Ultra-Fast Construction of Novel S-Scheme CuBi2O4/CuO Heterojunction for Selectively Photocatalytic CO2 Conversion to CO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterization
2.3. Photocatalytic Performance for CO2 Reduction
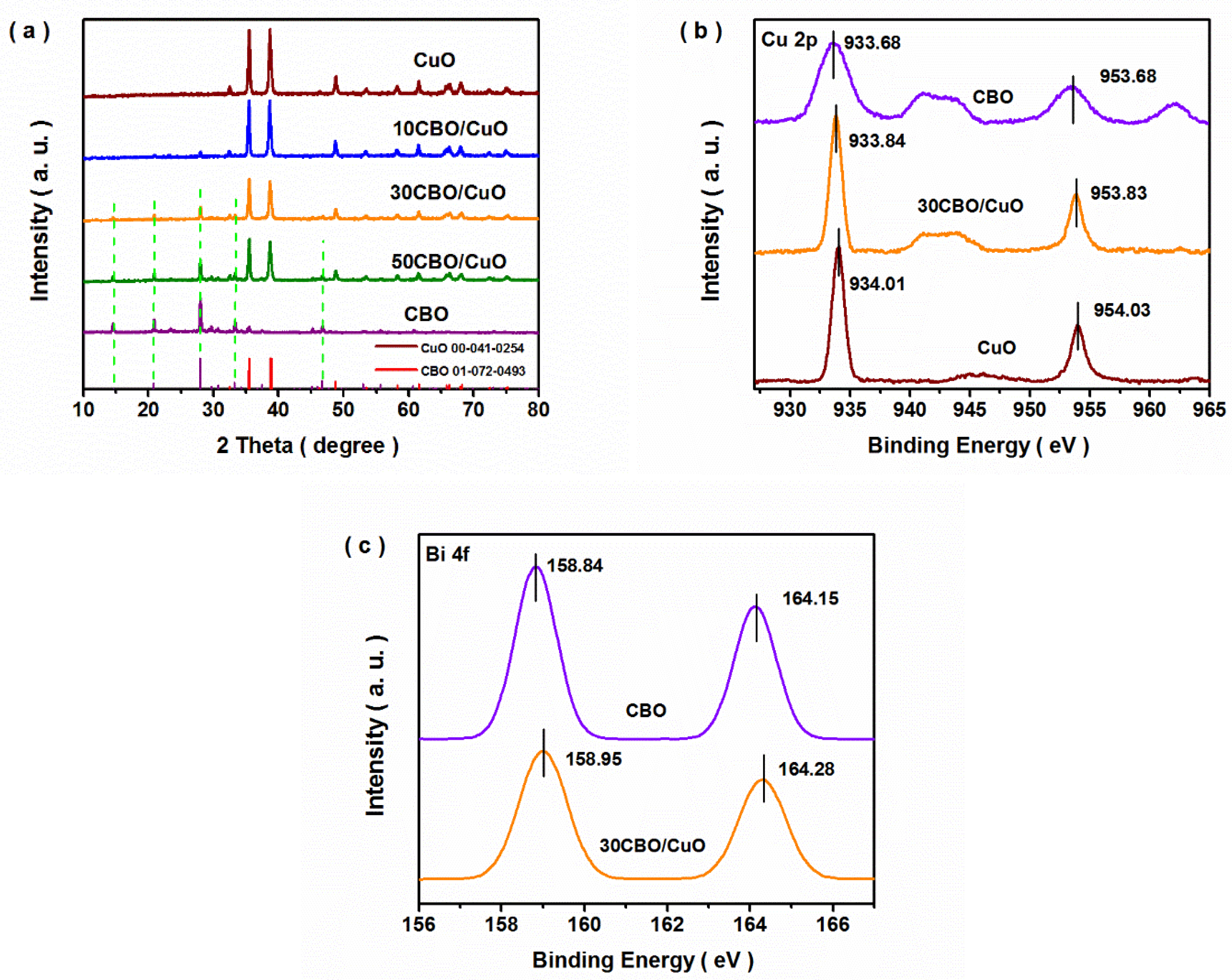
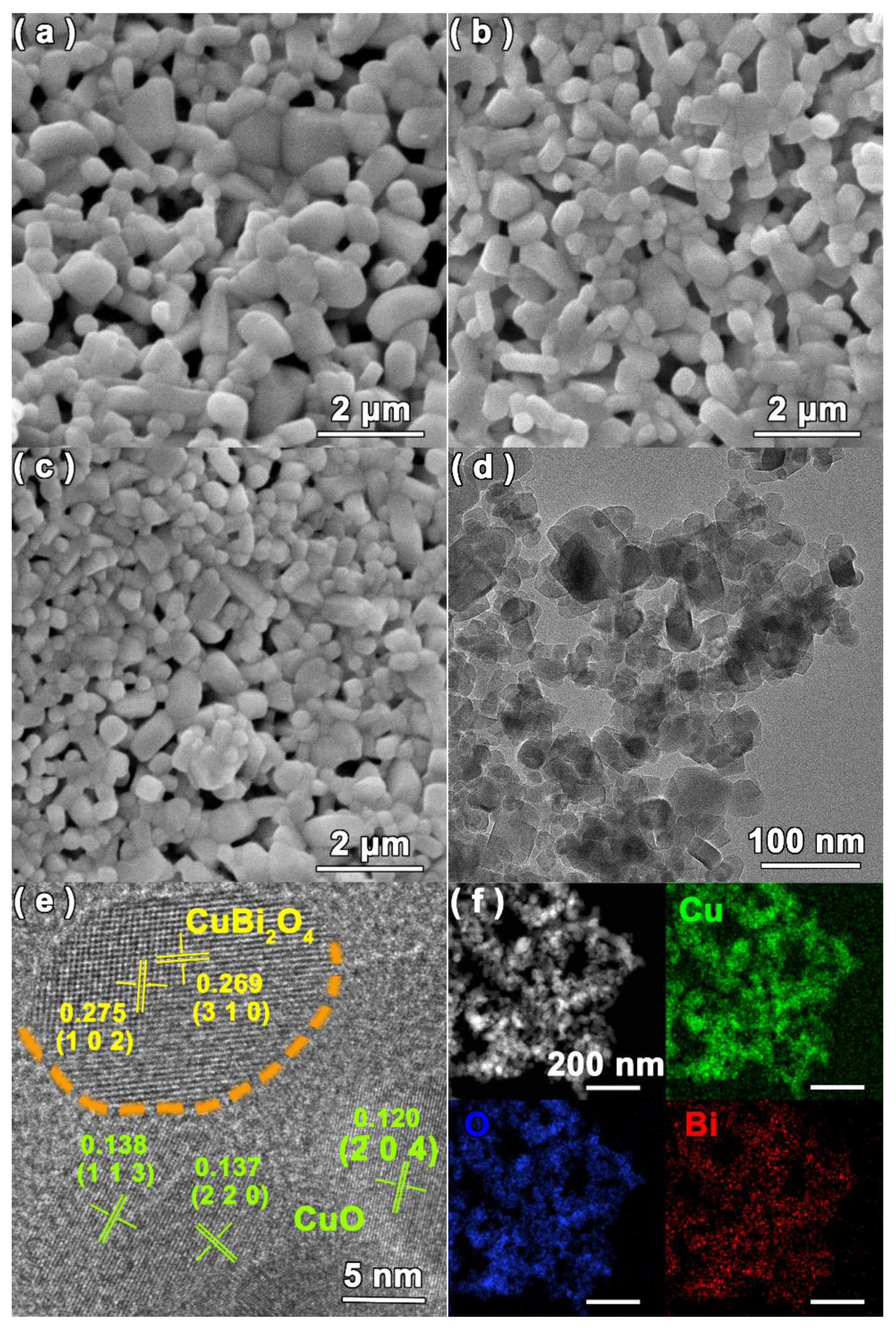
3. Results and Discussion
3.1. Structure, Composition and Morphology
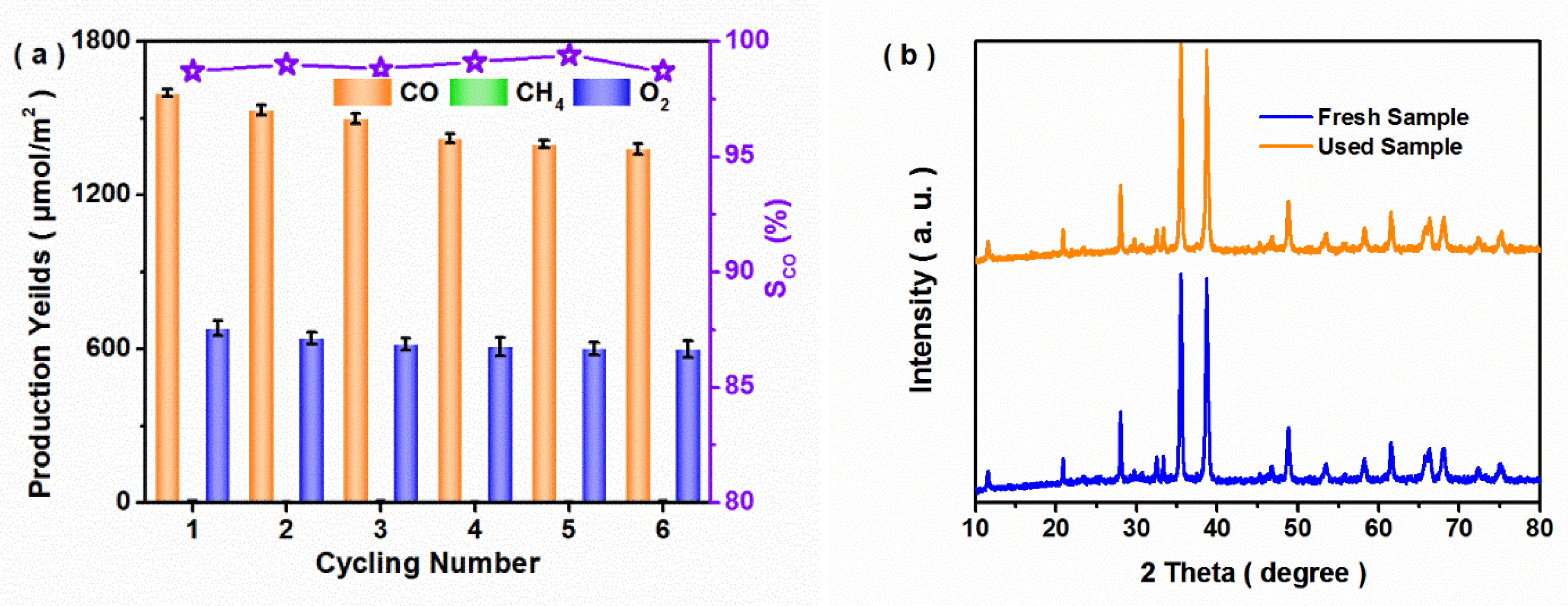
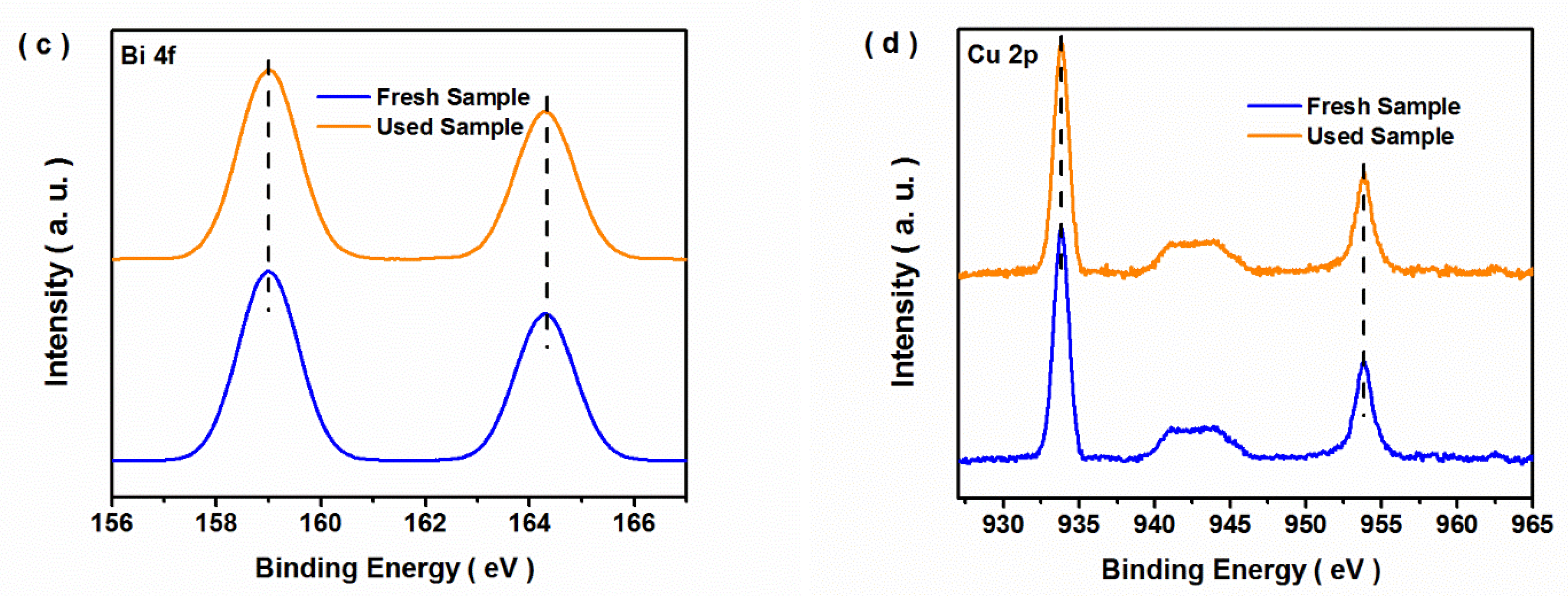
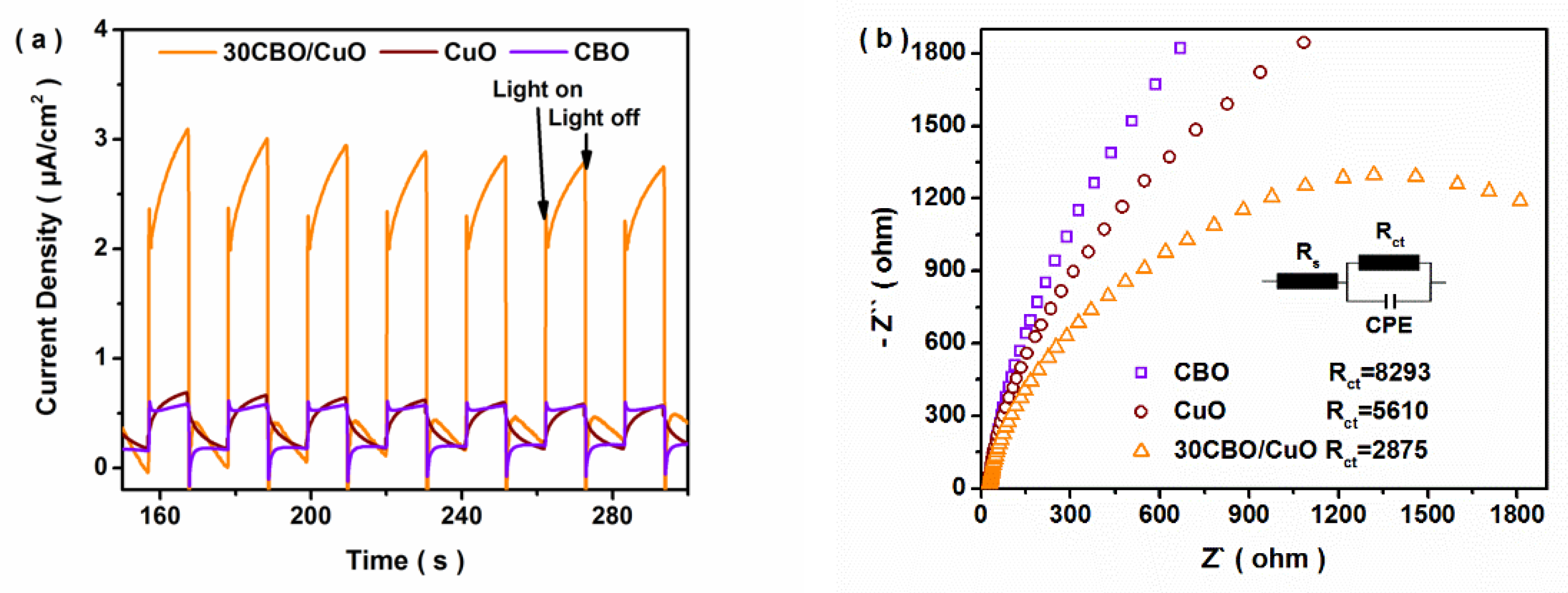
3.2. Photocatalytic Performance of CO2 Reduction
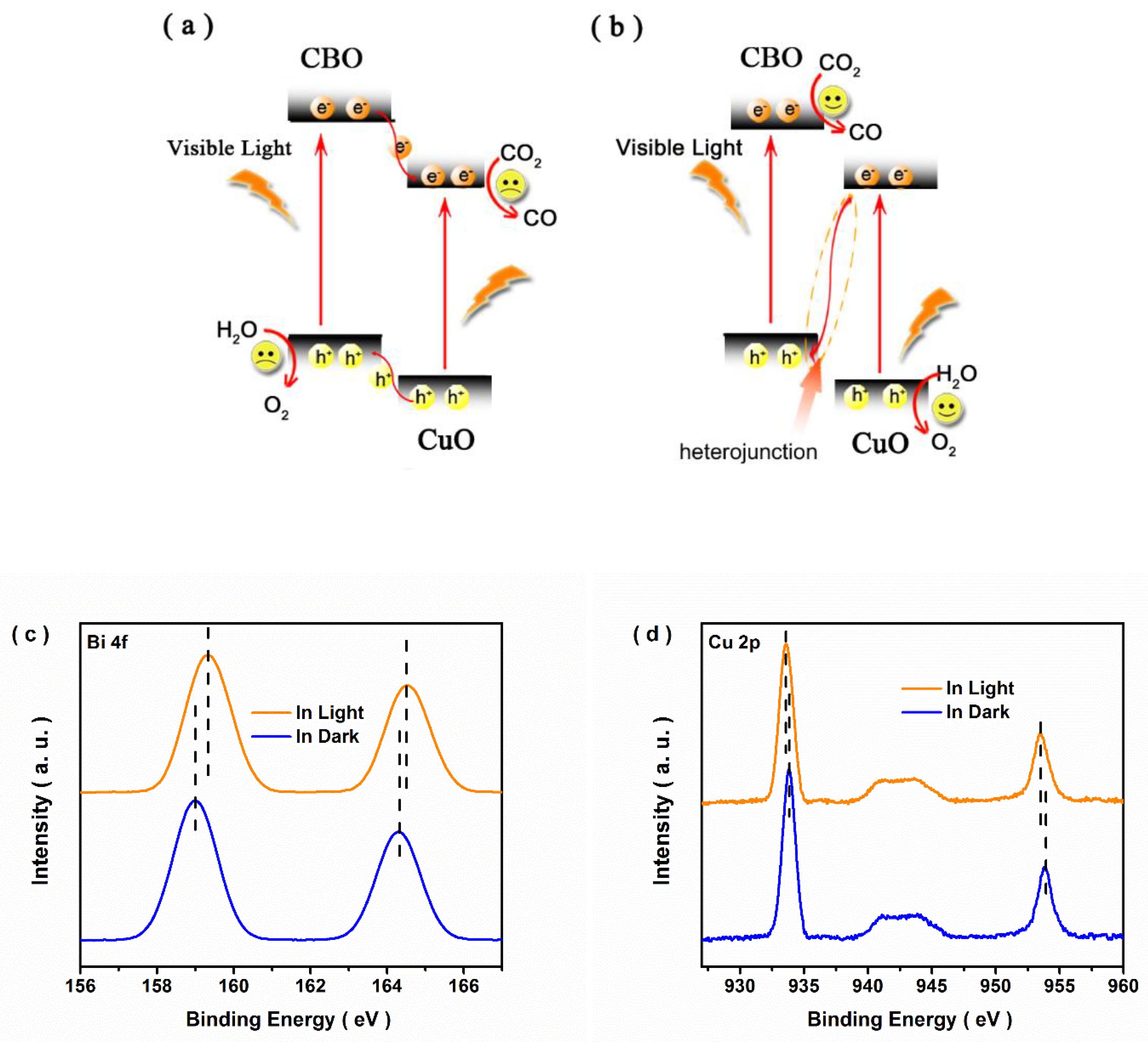
3.3. Possible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Ma, Y.; Liu, Y.; Qiu, W.; Wang, Q.; Yang, X.; Liu, M.; Qiu, X.; Li, W.; Li, J. Insights into the development of Cu-based photocathodes for carbon dioxide (CO2) conversion. Green Chem. 2021, 23, 3207–3240. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.-C.; Guo, X.; Tian, H.-L.; Zhang, W.; Gao, H.; Han, H.; Li, R.; Hou, Y. Ultra-fast construction of CuBi2O4 films supported Bi2O3 with dominant (0 2 0) facets for efficient CO2 photoreduction in water vapor. J. Alloys Compd. 2021, 890, 161919. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; He, T. Study on nanoporous CuBi2O4 photocathode coated with TiO2 overlayer for photoelectrochemical CO2 reduction. Chemosphere 2021, 264, 128508. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Bao, Y.; Song, S.; Yao, G.; Jiang, S. S-scheme photocatalytic systems. Sol. RRL 2021, 5, 2100118. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Xie, B.; Lovell, E.; Tan, T.H.; Jantarang, S.; Yu, M.; Scott, J.; Amal, R. Emerging material engineering strategies for amplifying photothermal heterogeneous CO2 catalysis. J. Energy Chem. 2021, 59, 108–125. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- Dou, X.; Chen, Y.; Shi, H. CuBi2O4/BiOBr composites promoted PMS activation for the degradation of tetracycline: S-scheme mechanism boosted Cu2+/Cu+ cycle. Chem. Eng. J. 2022, 431, 134054. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Yang, F.; Ning, X.; Zhan, L.; Wu, Z.; Zhou, X. Generating a captivating S-scheme CuBi2O4/CoV2O6 heterojunction with boosted charge spatial separation for efficiently removing tetracycline antibiotic from wastewater. J. Clean. Prod. 2022, 357, 131992. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Li, Y.; Yao, J.; Shu, S.; Huang, L.; Song, Y.; Tian, Q. Constructing an S-scheme CuBi2O4/Bi4O5I2 heterojunction for light emitting diode-driven pollutant degradation and bacterial inactivation. J. Colloid Interface Sci. 2022, 621, 295–310. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.-C.; Guo, X.; Qiao, X.; Liu, F.; Li, R.; Zhang, W.; Hou, Y.; Han, H. Controllable synthesized step-scheme heterojunction of CuBi2O4 decorated WO3 plates for visible-light-driven CO2 reduction. Nano Res. 2022, 15, 5962–5969. [Google Scholar] [CrossRef]
- Pulipaka, S.; Boni, N.; Ummethula, G.; Meduri, P. CuO/CuBi2O4 heterojunction photocathode: High stability and current densities for solar water splitting. J. Catal. 2020, 387, 17–27. [Google Scholar] [CrossRef]
- Edelmannová, M.; Lin, K.-Y.; Wu, J.C.S.; Troppová, I.; Čapek, L.; Kočí, K. Photocatalytic hydrogenation and reduction of CO2 over CuO/TiO2 photocatalysts. Appl. Surf. Sci. 2018, 454, 313–318. [Google Scholar] [CrossRef]
- Taraka, T.P.Y.; Gautam, A.; Jain, S.L.; Bojja, S.; Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO p-n heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 2019, 31, 207–214. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, K.; Xiong, J.; Wei, Y.; Han, C.; Li, W.; Cheng, G. 1D/2D WO3 nanostructure coupled with nanoparticulate CuO cocatalyst for enhancing solardriven CO2 photoreduction: The impact of the crystal facet. Sustain. Energy Fuels 2020, 4, 2593–2603. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, Y.; Li, D.; Chen, L.; Meng, S.; Jiang, D.; Chen, M. Construction of CuO quantum dots/WO3 nanosheets 0D/2D Z-scheme heterojunction with enhanced photocatalytic CO2 reduction activity under visible-light. J. Alloys Compd. 2021, 858, 157668. [Google Scholar] [CrossRef]
- Song, Y.; Ye, C.; Yu, X.; Tang, J.; Zhao, Y.; Cai, W. Electron-induced enhanced interfacial interaction of the CuO/BiOCl heterostructure for boosted CO2 photoreduction performance under simulated sunlight. Appl. Surf. Sci. 2022, 583, 152463. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Gu, E.; Song, W.; Zeng, D. Anchoring CuO nanospindles on g-C3N4 nanosheets for photocatalytic pollutant degradation and CO2 reduction. J. Alloys Compd. 2022, 914, 165339. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Silva, G.T.S.T.; Oliveira, J.A.; Lopes, O.F.; Torres, J.A.; Carmo, M.; Ribeiro, C. CuO decoration controls Nb2O5 photocatalyst selectivity in CO2 reduction. ACS Appl. Energy Mater. 2020, 3, 7629–7636. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, C.-Y.; Reisner, E. Photoelectrochemical reduction of aqueous protons with a CuO|CuBi2O4 heterojunction under visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 22462. [Google Scholar] [CrossRef]
- Hossain, M.K.; Samu, G.F.; Gandha, K.; Santhanagopalan, S.; Liu, J.P.; Janaky, C.; Rajeshwar, K. Solution combustion synthesis, characterization, and photocatalytic activity of CuBi2O4 and its nanocomposites with CuO and α-Bi2O3. J. Phys. Chem. C 2017, 121, 8252–8261. [Google Scholar] [CrossRef]
- Xu, Y.; Jian, J.; Li, F.; Liu, W.; Jia, L.; Wang, H. Porous CuBi2O4 photocathodes with rationally engineered morphology and composition towards high-efficiency photoelectrochemical performance. J. Mater. Chem. A 2019, 7, 21997–22004. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Goncalves, R.V.; Wender, H. Improved visible light photoactivity of CuBi2O4/CuO heterojunctions for photodegradation of methylene blue and metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Wu, C.-H.; Onno, E.; Lin, C.-Y. CuO nanoparticles decorated nano-dendrite-structured CuBi2O4 for highly sensitive and selective electrochemical detection of glucose. Electrochim. Acta 2017, 229, 129–140. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, F.; Guo, P.; Lei, Y.; Xi, Q.; Wang, F. Short-time hydrothermal synthesis of CuBi2O4 nanocolumn arrays for efficient visible-light photocatalysis. Nanomaterials 2019, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Shen, D.; Zhang, Q.; Zou, H.; Liu, Z.; Peng, F. Z-scheme Bi2WO6/CuBi2O4 heterojunction mediated by interfacial electric field for efficient visible-light photocatalytic degradation of tetracycline. Chem. Eng. J. 2019, 369, 292–301. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, H.; Gao, Z.; Liu, Y.; Jia, Y.; Liu, H. Boosting glucose oxidation by constructing Cu–Cu2O heterostructures. New J. Chem. 2020, 44, 18449–18456. [Google Scholar] [CrossRef]
- Shi, H.; Fan, J.; Zhao, Y.; Hu, X.; Zhang, X.; Tang, Z. Visible light driven CuBi2O4/Bi2MoO6 p-n heterojunction with enhanced photocatalytic inactivation of E. coli and mechanism insight. J. Hazard. Mater. 2020, 381, 121006. [Google Scholar] [CrossRef]
- Wang, L.; Huang, T.; Yang, G.; Lu, C.; Dong, F.; Li, Y.; Guan, W. The precursor-guided hydrothermal synthesis of CuBi2O4/WO3 heterostructure with enhanced photoactivity under simulated solar light irradiation and mechanism insight. J. Hazard. Mater. 2020, 381, 120956. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type II AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kramm, B.; Laufer, A.; Reppin, D.; Kronenberger, A.; Hering, P.; Polity, A.; Meyer, B.K. The band alignment of Cu2O/ZnO and Cu2O/GaN heterostructures. Appl. Phys. Lett. 2012, 100, 094102. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, Y.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Wang, L. S-scheme ZnO/WO3 heterojunction photocatalyst for efficient H2O2 production. J. Mater. Sci. Technol. 2022, 124, 193–201. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Polavarapu, L.; Feldmann, J.; Stolarczyk, J.K. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ irradiated XPS investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. S-scheme heterojunction TiO2/CdS nanocomposite nanofiber as H2-production photocatalyst. ChemCatChem 2019, 11, 6301–6309. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Zhan, P.; Yang, S.; Chu, M.; Zhu, Q.; Zhuang, Y.; Ren, C.; Chen, Z.; Lu, L.; Qin, P. Amorphous copper-modified gold interface promotes selective CO2 electroreduction to CO. ChemCatChem 2022, 14, e202200109. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Qiao, X.; Wang, J.; Zhao, M.; Ge, H.; Ma, J.; Liu, S.; Zhang, W. Ultra-Fast Construction of Novel S-Scheme CuBi2O4/CuO Heterojunction for Selectively Photocatalytic CO2 Conversion to CO. Nanomaterials 2022, 12, 3247. https://doi.org/10.3390/nano12183247
Shi W, Qiao X, Wang J, Zhao M, Ge H, Ma J, Liu S, Zhang W. Ultra-Fast Construction of Novel S-Scheme CuBi2O4/CuO Heterojunction for Selectively Photocatalytic CO2 Conversion to CO. Nanomaterials. 2022; 12(18):3247. https://doi.org/10.3390/nano12183247
Chicago/Turabian StyleShi, Weina, Xiu Qiao, Jichao Wang, Miao Zhao, Hongling Ge, Jingjing Ma, Shanqin Liu, and Wanqing Zhang. 2022. "Ultra-Fast Construction of Novel S-Scheme CuBi2O4/CuO Heterojunction for Selectively Photocatalytic CO2 Conversion to CO" Nanomaterials 12, no. 18: 3247. https://doi.org/10.3390/nano12183247




