Synthesis and Characterization of Reproducible Linseed Oil-Loaded Silica Nanoparticles with Potential Use as Oxygen Scavengers in Active Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Nanoparticle Synthesis
2.3. Nanoparticle Characterization
2.4. Performance Analysis
2.5. Reproducibility Evaluation
3. Results and Discussion
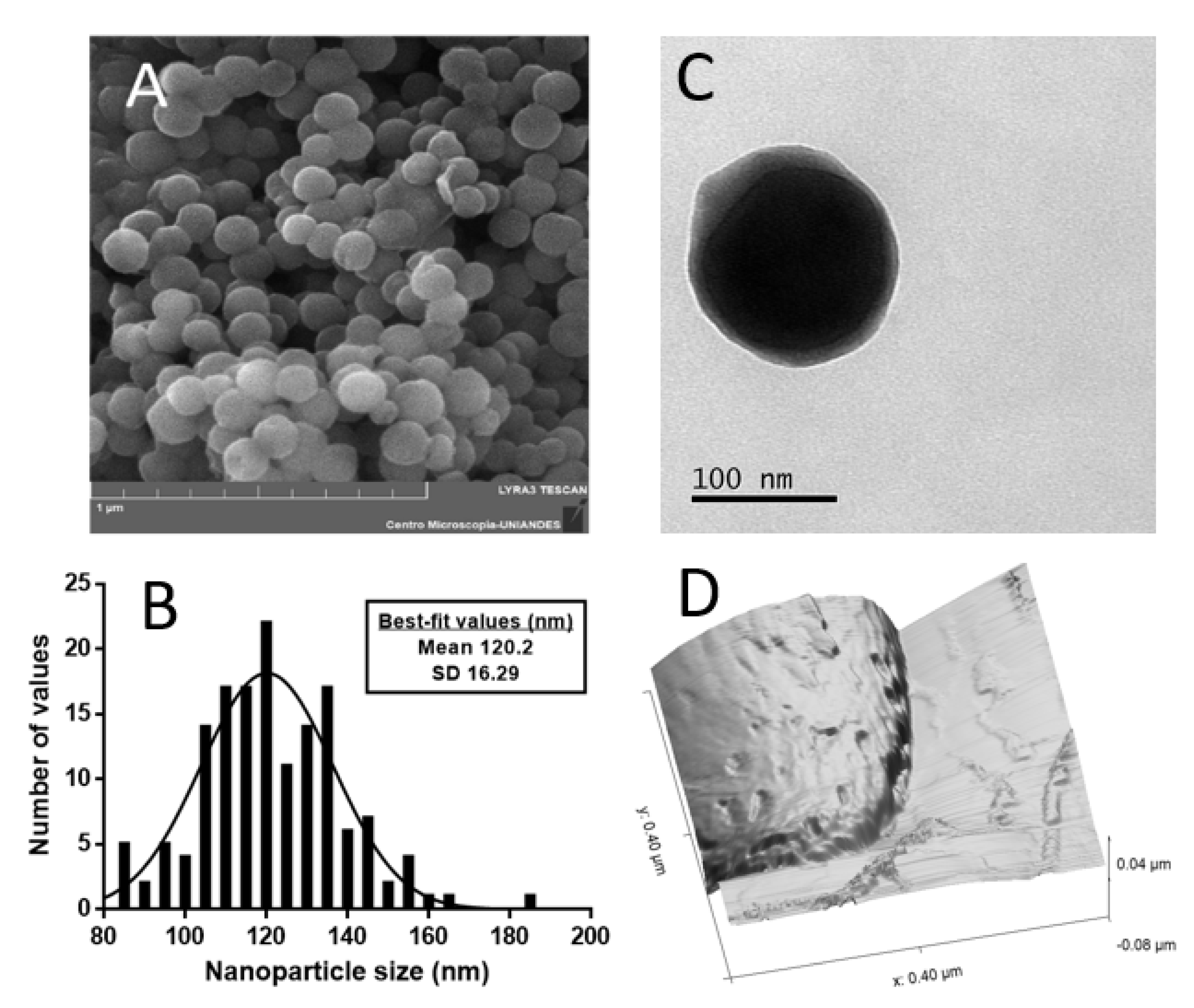
3.1. Nanoparticle Characterization
3.2. Performance Analysis
3.3. Reproducibillity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Frankel, E.N. Biological systems. In Lipid Oxid; Woodhead Publishing: Sawston, UK, 2012; pp. 391–455. [Google Scholar]
- Lee, J.S.; Chang, Y.; Lee, E.S.; Song, H.G.; Chang, P.S.; Han, J. Ascorbic Acid-Based Oxygen Scavenger in Active Food Packaging System for Raw Meatloaf. J. Food Sci. 2018, 83, 682–688. [Google Scholar] [CrossRef]
- Vermeiren, L.; Heirlings, L.; Devlieghere, F.; Debevere, J. Oxygen, ethylene and other scavengers. In Novel Food Packaging Techniques; Elsevier: Amsterdam, The Netherlands, 2003; pp. 22–49. [Google Scholar]
- Lim, L.-T. Enzymes for food-packaging applications. In Improving and Tailoring Enzymes for Food Quality and Functionality; Woodhead Publishing: Sawston, UK, 2015; pp. 161–178. [Google Scholar]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nielsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. In Comprehensive Reviews in Food Science and Food Safety; Blackwell Publishing Inc.: Oxford, UK, 2018; Volume 17, pp. 165–199. [Google Scholar]
- Souza, R.; Peruch, G.; Pires, A.C.d.S. Oxygen Scavengers: An Approach on Food Preservation. In Structure and Function of Food Engineering; InTech: London, UK, 2012. [Google Scholar]
- Tewari, G.; Jayas, D.S.; Jeremiah, L.E.; Holley, R.A. Absorption kinetics of oxygen scavengers. Int. J. Food Sci. Technol. 2002, 37, 209–217. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, Y.S. Effect of storage conditions on the absorption kinetics of non-metallic oxygen scavenger suitable for moist food packaging. J. Food Meas. Charact. 2017, 11, 965–971. [Google Scholar] [CrossRef]
- Pant, A.F.; Sängerlaub, S.; Müller, K. Gallic Acid as an Oxygen Scavenger in Bio-Based Multilayer Packaging Films. Mater. 2017, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Charles, F.; Sanchez, J.; Gontard, N. Absorption kinetics of oxygen and carbon dioxide scavengers as part of active modified atmosphere packaging. J. Food Eng. 2006, 72, 1–7. [Google Scholar] [CrossRef]
- Salehi, M.A.; Trey, S.M.; Henriksson, G.; Johansson, M. Effect of model lignin structures on the oxidation of unsaturated fatty acids. Polym. from Renew. Resour. 2010, 1, 69–90. [Google Scholar]
- Cosgrove, J.P.; Church, D.F.; Pryor, W.A. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 1987, 22, 299–304. [Google Scholar] [CrossRef]
- Richaud, E.; Audouin, L.; Fayolle, B.; Verdu, J.; Matisová-Rychlá, L.; Rychlý, J. Rate constants of oxidation of unsaturated fatty esters studied by chemiluminescence. Chem. Phys. Lipids 2012, 165, 753–759. [Google Scholar] [CrossRef]
- Zoukrami, F.; Haddaoui, N.; Sclavons, M.; Devaux, J.; Vanzeveren, C. Rheological properties and thermal stability of compatibilized polypropylene/untreated silica composites prepared by water injection extrusion process. Polym. Bull. 2018, 75, 5551–5566. [Google Scholar] [CrossRef]
- Zheng, J.Z.; Zhou, X.P.; Ying, J.R.; Xie, X.L.; Mai, Y.W. Enhanced mechanical properties of polypropylene/silica nanocomposites with surface modification of nano-silica via in situ copolymerization of methyl methacrylate and butyl acrylate. Chinese J. Polym. Sci. 2009, 27, 685–694. [Google Scholar] [CrossRef]
- Miguel, M.d.; Ollier, R.; Alvarez, V.; Vallo, C. Effect of the preparation method on the structure of linseed oil-filled poly(urea-formaldehyde) microcapsules. Prog. Org. Coatings 2016, 97, 194–202. [Google Scholar] [CrossRef]
- Röcker, B.; Mäder, G.; Monnard, F.W.; Jancikova, M.; Welker, M.; Schoelkopf, J.; Yildirim, S. Evaluation of the Potential of Modified Calcium Carbonate as a Carrier for Unsaturated Fatty Acids in Oxygen Scavenging Applications. Materials 2021, 14, 5000. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xu, Y.; Cai, N.; Jia, G. Polypropylene/silica nanocomposites prepared by in-situ melt ultrasonication. Polym. Compos. 2009, 30, 835–840. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horizons 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Lasio, J.; Allgeier, A.M.; Chan, C.D.; Londono, J.D.; Najafi, E.; Woerner, F.J. Control of Mechanical Stability of Hollow Silica Particles, and Its Measurement by Mercury Intrusion Porosimetry. Langmuir 2017, 33, 4666–4674. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Khan, M.U.; Gomes, V.G. Enhanced silica nanocomposite via dual step functionalisation and in-situ polymerisation. Int. J. Nanotechnol. 2013, 10, 1078–1092. [Google Scholar] [CrossRef]
- Niederberger, N.; Pinna, M. Aqueous and Nonaqueous Sol-Gel. In Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer: London, UK, 2009; pp. 7–18. [Google Scholar]
- Garcia, A. Kinetic Modeling of Oxygen Absorption by Unsaturated Esters and Linseed Oil to be Used as Oxygen Scavengers; Universidad de los Andes: Bogota, Colombia, 2015. [Google Scholar]
- Teng, Z.; Han, Y.; Li, J.; Yan, F.; Yang, W. Preparation of hollow mesoporous silica spheres by a sol-gel/emulsion approach. Microporous Mesoporous Mater. 2010, 127, 67–72. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, D.; Gong, F.; Gao, Y.; Yan, X.; Ma, G. Facile one-pot emulsion/sol-gel method for preparing wrinkled silica microspheres. Particuology 2021, 56, 33–42. [Google Scholar] [CrossRef]
- Arellano, A. Efectos Sobre las Propiedades Caracteristicas del Polipropileno (PP) Debido a la Incorporacion de Nano-Agentes Antioxidantes; Universidad de los Andes: Bogota, Colombia, 2019. [Google Scholar]
- Jabariyan, S.; Zanjanchi, M.A. A simple and fast sonication procedure to remove surfactant templates from mesoporous MCM-41. Ultrason. Sonochem. 2012, 19, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Jayanudin, M.; Fahrurrozi, S.K.; Rochmadi, W. Preparation of Chitosan Microcapsules Containing Red Ginger Oleoresin Using Emulsion Crosslinking Method. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001880991. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, T.; Rajeshwari, K.; Kanchana, V.; Sudha, P.N.; Parthasarathy, K. Impact of nanoparticle shape, size, and properties of the sustainable nanocomposites. In Sustainable Polymer Composites and Nanocomposites; Springer: Cham, Denmark, 2019; pp. 313–336. [Google Scholar]
- Ferdous, S. The Effects of Filler-Matrix Interface Strength, Filler Shape And Filler Dispersion On The Mechanical Properties of Polymer Nanocomposites. In Proceedings of the American Society for Composites, Arlington, TX, USA, 1–3 October 2012. [Google Scholar]
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Zeng, H.M.; Friedrich, K. Improvement of Tensile Properties of nano-SiO2/PP Composites in Relation to Percolation Mechanism. Polymer 2001, 57, 3301–3304. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Zhuang, T. Study on the rheological, crystallization, and mechanical properties of HDPE functionalized by nano-SiO 2. Synth. React. Inorganic Met. Nano-Metal Chem. 2012, 42, 1267–1272. [Google Scholar] [CrossRef]
- Gómez Alfonzo, R.H. Aceite de Linaza Encapsulado Mediante el Método Sol-Gel Para Uso Potencial en Empaques Activos Absorbedores de Oxígeno; Universidad de los Andes: Bogota, Colombia, 2018. [Google Scholar]
- Bogush, G.H.; Tracy, M.A.; Zukoski, C. F, IV. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non. Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, T.H.; Lee, M.H.; Song, H.K. Catalytic carbonization of an uncarbonizable precursor by transition metals in olivine cathode materials of lithium ion batteries. J. Mater. Chem. 2012, 22, 20305–20310. [Google Scholar] [CrossRef]
- Sharma, V.; Banait, J.S.; Larock, R.C.; Kundu, P.P. Morphological and thermal characterization of linseed-Oil based polymers from cationic and thermal polymerization. J. Polym. Environ. 2010, 18, 235–242. [Google Scholar] [CrossRef]
- Levi, S.; Rac, V.; Manojlovi, V.; Raki, V.; Bugarski, B.; Flock, T.; Krzyczmonik, K.E.; Nedovi, V. Limonene encapsulation in alginate/poly (vinyl alcohol). Procedia Food Sci. 2011, 1, 1816–1820. [Google Scholar] [CrossRef]
- Himed, L.; Merniz, S.; Monteagudo-olivan, R.; Barkat, M.; Coronas, J. Antioxidant activity of the essential oil of Citrus limon before and after its encapsulation in amorphous SiO2. Sci. African 2019, 6, e00181. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, G.; Huang, S.; Li, S.; Sun, J.; Zhang, D.; Qiu, S. Controlled release of Captopril by regulating the pore size and morphology of ordered mesoporous silica. Microporous Mesoporous Mater. 2006, 92, 1–9. [Google Scholar] [CrossRef]
- Hu, L.; Sun, C.; Song, A.; Chang, D.; Zheng, X.; Gao, Y.; Jiang, T.; Wang, S. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian J. Pharm. Sci. 2014, 9, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.F.; Tsui, G.C.P.; Tang, C.Y.; Yang, M. Optimization strategy for encapsulation efficiency and size of drug loaded silica xerogel/polymer core-shell composite nanoparticles prepared by gelation-emulsion method. Polym. Eng. Sci. 2018, 58, 742–751. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, H.M.; Lee, W.J.; Lee, C.W.; Kim, T.I.; Lee, J.K.; Jeong, J.; Paek, S.M.; Oh, J.M. Surface treatment of silica nanoparticles for stable and charge-controlled colloidal silica. Int. J. Nanomed. 2014, 9 (Suppl. 2), 29–40. [Google Scholar]
- Xu, P.; Wang, H.; Tong, R.; Du, Q.; Zhong, W. Preparation and morphology of SiO2/PMMA nanohybrids by microemulsion polymerization. Colloid Polym. Sci. 2006, 284, 755–762. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Jeziórska, R.; Zielecka, M.; Gutarowska, B.; Zakowska, Z. High-density polyethylene composites filled with nanosilica containing immobilized nanosilver or nanocopper: Thermal, mechanical, and bactericidal properties and morphology and interphase characterization. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Selvamani, V. Stability Studies on Nanomaterials Used in Drugs. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 425–444. [Google Scholar]
- Bracho, D.; Dougnac, V.N.; Palza, H.; Quijada, R. Functionalization of silica nanoparticles for polypropylene nanocomposite applications. J. Nanomater. 2012, 2012, 263915. [Google Scholar] [CrossRef]
- Orellana, F.; Lisperguer, J.; Nuñez, C. Synthesis and characterization of polypropylene-silica, alumina and titania nanoparticles, prepared by melting. J. Chil. Chem. Soc. 2014, 59, 2389–2393. [Google Scholar] [CrossRef]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of functionalized silica nanoparticles on the mass transfer process in active PLA nanocomposite films obtained by supercritical impregnation for sustainable food packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Burris, D.L.; Zhao, S.; Duncan, R.; Lowitz, J.; Perry, S.S.; Schadler, L.S.; Sawyer, W.G. A route to wear resistant PTFE via trace loadings of functionalized nanofillers. Wear 2009, 267, 653–660. [Google Scholar] [CrossRef]
- Sikora, A.; Bartczak, D.; Geissler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard Alexander, G.; Goenaga-Infante, H.; et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnology 2011, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinosos, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Fuertes, A.B.; Sevilla, M.; Titirici, M.M. The influence of pore size distribution on the oxygen reduction reaction performance in nitrogen doped carbon microspheres. J. Mater. Chem. A 2016, 4, 2581–2589. [Google Scholar] [CrossRef]
- Miltz, J.; Perry, M. Evaluation of the performance of iron-based oxygen scavengers, with comments on their optimal applications. Packag. Technol. Sci. 2005, 18, 21–27. [Google Scholar] [CrossRef]
- Brown, C.E. Coefficient of Variation. In Applied Multivariate Statistics in Geohydrology and Related Sciences; Springer: Berlin/Heidelberg, Germany, 1998; pp. 155–157. [Google Scholar]





| Parameter | Mean | Standard Deviation | CV α | Criteria Fulfilled |
|---|---|---|---|---|
| Mean(ND) (size) (nm) β | 122.67 | 2.69 | 0.03 | Yes |
| SD(ND) (size) (nm) β | 17.27 | 0.74 | 0.04 | Yes |
| Oil loading (%) | 34.03 | 2.34 | 0.07 | Yes |
| Oil retention (%) | 57.35 | 3.23 | 0.06 | Yes |
| Encapsulation efficiency (%) | 33.92 | 1.46 | 0.04 | Yes |
| ζ-potential (mV) | −56.05 | 1.19 | 0.02 | Yes |
| Mean pore diam. (nm) | 3.66 | 0.08 | 0.02 | Yes |
| Mean pore vol. (cc/g) | 0.64 | 0.08 | 0.12 | Yes |
| BET Area (m2/g) | 747.23 | 104.77 | 0.14 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, J.F.; Rozo, D.F.; Chaparro, L.M.; Medina, J.A.; Salcedo-Galán, F. Synthesis and Characterization of Reproducible Linseed Oil-Loaded Silica Nanoparticles with Potential Use as Oxygen Scavengers in Active Packaging. Nanomaterials 2022, 12, 3257. https://doi.org/10.3390/nano12183257
Alvarado JF, Rozo DF, Chaparro LM, Medina JA, Salcedo-Galán F. Synthesis and Characterization of Reproducible Linseed Oil-Loaded Silica Nanoparticles with Potential Use as Oxygen Scavengers in Active Packaging. Nanomaterials. 2022; 12(18):3257. https://doi.org/10.3390/nano12183257
Chicago/Turabian StyleAlvarado, Juan Felipe, Daniel Fernando Rozo, Luis Miguel Chaparro, Jorge Alberto Medina, and Felipe Salcedo-Galán. 2022. "Synthesis and Characterization of Reproducible Linseed Oil-Loaded Silica Nanoparticles with Potential Use as Oxygen Scavengers in Active Packaging" Nanomaterials 12, no. 18: 3257. https://doi.org/10.3390/nano12183257
APA StyleAlvarado, J. F., Rozo, D. F., Chaparro, L. M., Medina, J. A., & Salcedo-Galán, F. (2022). Synthesis and Characterization of Reproducible Linseed Oil-Loaded Silica Nanoparticles with Potential Use as Oxygen Scavengers in Active Packaging. Nanomaterials, 12(18), 3257. https://doi.org/10.3390/nano12183257







