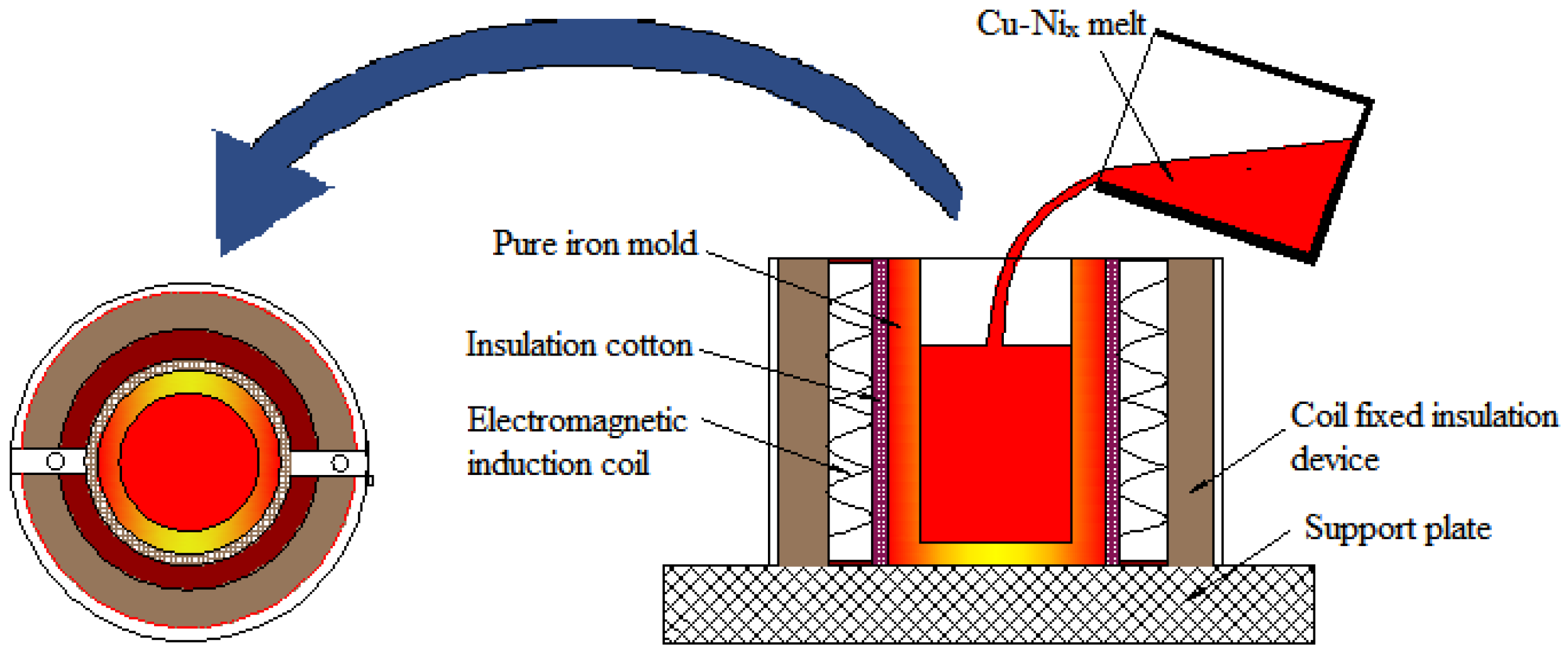
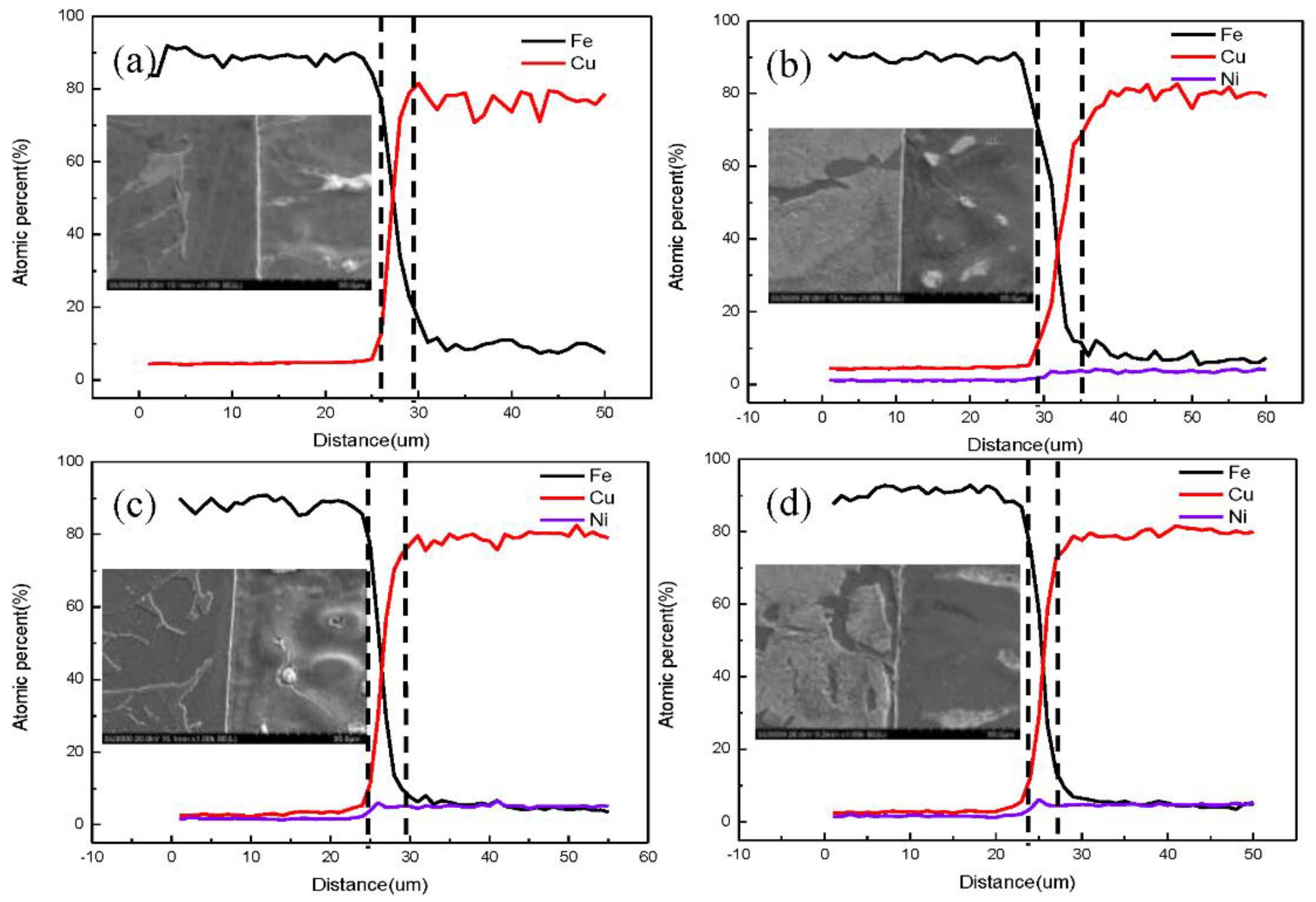
2.2. Experimental Method
2.3. MD Foundation
3. Results and Discussion
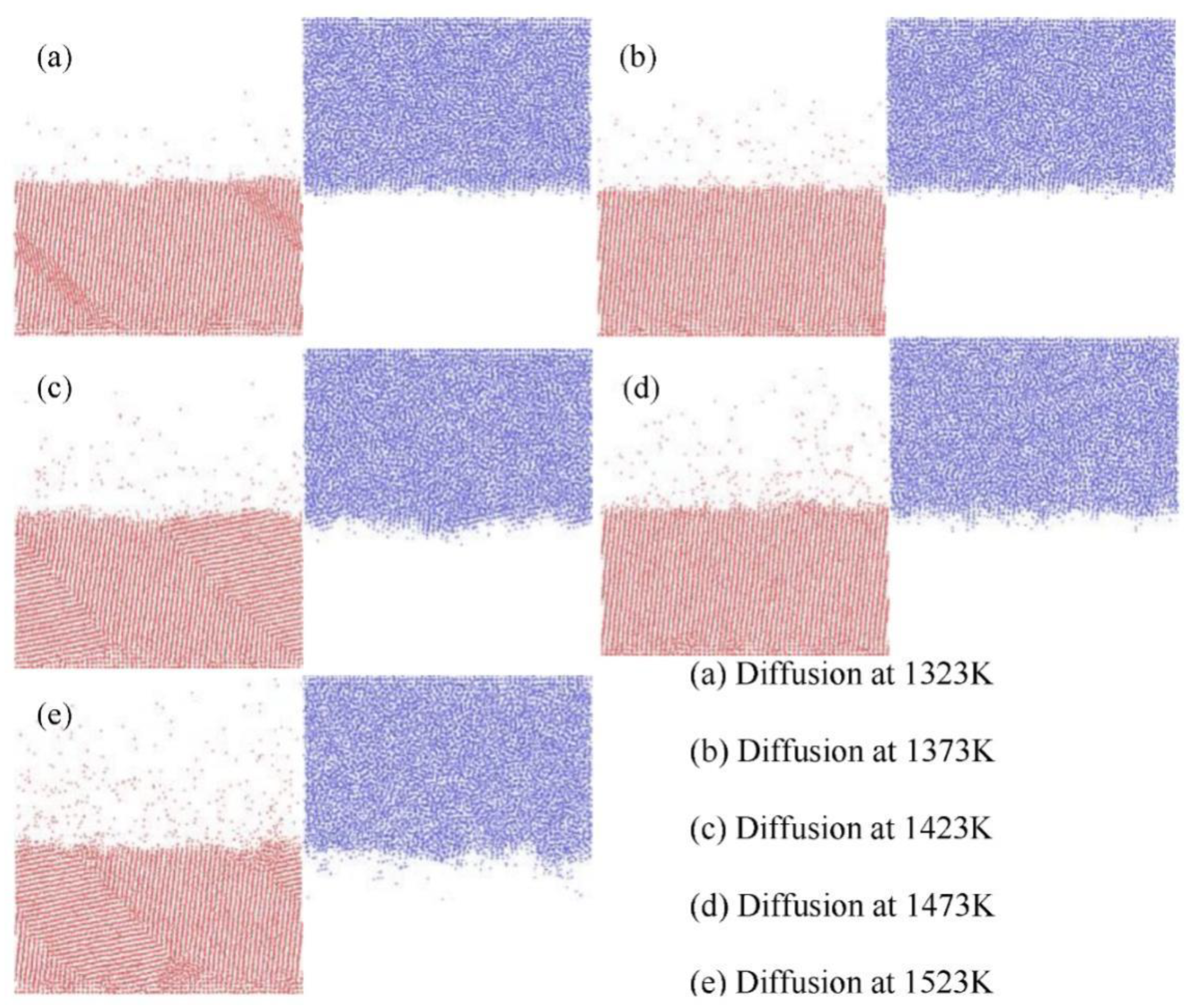
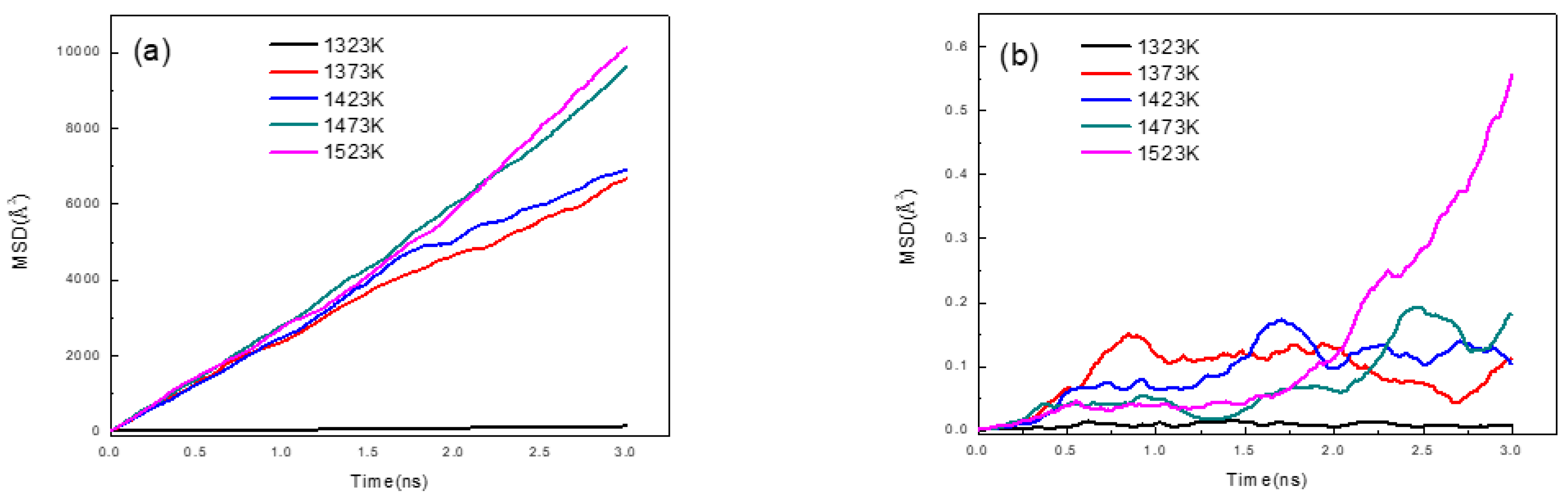
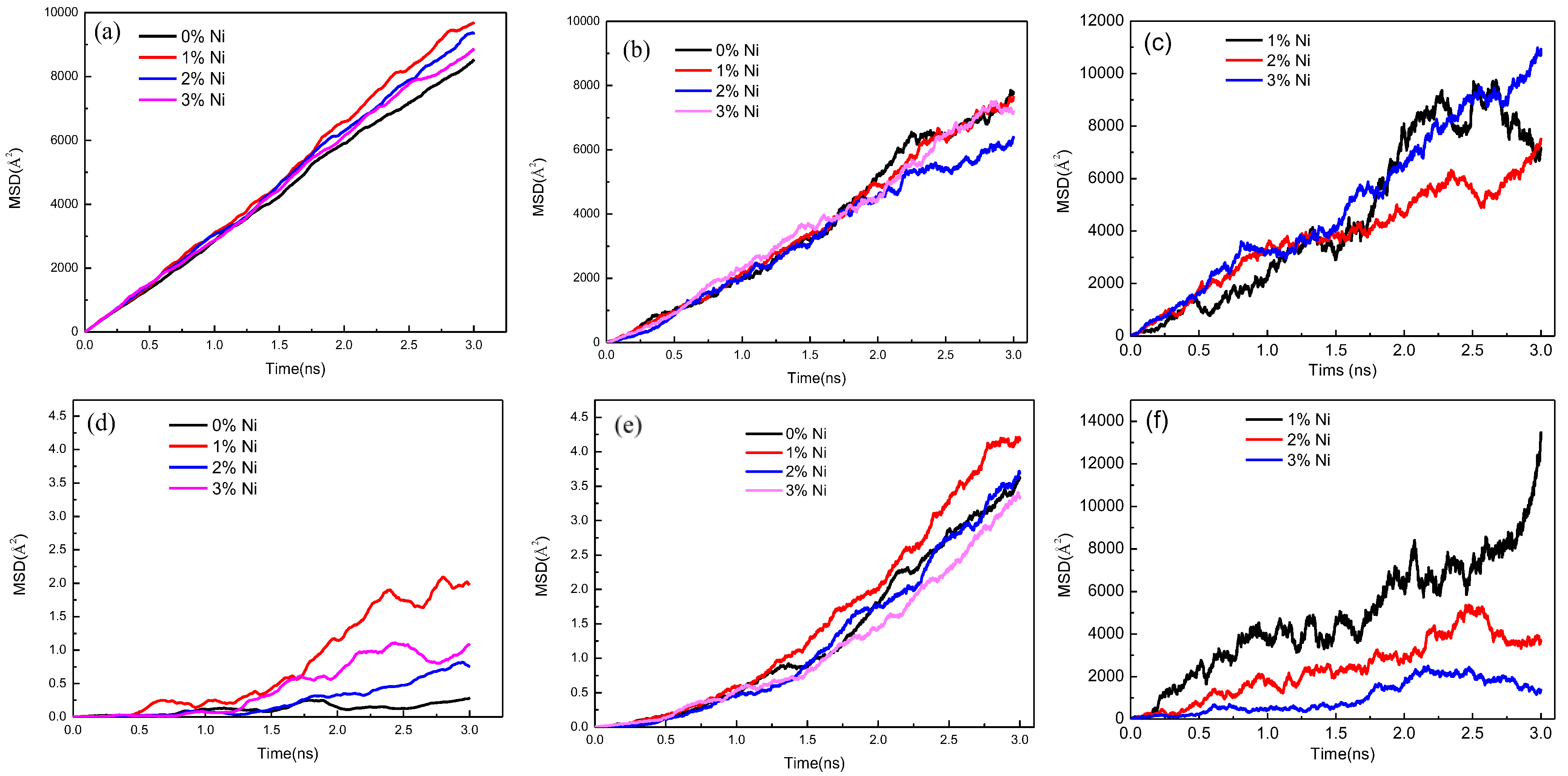
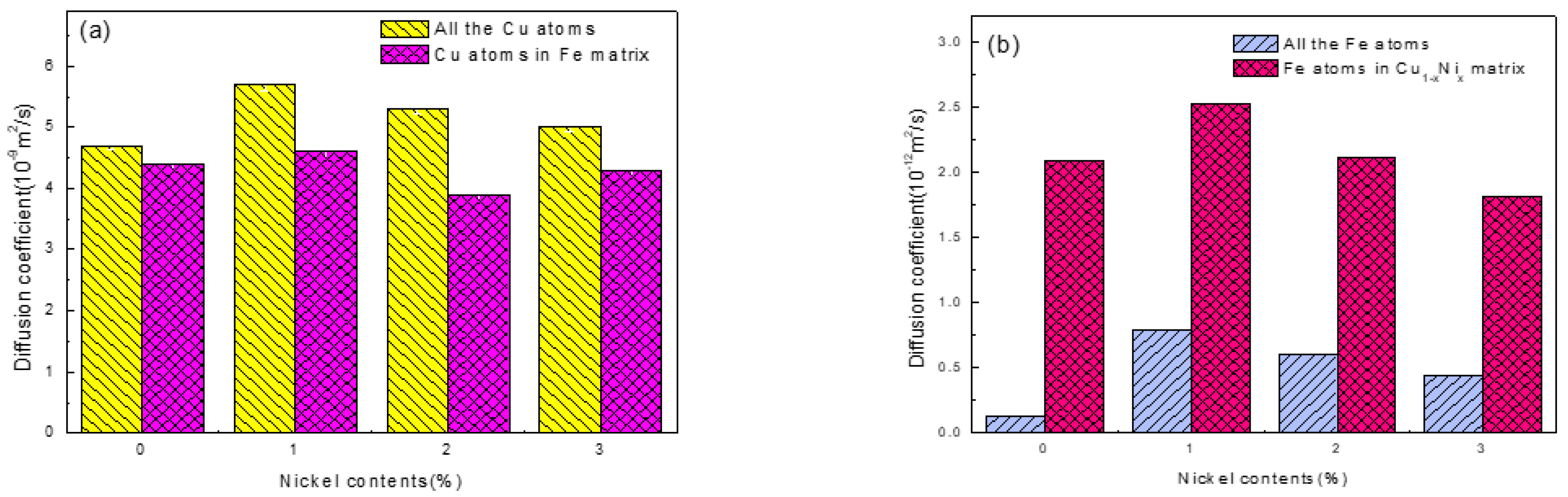
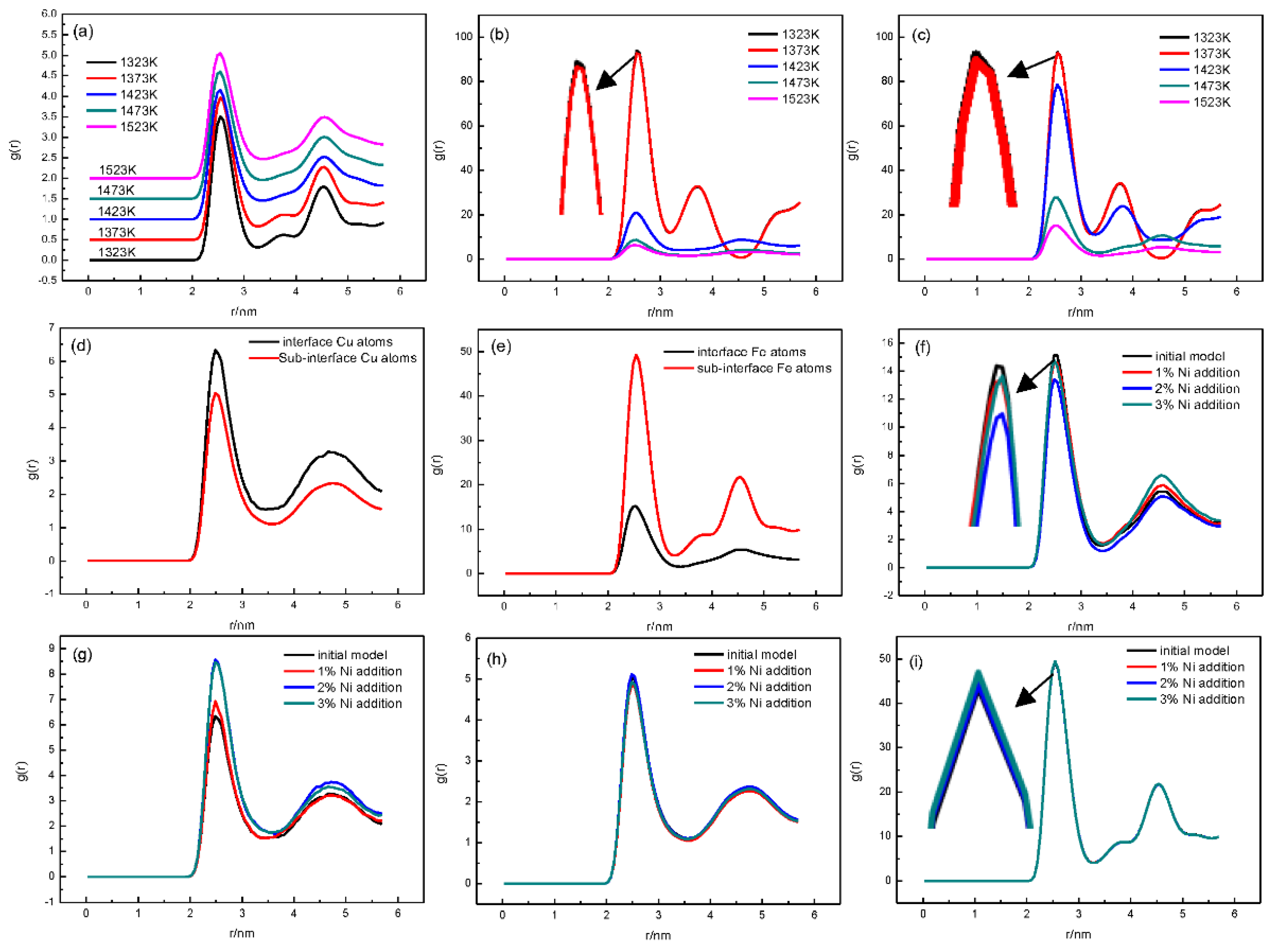
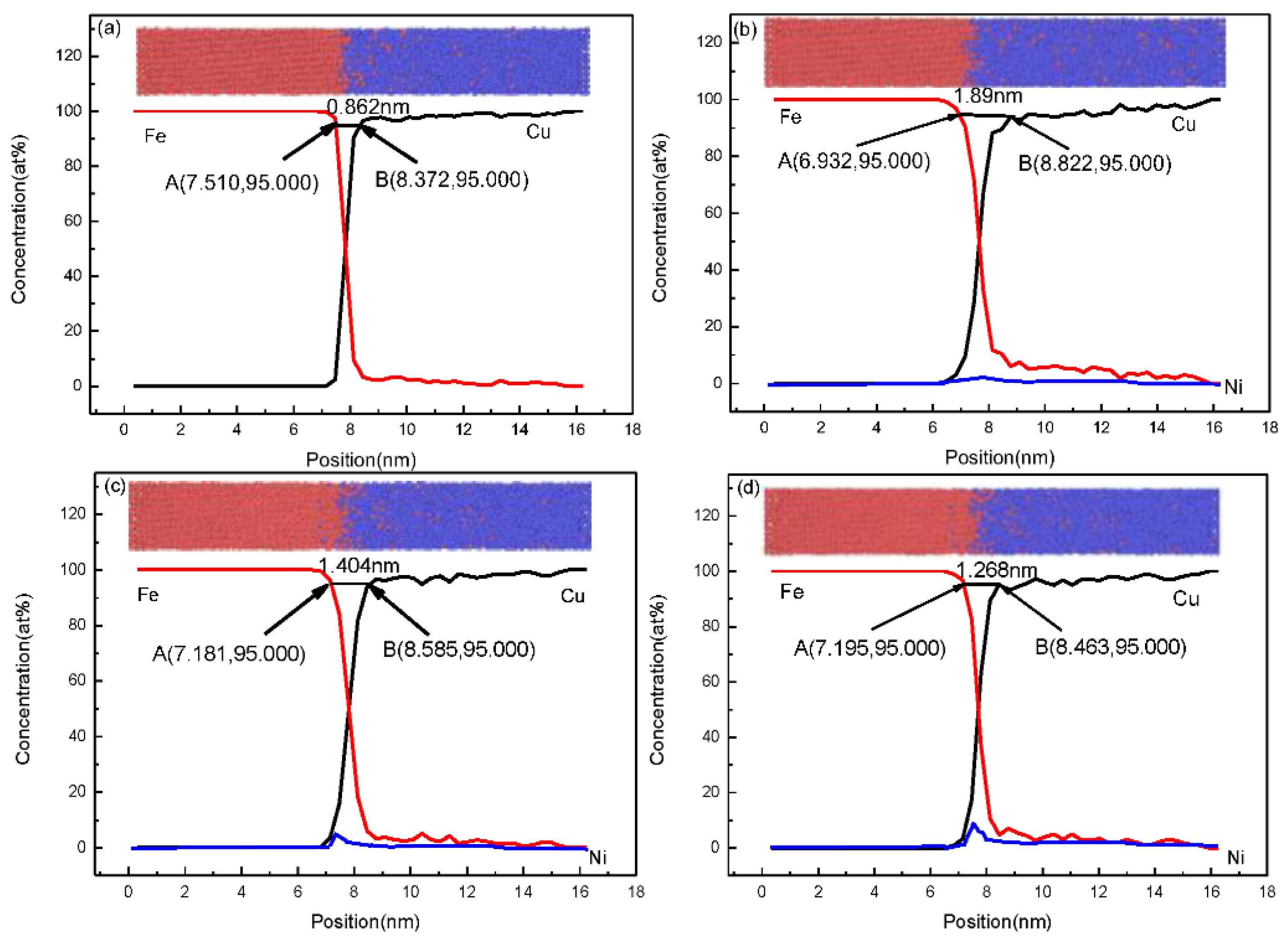
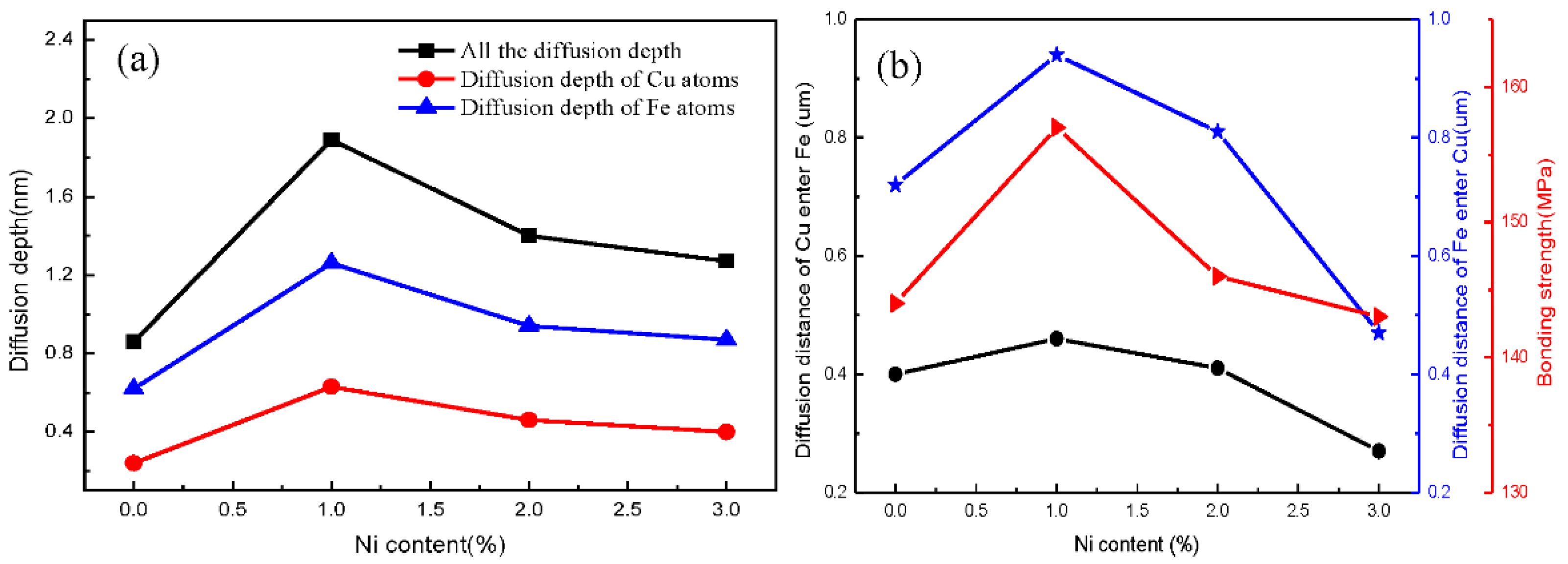
3.1. Fe/Cu1−x-Nix Interface Simulation and Experimental Results
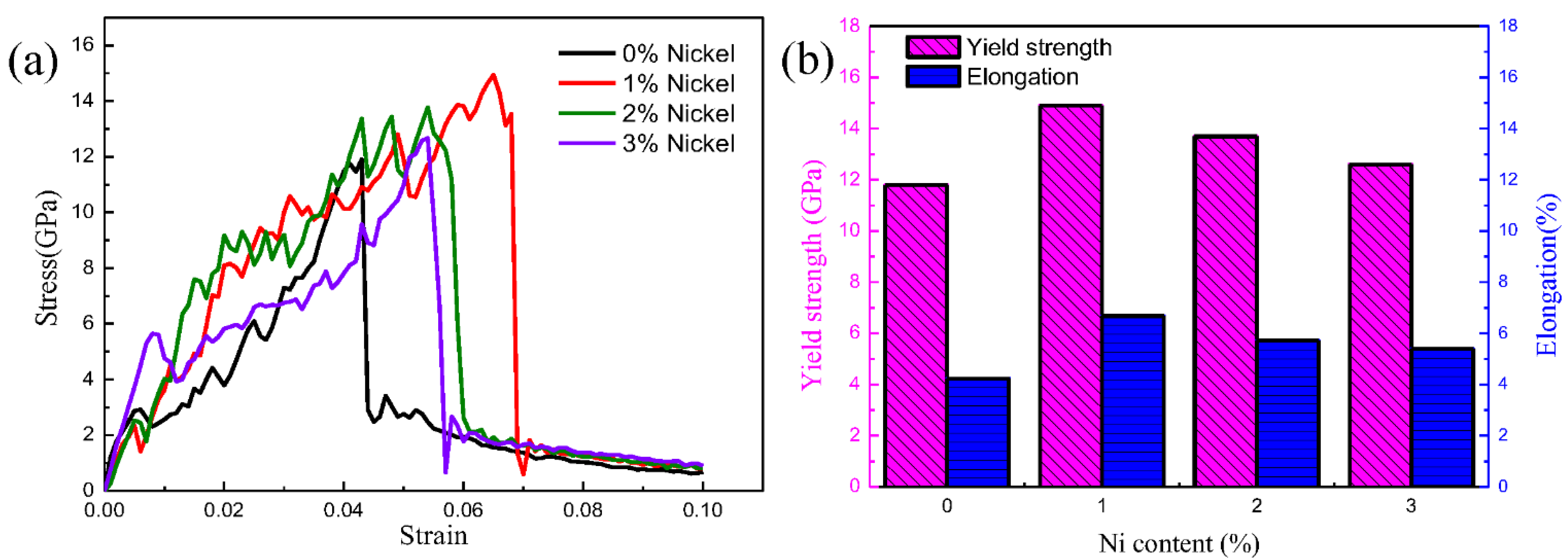
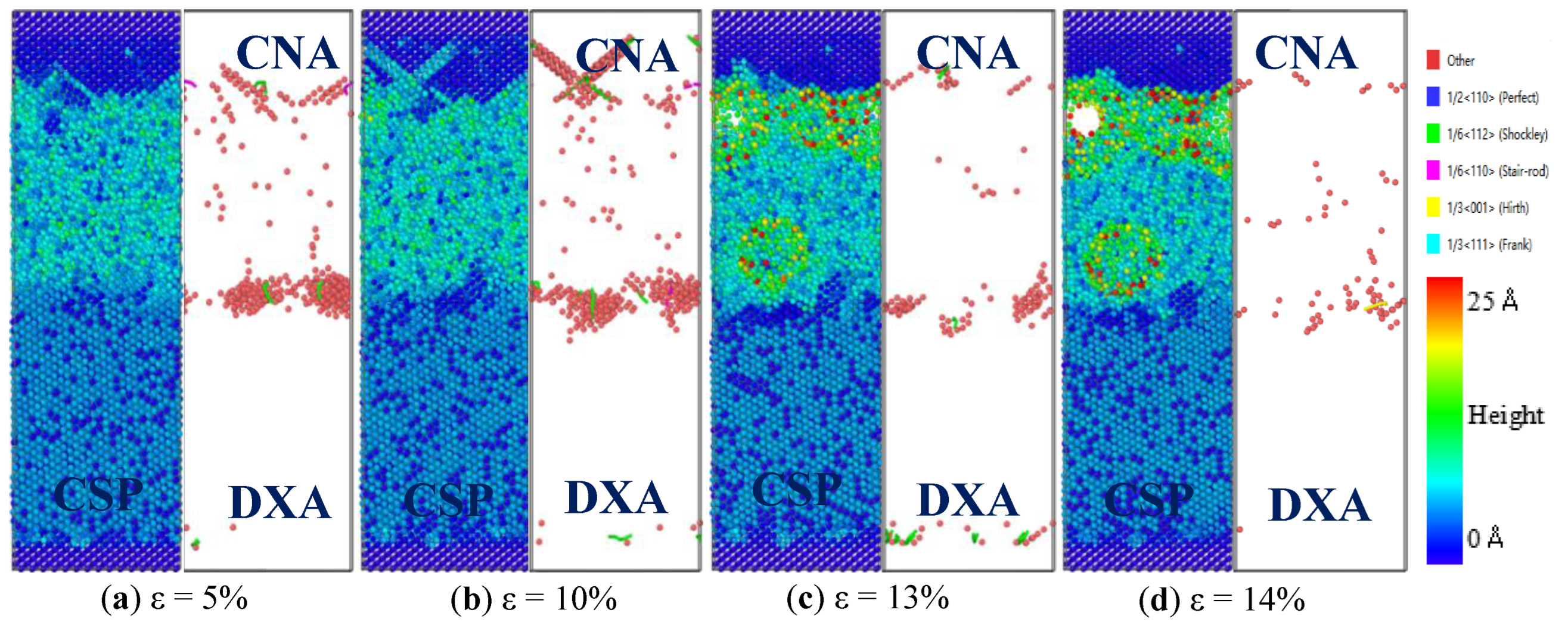
3.2. Mechanical Properties of Fe/Cu1−x-Nix Bimetallic Interface
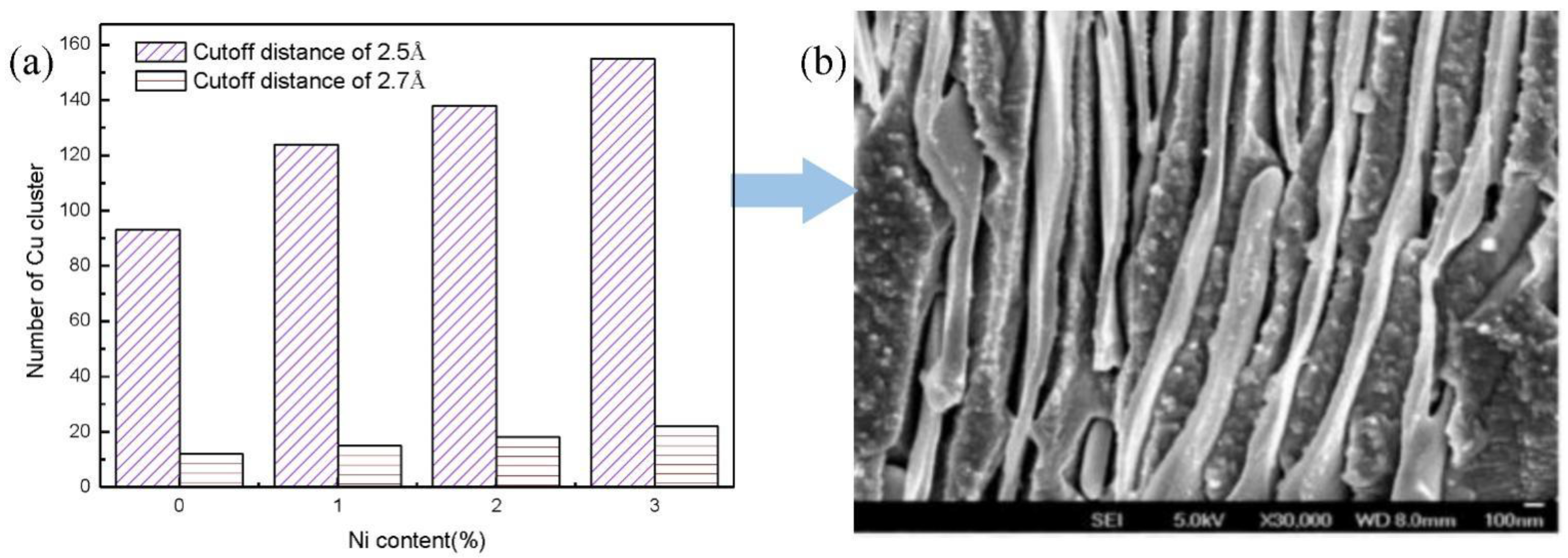
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, H.B.; Zhao, N.B. Interfacial layer simulation and effect on Cu-Ar manofluids thermal conductivity using molecular dynamics method. J. Mol. Liq. 2018, 259, 40–47. [Google Scholar] [CrossRef]
- Izumi, T.; Kametani, N.; Miyake, S.; Kanetsuki, S.; Namazu, T. New local joining technique for metal materials using exothermic heat of Al/Ni multilayer powder. Jpn. J. Appl. Phys. 2018, 57, 06HJ10. [Google Scholar] [CrossRef]
- Sakamaki, M.; Amemiya, K. Element Specific Magnetic Anisotropy Energy of Alternately Layered FeNi Thin Films. Appl. Phys. Express 2017, 4, 012043. [Google Scholar] [CrossRef]
- Tao, X.M.; Yao, P.; Wei, W.W.; Chen, H.M.; Ouyang, Y.F.; Du, Y.; Yuan, Y.; Peng, Q. An experimental study on the interdiffusion behaviors and mechanical properties of Ni-Zr system. J. Alloys Compd. 2018, 752, 412–419. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Pang, T.K.; He, J.X.; Tao, X.M.; Chen, H.M.; Ouyang, Y.F.; Du, Y. Interdiffusion behaviors and mechanical properties of Cu-Zr system. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2018, 61, 92–97. [Google Scholar] [CrossRef]
- Mao, A.; Zhang, J.; Yao, S.; Wang, A.; Wang, W.; Li, Y.; Qiao, C.; Xie, J.; Jia, Y. The diffusion behaviors at the Cu-Al solid-liquid interface: A molecular dynamics study. Results Phys. 2020, 16, 102998. [Google Scholar] [CrossRef]
- Zhang, R.F.; Germann, T.C.; Wang, J.; Liu, X.Y.; Beyerlein, I.J. Role of interface structure on the plastic response of Cu/Nb nanolaminates under shock compression: Non-equilibrium molecular dynamics simulations. Scr. Mater. 2013, 68, 114–117. [Google Scholar] [CrossRef]
- Xu, S.; Fan, X.; Gu, C.; Shi, Y.; Singh, D.J.; Zheng, W. First principles and molecular dynamics study of Li wetting and diffusion on W surfaces. J. Nucl. Mater. 2020, 539, 152345. [Google Scholar] [CrossRef]
- Aguirre, R.; Chavez, J.J.; Li, J.; Zhou, X.W.; Almeida, S.F.; Wolden, C.; Zubia, D. Crystal Growth and Atom Diffusion in (Cu)ZnTe/CdTe via Molecular Dynamics. IEEE J. Photovolt. 2018, 8, 594–599. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Tao, X.; Chen, H.; Ouyang, Y. Molecular dynamics simulation of diffusion bonding of Al-Cu interface. Model. Simul. Mater. Sci. Eng. 2014, 22, 396–397. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Wang, W.-X.; Zhou, J.; Cao, X.-Q.; Xie, R.-S.; Wei, Y. Molecular Dynamics Simulations and Experimental Investigations of Atomic Diffusion Behavior at Bonding Interface in an Explosively Welded Al/Mg Alloy Composite Plate. Acta Metall. Sin. Engl. Lett. 2017, 30, 983–991. [Google Scholar] [CrossRef]
- Wei, J.; Xu, C.; Li, P.; Deng, H.; Xiao, S.; Hu, W. Molecular dynamics simulations of the diffusion characteristics on the Fe-W interfaces system. Fusion Eng. Des. 2020, 159, 11850. [Google Scholar] [CrossRef]
- Luo, M.; Liang, L.; Lang, L.; Xiao, S.; Hu, W.; Deng, H. Molecular dynamics simulations of the characteristics of Mo/Ti interfaces. Comput. Mater. Sci. 2018, 141, 293–301. [Google Scholar] [CrossRef]
- di Luozzoa, N.; Boudardb, M.; Fontanaa, M.; Arcondoa, B. Effective diffusion coefficient for Cu in steel joined by transient liquid phase bonding. Mater. Des. 2016, 92, 760–766. [Google Scholar] [CrossRef]
- Lee, S.-G.; Chung, Y.-C. The early stage of deposition process for Fe-Cu magnetic multilayer systems: Molecular dynamics simulation. J. Physics.D Appl. Phys. 2009, 42, 305–309. [Google Scholar] [CrossRef]
- Lee, S.-G.; Chung, Y.-C. Atomic Investigation of Fe-Cu Magnetic Thin Films by Molecular Dynamics Simulation. Jpn. J. Appl. Phys. 2007, 46, 6309–6311. [Google Scholar] [CrossRef]
- Zhang, G.; Kang, Y.; Wang, M.; Xu, H.; Jia, H. Atomic diffusion behavior and diffusion mechanism in Fe–Cu bimetal casting process studied by molecular dynamics simulation and experiment. Mater. Res. Express 2020, 7, 096519. [Google Scholar] [CrossRef]
- Zhang, S.X.; Li, G.P.; Gong, H.F.; Gao, N.; Chen, X.Z.; Wang, Z.G. Molecular dynamics simulation of energetic Cu55 clusters deposition on a Fe (0 0 1) surface. Comput. Mater. Sci. 2014, 97, 165–171. [Google Scholar] [CrossRef]
- Molnar, D.; Mukherjee, R.; Choudhury, A.; Mora, A.; Binkele, P.; Selzer, M.; Nestler, B.; Schmauder, S. Multiscale simulations on the coarsening of Cu-rich precipitates in alpha-Fe using kinetic Monte Carlo, molecular dynamics and phase-field simulations. Acta Mater. 2012, 60, 6961–6971. [Google Scholar] [CrossRef]
- Fujii, K.; Fukuya, K.; Kasada, R.; Kimura, A.; Ohkubo, T. Effects of tensile stress on Cu clustering in irradiated Fe–Cu alloy. J. Nucl. Mater. 2015, 458, 281–287. [Google Scholar] [CrossRef]
- Lv, G.C.; Su, Y.J. Molecular dynamics simulation and first principles calculations of radiation-induced Cu clusters in Fe-3 at.% Cu alloy. Comput. Phys. Commun. 2017, 211, 41–44. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Xu, H. Atomistic Simulations and Experimental Investigations of the Diffusion Behavior of Steel/ZCuPb20Sn5 Bimetals. Coatings 2020, 10, 549. [Google Scholar] [CrossRef]
- Tao, Q.B.; Benabou, L.; Vivet, L.; Le, V.N.; Ouezdou, F.B. Effect of Ni and Sb additions and testing conditions on the mechanical properties and microstructures of lead-free solder joints. Mater. Sci. Eng. A 2016, 669, 403–416. [Google Scholar] [CrossRef]
- Hu, X.W.; Qiu, H.Y.; Jiang, X.X. Effect of Ni addition into the Cu substrate on the interfacial IMC growth during the liquid-state reaction with Sn-58Bi solder. J. Mater. Sci. 2019, 30, 1907–1918. [Google Scholar] [CrossRef]
- Yang, L.; Ge, J.G.; Zhang, Y.C.; Dai, J. Interfacial IMC Layer and Tensile Properties of Ni-Reinforced Cu/Sn–0.7Cu–0.05Ni/Cu Solder Joint: Effect of Aging Temperature. Trans. Indian Inst. Met. 2017, 70, 2429–2439. [Google Scholar] [CrossRef]
- Ma, G.C.; Fan, J.L.; Gong, H.R. Mechanical behavior of Cu-W interface systems upon tensile loading from molecular dynamics simulations. Comput. Mater. Sci. 2018, 152, 165–168. [Google Scholar] [CrossRef]
- Liu, H.; Ke, F.J.; Pan, H.; Zhou, M. Molecular dynamics simulation of the diffusion bonding and tensile behavior of a Cu-Al interface. Acta Phys. Sin. 2007, 56, 407–412. [Google Scholar] [CrossRef]
- Hua, J.; Duan, Z.; Song, C.; Liu, Q. Molecular dynamics study on the tensile properties of graphene/Cu nanocomposite. Int. J. Comput. Mater. Sci. Eng. 2017, 6, 1750021. [Google Scholar] [CrossRef]
- Wei, W.; Yu, X. Molecular dynamics study of the effect of lithium on the tensile behaviors of bcc iron. Mater. Today Commun. 2020, 24, 101217. [Google Scholar] [CrossRef]
- Yan, H.; Liu, L.; Zhuang, J.; Zhou, P. Molecular Dynamics Simulation of Carbon Effect on the Thermal Physical Properties of the Molten Iron. ISIJ Int. 2019, 59, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.Y.; Liu, Y.; Wang, R.S.; Wang, J.T. Effect of Ni on Cu precipitation kinetics in α-Fe by AKMC study. arXiv 2012, arXiv:1212.6900. [Google Scholar]
- Bonny, G.; Pasianot, R.C.; Castin, N.; Malerba, L. Ternary Fe–Cu–Ni many-body potential to model reactor pressure vessel steels: First validation by simulated thermal annealing. Philos. Mag. 2009, 89, 3531–3546. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, G.W.; Xu, H.; Dong, Y.; Sun, F.E.; Fu, Y.Z. Density functional theory investigation on the stability, adhesion strength, tensile properties and fracture behavior of γ-Fe/Cu heterogeneous nucleation interface. Results Phys. 2021, 24, 104143. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Zhang, G.W.; Xu, H. Effect of phosphorus on interface characterization of steel–copper bimetals in solid–liquid composite casting. Mater. Today Commun. 2022, 30, 103037. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 1, 015012. [Google Scholar] [CrossRef]
- Zhang, K.; Zhan, Y. Adhesion strength and stability of Cu(111)/TiC(111) interface in composite coatings by first principles study. Vacuum 2019, 163, 215–222. [Google Scholar] [CrossRef]
- Brandes, E.A.; Brook, G.B.; Paufler, P. Smithells Metals Reference Book; Butter-Worths: London, UK, 1983. [Google Scholar]
- Yu, J.; Lin, X.; Wang, J.J.; Chen, J.; Huang, W.D. First-principles study of the relaxation and energy of bcc-Fe, fcc-Fe and AISI-304 stainless steel surfaces. Appl. Surf. Sci. 2009, 255, 9032–9039. [Google Scholar] [CrossRef]
- Acet, M.; Zähres, H.; Wassermann, E.F.; Pepperhoff, W. High-temperature moment-volume instability and anti-Invar of γ-Fe. Phys. Rev. B 1994, 49, 6012. [Google Scholar] [CrossRef]
- Song, J.; Gao, Z.B.; Zhang, L.; Wu, W.H.; He, B.B.; Lu, L. Prediction on elastic properties of Nb-doped Ni systems. Mol. Simul. 2019, 45, 935–941. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data; ASM International: Materials Park, OH, USA, 2011. [Google Scholar]
- Meis, C.; Fleche, J.L. Study of the solubility limit of oxygen vacancies in TiO2−x using molecular dynamics. Solid State Ion. 1997, 1, 333–335. [Google Scholar] [CrossRef]
- Chen, S.; Ke, F.; Zhou, M. Atomistic investigation of the effects of temperature and surface roughness on diffusion bonding between Cu and Al. Acta Mater. 2007, 55, 3169–3175. [Google Scholar] [CrossRef]
- Zeng, Y.Z.; Li, Q.X.; Bai, K.W. Prediction of interstitial diffusion activation energies of nitrogen, oxygen, boron and carbon in bcc, fcc, and hcp metals using machine Learning. Comput. Mater. Sci. 2018, 144, 232–247. [Google Scholar] [CrossRef]
- Du, S.M.; Wang, X.C.; Li, Z.; Yang, Z.H.; Wang, J.B. Effect of Ni Content on Microstructure and Characterization of Cu-Ni-Sn Alloys. Materials 2018, 11, 1108. [Google Scholar] [CrossRef] [PubMed]

| Method | A(Å) | C11 | C12 | C44 | B | G | ||
|---|---|---|---|---|---|---|---|---|
| Cu | Present work | EAM | 3.614 | 185.8 | 128.6 | 86.5 | 148.5 | 63.3 |
| Other | DFT [37] | 3.631 | 162.2 | 125.1 | 84.1 | 137.4 | 57.8 | |
| Exp [38] | 3.615 | 137.0 | ||||||
| γ-Fe | Present work | EAM | 3.462 | 342.6 | 117.9 | 226.1 | 192.8 | 180.6 |
| Other | DFT [39] | 3.474 | 338.9 | 120.5 | 215.8 | 193.3 | 173.6 | |
| Exp [40] | 3.645 | 193.5 | ||||||
| Ni | Present work | EAM | 3.460 | 269.7 | 155.8 | 129.5 | 193.8 | 100.5 |
| Other | DFT [41] | 3.521 | 276.4 | 159.6 | 132.0 | 198.5 | 102.6 | |
| Exp [42] | 3.524 | 194.0 |
| Interface Models | Method | Interface Energy | Surface Energy (J/m2) | ||
|---|---|---|---|---|---|
| Cu Slab | Fe Slab | ||||
| Fe(100)/Cu(100) | Present work | EAM | 1.71 | 1.46 | 3.39 |
| Other [34] | DFT | 1.64 | 1.45 | 3.36 | |
| Fe(110)/Cu(110) | Present work | EAM | 1.95 | 1.59 | 3.55 |
| Other [34] | DFT | 1.88 | 1.58 | 3.49 | |
| Fe(111)/Cu(111) | Present work | EAM | 1.26 | 1.49 | 2.91 |
| Other [34] | DFT | 1.23 | 1.36 | 2.92 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, M.; Yu, H.; Xu, H.; Wan, A. Molecular Dynamics and Experimental Investigation on the Interfacial Binding Mechanism in the Fe/Cu1−x-Nix Bimetallic Interface. Nanomaterials 2022, 12, 3245. https://doi.org/10.3390/nano12183245
Zhang G, Wang M, Yu H, Xu H, Wan A. Molecular Dynamics and Experimental Investigation on the Interfacial Binding Mechanism in the Fe/Cu1−x-Nix Bimetallic Interface. Nanomaterials. 2022; 12(18):3245. https://doi.org/10.3390/nano12183245
Chicago/Turabian StyleZhang, Guowei, Mingjie Wang, Huan Yu, Hong Xu, and An Wan. 2022. "Molecular Dynamics and Experimental Investigation on the Interfacial Binding Mechanism in the Fe/Cu1−x-Nix Bimetallic Interface" Nanomaterials 12, no. 18: 3245. https://doi.org/10.3390/nano12183245
APA StyleZhang, G., Wang, M., Yu, H., Xu, H., & Wan, A. (2022). Molecular Dynamics and Experimental Investigation on the Interfacial Binding Mechanism in the Fe/Cu1−x-Nix Bimetallic Interface. Nanomaterials, 12(18), 3245. https://doi.org/10.3390/nano12183245





