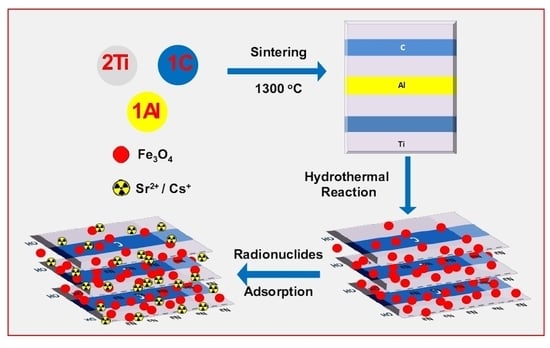
A Hydrofluoric Acid-Free Green Synthesis of Magnetic M.Ti2CTx Nanostructures for the Sequestration of Cesium and Strontium Radionuclide
Abstract
:Highlights
- An environmentally friendly MAX phase etching methodology was established
- Sodium hydroxide produced magnetic layered M.Ti2CTx nanostructure
- M.Ti2C-AIII exhibited exceptional Sr2+ and Cs+ removal capacities of 376 and 142.88 mg/g
- Highly efficient magnetic nanostructures permitted selective radionuclide removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of M.Ti2CTx
2.2. Characterization
2.3. Adsorption Experiments
3. Results and Discussions
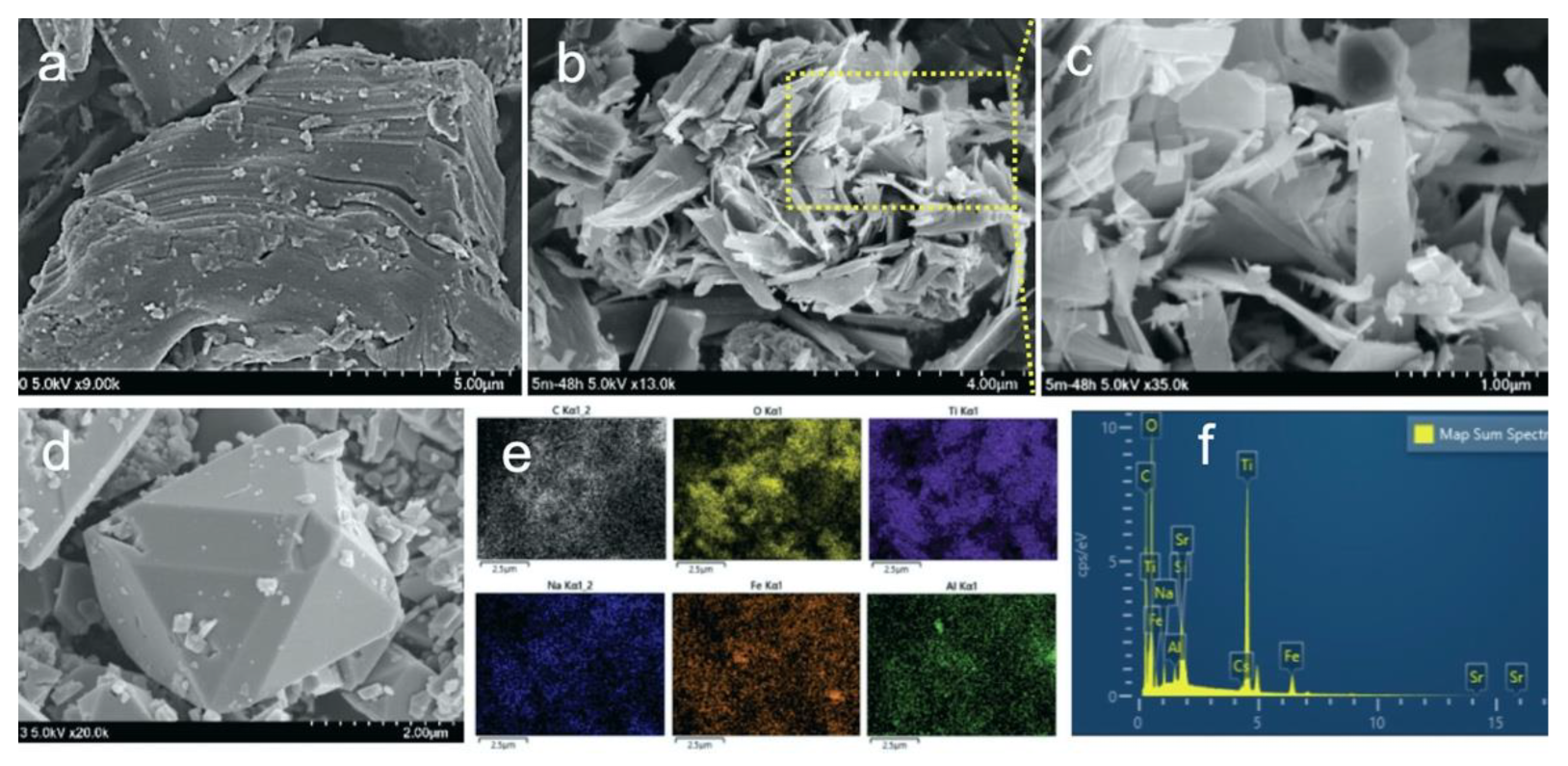
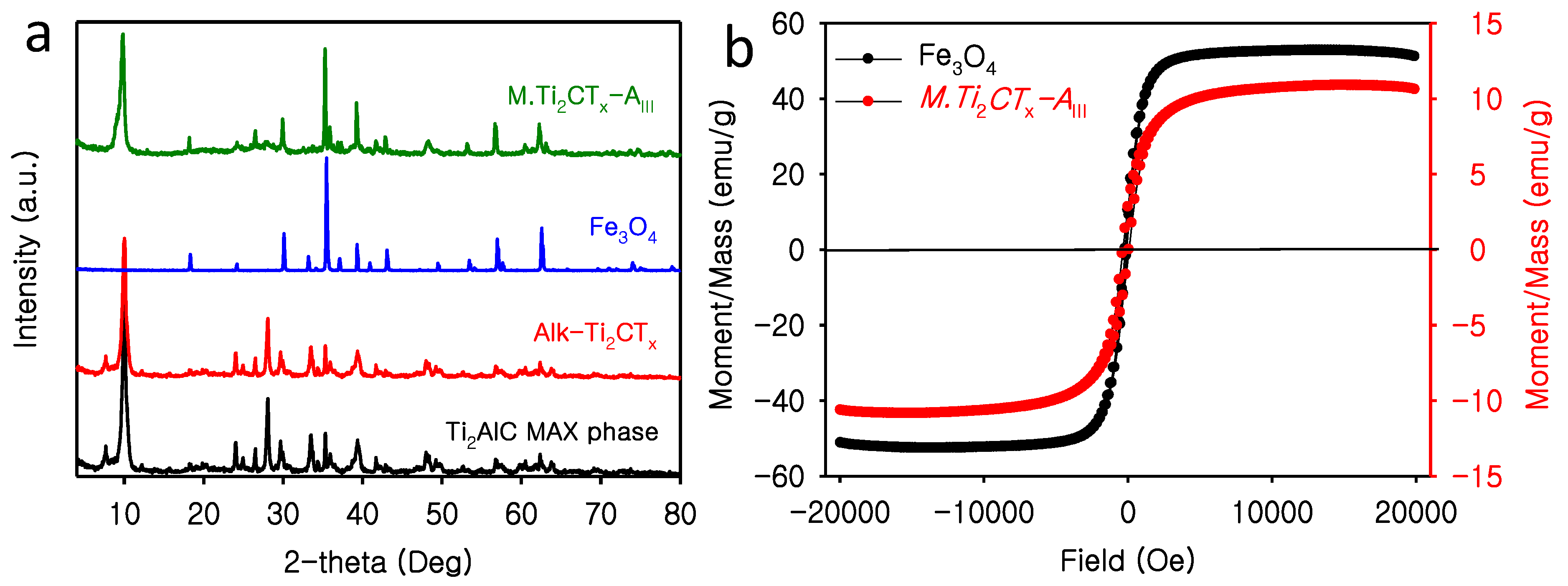
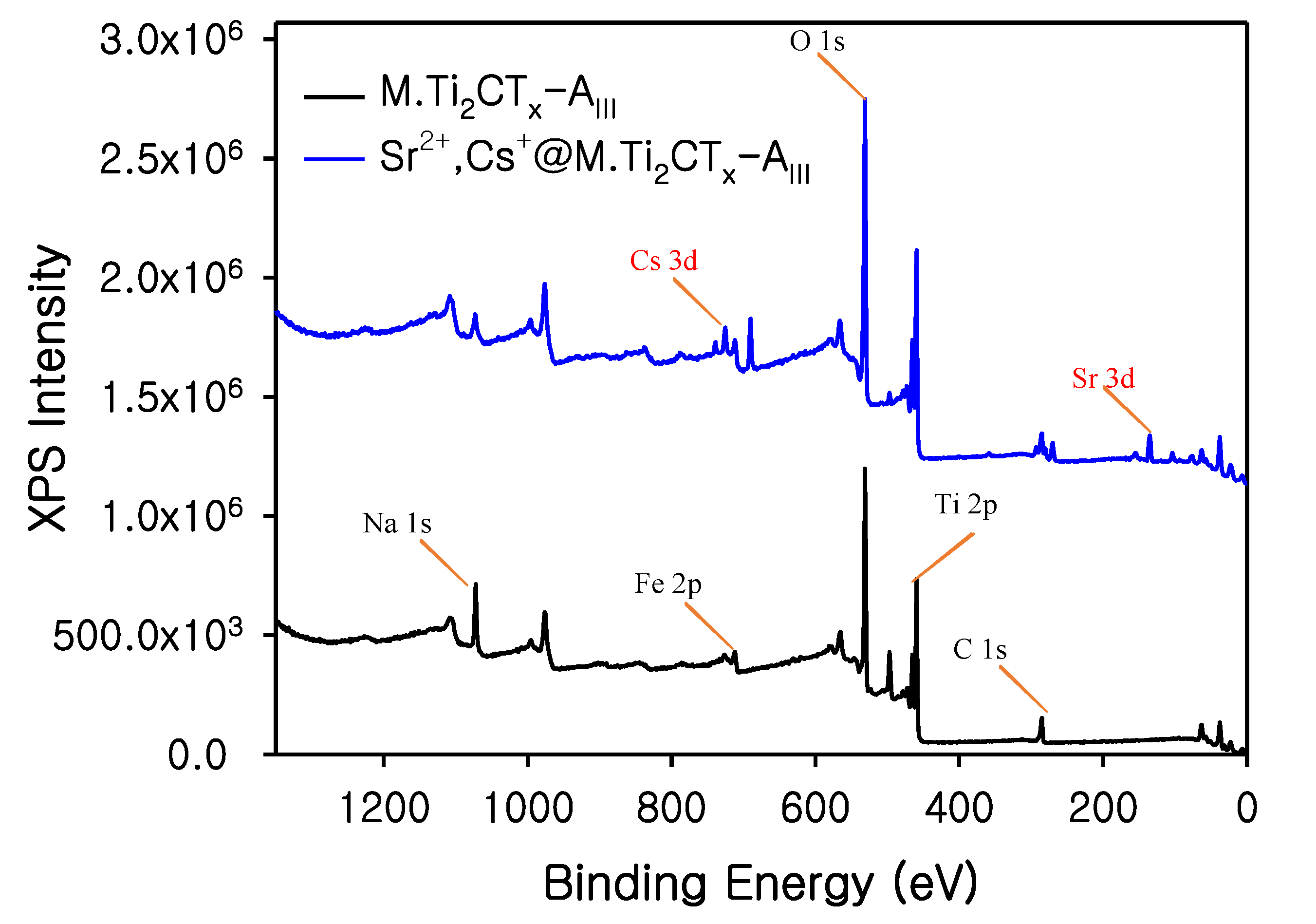
3.1. Characterization of M.Ti2CTx-AIII
3.2. Radionuclide Adsorption
3.2.1. Adsorptive Behavior of M.Ti2CTx
3.2.2. Comparison with Other Materials
3.2.3. Effect of Solution pH
3.2.4. Effect of Contact Time
3.2.5. Adsorption Isotherm
3.2.6. Practical Application of Alk-Ti2Csheet
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parsons, J.; Buongiorno, J.; Corradini, M.; Petti, D. A Fresh Look at Nuclear Energy. Science 2019, 363, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, H.S.; Jeong, H.; Park, M.; Chung, D.; Lee, K.; Lee, E.; Lim, W.T. Selective Removal of Radioactive Cesium from Nuclear Waste by Zeolites: On the Origin of Cesium Selectivity Revealed by Systematic Crystallographic Studies. J. Phase Equilibria 2017, 121, 10594–10608. [Google Scholar] [CrossRef]
- Vandeperre, L.; Qi, J.; Branford, W.R.; Ryan, M.P.; Preedy, O.; Boldrin, D.; Chen, S.; Calì, E. Functionalised Magnetic Nanoparticles for Uranium Adsorption with Ultra-High Capacity and Selectivity. J. Mater. Chem. A 2018, 6, 3063–3073. [Google Scholar] [CrossRef]
- Ueki, Y.; Hoshina, H.; Saiki, S.; Kasai, N.; Iwanade, A.; Seko, N. Hybrid Grafted Ion Exchanger for Decontamination of Radioactive Cesium in Fukushima Prefecture and Other Contaminated Areas. J. Radioanal. Nucl. Chem. 2012, 293, 703–709. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Tan, L.; Liu, H.; Ye, X.; Hayat, T.; Wang, X. Core–Shell Hierarchical C@Na2Ti3O7·9H2O Nanostructures for the Efficient Removal of Radionuclides. Environ. Sci. Nano 2018, 5, 1140–1149. [Google Scholar] [CrossRef]
- Hong, H.J.; Kim, B.G.; Ryu, J.; Park, I.S.; Chung, K.S.; Lee, S.M.; Lee, J.B.; Jeong, H.S.; Kim, H.; Ryu, T. Preparation of Highly Stable Zeolite-Alginate Foam Composite for Strontium(90Sr) Removal from Seawater and Evaluation of Sr Adsorption Performance. J. Environ. Manag. 2018, 205, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.H.; Li, C.-W.; Choo, K.-H. Comparison of Low-Pressure Reverse Osmosis Filtration and Polyelectrolyte-Enhanced Ultrafiltration for the Removal of Co and Sr from Nuclear Plant Wastewater. Sep. Purif. Technol. 2016, 157, 209–214. [Google Scholar] [CrossRef]
- Hodkin, D.J.; Stewart, D.I.; Graham, J.T.; Burke, I.T. Coprecipitation of 14C and Sr with Carbonate Precipitation: The Importance of Reaction Kinetics and Recrystallization Pathways. Sci. Total Environ. 2016, 562, 335–343. [Google Scholar] [CrossRef]
- Wang, J. Co-Extraction of Strontium and Cesium from Simulated High-Level Liquid Waste (HLLW) by Calixcrown and Crown Ether. J. Nucl. Sci. Technol. 2015, 52, 171–177. [Google Scholar] [CrossRef]
- Yang, H.M.; Hwang, J.R.; Lee, D.Y.; Kim, K.B.; Park, C.W.; Kim, H.R.; Lee, K.W. Eco-Friendly One-Pot Synthesis of Prussian Blue-Embedded Magnetic Hydrogel Beads for the Removal of Cesium from Water. Sci. Rep. 2018, 8, 11476. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Huang, L.; Ma, L.; Shim, Y.; Islam, S.M.; Wang, P.; Zhao, L.D.; Wang, S.; Sun, G.; Yang, X.; et al. Efficient Uranium Capture by Polysulfide/Layered Double Hydroxide Composites. J. Am. Chem. Soc. 2015, 137, 3670–3677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Li, J.; Xu, J.; Wang, X. Fabrication of Fe/Fe3C@porous Carbon Sheets from Biomass and Their Application for Simultaneous Reduction and Adsorption of Uranium(vi) from Solution. Inorg. Chem. Front. 2014, 1, 641–648. [Google Scholar] [CrossRef]
- Shahzad, A.; Oh, J.M.; Rasool, K.; Jang, J.; Kim, B.; Lee, D.S. Strontium Ions Capturing in Aqueous Media Using Exfoliated Titanium Aluminum Carbide (Ti2AlC MAX Phase). J. Nucl. Mater. 2021, 549, 152916. [Google Scholar] [CrossRef]
- Kaewmee, P.; Manyam, J.; Opaprakasit, P.; Truc Le, G.T.; Chanlek, N.; Sreearunothai, P. Effective Removal of Cesium by Pristine Graphene Oxide: Performance, Characterizations and Mechanisms. RSC Adv. 2017, 7, 38747–38756. [Google Scholar] [CrossRef]
- Liu, H.; Yonezawa, A.; Kumagai, K.; Sano, M.; Miyake, T. Cs and Sr Removal over Highly Effective Adsorbents ETS-1 and ETS-2. J. Mater. Chem. A 2015, 3, 1562–1568. [Google Scholar] [CrossRef]
- Borai, E.H.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient Removal of Cesium from Low-Level Radioactive Liquid Waste Using Natural and Impregnated Zeolite Minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C.; Yantasee, W. Selective Capture of Cesium and Thallium from Natural Waters and Simulated Wastes with Copper Ferrocyanide Functionalized Mesoporous Silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef]
- Delchet, C.; Tokarev, A.; Dumail, X.; Toquer, G.; Barré, Y.; Guari, Y.; Guerin, C.; Larionova, J.; Grandjean, A. Extraction of Radioactive Cesium Using Innovative Functionalized Porous Materials. RSC Adv. 2012, 2, 5707–5716. [Google Scholar] [CrossRef]
- Causse, J.; Tokarev, A.; Ravaux, J.; Moloney, M.; Barré, Y.; Grandjean, A. Facile One-Pot Synthesis of Copper Hexacyanoferrate Nanoparticle Functionalised Silica Monoliths for the Selective Entrapment of 137Cs. J. Mater. Chem. A 2014, 2, 9461–9464. [Google Scholar] [CrossRef]
- Come, J.; Naguib, M.; Rozier, P.; Barsoum, M.W.; Gogotsi, Y.; Taberna, P. A Non-Aqueous Asymmetric Cell with a Ti2C-Based Two-Dimensional Negative Electrode. J. Electrochem. Soc. 2012, 159, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Jin-Cheng, L.; Zhang, X.; Zhou, Z. Recent Advances in MXene: Preparation, Properties, and Applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Wang, H.W.; Naguib, M.; Page, K.; Wesolowski, D.J.; Gogotsi, Y. Resolving the Structure of Ti3C2Tx MXenes through Multilevel Structural Modeling of the Atomic Pair Distribution Function. Chem. Mater. 2016, 28, 349–359. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y. Synthesis of Two-Dimensional Materials by Selective Extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water Treatment and Environmental Remediation Applications of Two-Dimensional Metal Carbides (MXenes). Mater. Today 2019, 30, 82–102. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water. ACS Sustain. Chem. Eng. 2017, 5, 11481–11488. [Google Scholar] [CrossRef]
- Shahzad, A.; Oh, J.M.; Azam, M.; Iqbal, J.; Hussain, S.; Miran, W.; Rasool, K. Advances in the Synthesis and Application of Anti-Fouling Membranes Using Two-Dimensional Nanomaterials. Membranes 2021, 11, 605. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Huo, C.; Wang, S.; Cui, C. Thermal Stability of Two-Dimensional Ti2C Nanosheets. Ceram. Int. 2015, 41, 2631–2635. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+1AXN Phases: A New Class of Solids; Thermodynamically Stable Nanolaminates. In Progress in Solid State Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 28, pp. 201–281. [Google Scholar]
- Wang, N.; Zhu, L.; Wang, D.; Wang, M.; Lin, Z.; Tang, H. Sono-Assisted Preparation of Highly-Efficient Peroxidase-like Fe3O4 Magnetic Nanoparticles for Catalytic Removal of Organic Pollutants with H2O2. Ultrason. Sonochem. 2010, 17, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Du, B.; Wei, Q.; Yang, J.; Hu, L.; Yan, L.; Xu, W. Synthesis of Amino Functionalized Magnetic Graphenes Composite Material and Its Application to Remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from Contaminated Water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef]
- Parham, H.; Zargar, B.; Shiralipour, R. Fast and Efficient Removal of Mercury from Water Samples Using Magnetic Iron Oxide Nanoparticles Modified with 2-Mercaptobenzothiazole. J. Hazard. Mater. 2012, 205–206, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Nawaz, M.; Moztahida, M.; Tahir, K.; Kim, J.; Lim, Y.; Kim, B.; Jang, J.; Lee, D.S. Exfoliation of Titanium Aluminum Carbide (211 MAX Phase) to Form Nanofibers and Two-Dimensional Nanosheets and Their Application in Aqueous-Phase Cadmium Sequestration. ACS Appl. Mater. Interfaces 2019, 11, 19156–19166. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, C.A.; Song, Y.; Huang, Y. A Novel Simple Method to Stably Synthesize Ti3AlC2 Powder with High Purity. Mater. Sci. Eng. A 2006, 428, 54–58. [Google Scholar] [CrossRef]
- Fatima, H.; Lee, D.W.; Yun, H.J.; Kim, K.S. Shape-Controlled Synthesis of Magnetic Fe3O4 Nanoparticles with Different Iron Precursors and Capping Agents. RSC Adv. 2018, 8, 22917–22923. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for Photocatalytic Degradation of Antiepileptic Drug Carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Shahzad, A.; Miran, W.; Rasool, K.; Nawaz, M.; Jang, J.; Lim, S.-R.; Lee, D.S. Heavy Metals Removal by EDTA-Functionalized Chitosan Graphene Oxide Nanocomposites. RSC Adv. 2017, 7, 9764–9771. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A.; Navratil, J.D. Adsorption of Cesium, Strontium, and Cobalt Ions on Magnetite and a Magnetite—Silica Composite. Ind. Eng. Chem. Res. 2001, 40, 1615–1623. [Google Scholar] [CrossRef]
- Luo, W.; Huang, Q.; Antwi, P.; Guo, B.; Sasaki, K. Synergistic Effect of ClO4− and Sr2+ Adsorption on Alginate-Encapsulated Organo-Montmorillonite Beads: Implication for Radionuclide Immobilization. J. Colloid Interface Sci. 2019, 560, 338–348. [Google Scholar] [CrossRef]
- Koilraj, P.; Kamura, Y.; Sasaki, K. Cosorption Characteristics of SeO42− and Sr2+ Radioactive Surrogates Using 2D/2D Graphene Oxide-Layered Double Hydroxide Nanocomposites. ACS Sustain. Chem. Eng. 2018, 6, 13854–13866. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Zhai, J.; Li, H.; Zhao, Y.; Yu, H. In Situ Controllable Synthesis of Magnetic Prussian Blue/Graphene Oxide Nanocomposites for Removal of Radioactive Cesium in Water. J. Mater. Chem. A 2014, 2, 326–332. [Google Scholar] [CrossRef]
- Vipin, A.K.; Hu, B.; Fugetsu, B. Prussian Blue Caged in Alginate/Calcium Beads as Adsorbents for Removal of Cesium Ions from Contaminated Water. J. Hazard. Mater. 2013, 258–259, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, X.; Luo, M.; Meng, M.; Ni, L.; Qiu, J.; Hu, Z.; Liu, F.; Zhong, G.; Liu, Z.; et al. Polymer Based on Graphene Oxide for Removal of Strontium from Aqueous Solution. J. Mater. Chem. A 2015, 1287–1297. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.S. Three-Dimensional Barium-Sulfate-Impregnated Reduced Graphene Oxide Aerogel for Removal of Strontium from Aqueous Solutions. J. Nucl. Mater. 2018, 504, 206–214. [Google Scholar] [CrossRef]
- Mu, W.; Yu, Q.; Li, X.; Wei, H.; Jian, Y. Applied Surface Science Porous Three-Dimensional Reduced Graphene Oxide Merged with WO3 for Efficient Removal of Radioactive Strontium. Appl. Surf. Sci. 2017, 423, 1203–1211. [Google Scholar] [CrossRef]
- Amer, H.; Moustafa, W.M.; Farghali, A.A.; El Rouby, M.A.; Khalil, W.F. Efficient Removal of Cobalt (II) and Strontium (II) Metals from Water Using Ethylene Diamine Tetra-Acetic Acid Functionalized Graphene Oxide. Z. Anorg. Allg. Chem. 2017, 643, 1776–1784. [Google Scholar] [CrossRef]
- Zhaoa, X.; Meng, Q.; Chen, G.; Wu, Z.; Sun, G.; Yu, G.; Sheng, L.; Weng, H.; Lin, M. An Acid-Resistant Magnetic Nb-Substituted Crystalline Silicotitanate for Selective Separation of Strontium and/or Cesium Ions from Aqueous Solution. Chem. Eng. J. 2018, 352, 133–142. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Q.; Fu, H.; Zou, G.; Zhang, Q. Heavy-Metal Adsorption Behavior of Two-Dimensional Alkalization-Intercalated MXene by First-Principles Calculations. J. Phys. Chem. C 2015, 119, 20923–20930. [Google Scholar] [CrossRef]


| No. | Material Code | Synthesis Conditions |
|---|---|---|
| 1 | M.Ti2CTx-AI | Synthesized at 5 M NaOH, 200 °C for 12 h |
| 2 | M.Ti2CTx-AII | Synthesized at 5 M NaOH, 200 °C for 24 h |
| 3 | M.Ti2CTx-AIII | Synthesized at 5 M NaOH, 200 °C for 48 h |
| 4 | M.Ti2CTx-BI | Synthesized at 10 M NaOH, 200 °C for 12 h |
| 5 | M.Ti2CTx-BII | Synthesized at 10 M NaOH, 200 °C for 24 h |
| 6 | M.Ti2CTx-BIII | Synthesized at 10 M NaOH, 200 °C for 48 h |
| 7 | M.Ti2CTx-CI | Synthesized at 15 M NaOH, 200 °C for 12 h |
| 8 | M.Ti2CTx-CII | Synthesized at 15 M NaOH, 200 °C for 24 h |
| 9 | M.Ti2CTx-CIII | Synthesized at 15 M NaOH, 200 °C for 48 h |
| Type of Adsorbent | Cs+ Concentration (ppm) | Removal (%) | Sr2+ Concentration (ppm) | Removal (%) | ||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | |||
| M.Ti2CTx-AI | 12.181 | 3.290 | 72.99 | 10.911 | 0.006 | 99.94 |
| M.Ti2CTx-AII | 12.181 | 3.022 | 75.19 | 10.911 | 0.011 | 99.89 |
| M.Ti2CTx-AIII | 12.181 | 2.505 | 79.43 | 10.911 | 0.009 | 99.91 |
| M.Ti2CTx-BI | 12.181 | 4.857 | 60.12 | 10.911 | 0.007 | 99.93 |
| M.Ti2CTx-BII | 12.181 | 8.643 | 29.05 | 10.911 | 0.022 | 99.79 |
| M.Ti2CTx-BIII | 12.181 | 9.484 | 22.14 | 10.911 | 0.022 | 99.80 |
| M.Ti2CTx-CI | 12.181 | 9.752 | 19.94 | 10.911 | 0.048 | 99.56 |
| M.Ti2CTx-CIII | 12.181 | 12.017 | 1.35 | 10.911 | 0.434 | 96.02 |
| Adsorbent | Radionuclide | Adsorption Capacity (mg/g) | Effective pH | References |
|---|---|---|---|---|
| magnetite−silica composite | Sr2+ Cs+ | 343.48 93.42 | 7 6 | [40] |
| OMt/alginate | Sr2+ | 42.41 | 6–11 | [41] |
| PB-MHBs-3 | Cs+ | 41.15 | 7 | [10] |
| MgAl-LDH/GO | Sr2+ | 213.35 | 4–10 | [42] |
| PB/Fe3O4/GO | Cs+ | 55.56 | 7 | [43] |
| PB + CNTs | Cs+ | 142.85 | 4–8 | [44] |
| RAFT-IIP | Sr2+ | 145.77 | 6–8 | [45] |
| BaSO4/rGO | Sr2+ | 129.37 | 7–11 | [46] |
| RGO/WO3 | Sr2+ | 149.56 | 4–11 | [47] |
| GO | Sr2+ | 140 | 6 | [48] |
| GO-EDTA | Sr2+ | 158 | 6 | |
| Magnetic Nb-CST | Sr2+ Cs+ | 14.38 11.18 | 9 4 | [49] |
| M.Ti2CTx-AIII | Sr2+ Cs+ | 376.05 142.88 | 3–11 6 | This work |
| Isotherm Model | Parameters | Values | |
|---|---|---|---|
| Sr2+@M.Ti2CTx-AIII | Cs+@M.Ti2CTx-AIII | ||
| Langmuir (Qe = QmKaCe/1 + KaCe) | qmax (mg/g) | 376.05 | 142.88 |
| kL | 0.056 | 0.077 | |
| R2 | 0.976 | 0.987 | |
| Freundlich (Qe = KFCe(1/n)) | kF (mg/g) | 84.29 | 35.46 |
| 1/n | 0.240 | 1.029 | |
| R2 | 0.882 | 0.892 | |
| Radionuclide | Parameters | Matrices | |||||
|---|---|---|---|---|---|---|---|
| Single-Element Solution | (Binary Solution: Sr2+ + Cs+) | ||||||
| Deionized Water | Tap Water | Simulated Seawater | Deionized Water | Tap Water | Simulated Seawater | ||
| Sr2+@ M.Ti2CTx-AIII | Initial Conc. (µg/L) | 1100.23 | 1100.23 | 1100.23 | 1005 | 1005 | 1005 |
| Final Conc. (µg/L) | 12.37 | 1.59 | 86.21 | 15.09 | 1.53 | 105.08 | |
| Removal (Conc. (µg/g) | 1087.87 | 1098.64 | 1014.02 | 989.91 | 1003.47 | 899.92 | |
| Removal (%) | 98.88 | 99.86 | 92.16 | 98.50 | 99.85 | 89.54 | |
| Cs+@ M.Ti2CTx-AIII | Initial Conc. (µg/L) | 1042.67 | 1042.67 | 1042.67 | 998.87 | 998.87 | 998.87 |
| Final Conc. (µg/L) | 58.04 | 310.85 | 717.071 | 61.41 | 323.91 | 886.75 | |
| Removal (Conc. (µg/g) | 984.63 | 731.81 | 325.59 | 938.52 | 674.96 | 112.12 | |
| Removal (%) | 94.43 | 70.19 | 31.23 | 93.85 | 67.57 | 11.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, J.; Rasool, K.; Howari, F.; Nazzal, Y.; Sarkar, T.; Shahzad, A. A Hydrofluoric Acid-Free Green Synthesis of Magnetic M.Ti2CTx Nanostructures for the Sequestration of Cesium and Strontium Radionuclide. Nanomaterials 2022, 12, 3253. https://doi.org/10.3390/nano12183253
Iqbal J, Rasool K, Howari F, Nazzal Y, Sarkar T, Shahzad A. A Hydrofluoric Acid-Free Green Synthesis of Magnetic M.Ti2CTx Nanostructures for the Sequestration of Cesium and Strontium Radionuclide. Nanomaterials. 2022; 12(18):3253. https://doi.org/10.3390/nano12183253
Chicago/Turabian StyleIqbal, Jibran, Kashif Rasool, Fares Howari, Yousef Nazzal, Tapati Sarkar, and Asif Shahzad. 2022. "A Hydrofluoric Acid-Free Green Synthesis of Magnetic M.Ti2CTx Nanostructures for the Sequestration of Cesium and Strontium Radionuclide" Nanomaterials 12, no. 18: 3253. https://doi.org/10.3390/nano12183253
APA StyleIqbal, J., Rasool, K., Howari, F., Nazzal, Y., Sarkar, T., & Shahzad, A. (2022). A Hydrofluoric Acid-Free Green Synthesis of Magnetic M.Ti2CTx Nanostructures for the Sequestration of Cesium and Strontium Radionuclide. Nanomaterials, 12(18), 3253. https://doi.org/10.3390/nano12183253