Adverse Outcome Pathways Associated with the Ingestion of Titanium Dioxide Nanoparticles—A Systematic Review
Abstract
:1. Introduction
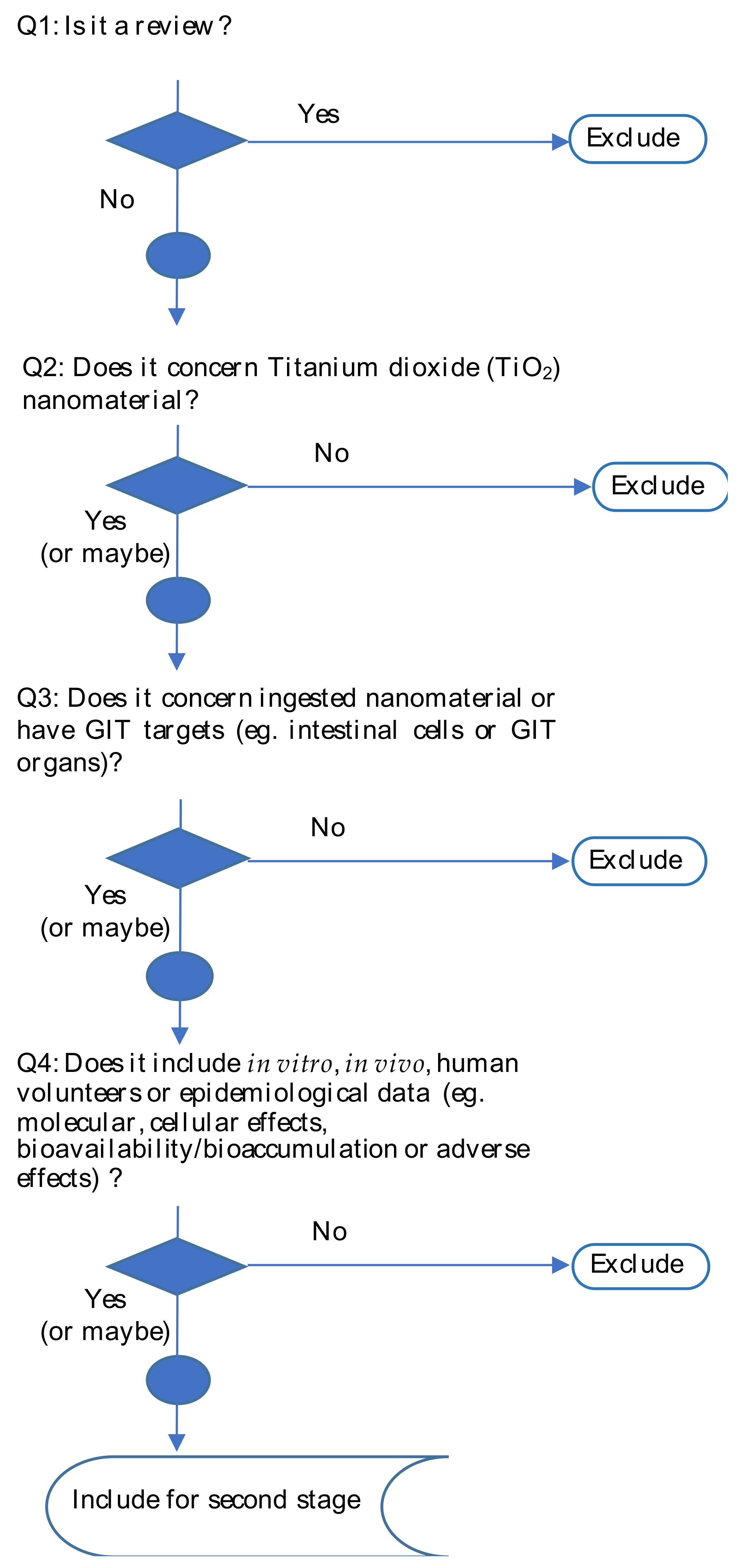
2. Materials and Methods
2.1. First Stage Screening
2.2. Stage II Screening
2.3. Setting up an AOP
3. Results and Discussion
3.1. Overview of the Results
3.2. Molecular and Cellular Effects
3.2.1. Cellular and Systemic Uptake
3.2.2. Oxidative Stress
3.2.3. Cell Death and Proliferation
3.2.4. Cell Signaling
3.2.5. Inflammation
3.2.6. Genotoxicity
3.2.7. Carcinogenicity
3.2.8. Biochemical and Other Physiological Parameters
3.2.9. Transcriptomics, Epigenomics and Proteomics
3.3. Adverse Outcomes
3.4. Towards an AOP Model for Ingested TiO2-NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vieira, A.; Gramacho, A.; Rolo, D.; Vital, N.; Silva, M.J.; Louro, H. Cellular and Molecular Mechanisms of Toxicity of Ingested Titanium Dioxide Nanomaterials. In Nanotoxicology in Safety Assessment of Nanomaterials; Springer Nature: Cham, Switzerland, 2022; pp. 225–257. [Google Scholar]
- Shi, H.B.; Magaye, R.; Castranova, V.; Zhao, J.S. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Comera, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Tache, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). EFSA Statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, 11. [Google Scholar] [CrossRef]
- European Commission. European Commission Regulation (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171) (Text with EEA Relevance); European Commission: Brussels, Belgium, 2022.
- EFSA Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585.
- Urrutia-Ortega, I.M.; Garduno-Balderas, L.G.; Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; Gonzalez-Robles, A.; Pedraza-Chaverri, J.; Hernandez-Pando, R.; Rodriguez-Sosa, M.; Leon-Cabrera, S.; et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef]
- Proquin, H.; Jetten, M.J.; Jonkhout, M.C.M.; Garduno-Balderas, L.G.; Briede, J.J.; de Kok, T.M.; Chirino, Y.I.; van Loveren, H. Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171). Food Chem. Toxicol. 2018, 111, 153–165. [Google Scholar] [CrossRef]
- Rompelberg, C.; Heringa, M.B.; van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; van Bemmel, G.; Brand, W.; Oomen, A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef]
- OECD. Users’ Handbook supplement to the Guidance Document for developing and assessing Adverse Outcome Pathways. In OECD Series on Adverse Outcome Pathways; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; LaLone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse outcome pathway development II: Best practices. Toxicol. Sci. 2014, 142, 321–330. [Google Scholar] [CrossRef]
- Louro, H.; Borges, T.; Silva, M.J. Manufactured nanomaterials—New challenges for public health. Rev. Port. Saúde Pública 2013, 31, 145–157. [Google Scholar]
- Rolo, D.; Tavares, A.; Vital, N.; Silva, M.J.; Louro, H. Overview of Adverse Outcome Pathways and Current Applications on Nanomaterials. In Nanotoxicology in Safety Assessment of Nanomaterials; Springer Nature: Cham, Switzerland, 2022; pp. 415–439. [Google Scholar]
- Braakhuis, H.M.; Gosens, I.; Heringa, M.B.; Oomen, A.G.; Vandebriel, R.J.; Groenewold, M.; Cassee, F.R. Mechanism of action of TiO2: Recommendations to reduce uncertainties related to carcinogenic potential. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 203–223. [Google Scholar] [CrossRef]
- Brand, W.; Peters, R.J.B.; Braakhuis, H.M.; Maślankiewicz, L.; Oomen, A.G. Possible effects of titanium dioxide particles on human liver, intestinal tissue, spleen and kidney after oral exposure. Nanotoxicology 2020, 14, 985–1007. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, S.; Mir, Z.M. Considerations for conducting systematic reviews: Evaluating the performance of different methods for de-duplicating references. Syst. Rev. 2021, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Jornod, F.; Jaylet, T.; Blaha, L.; Sarigiannis, D.; Tamisier, L.; Audouze, K. AOP-helpFinder webserver: A tool for comprehensive analysis of the literature to support adverse outcome pathways development. Bioinformatics 2022, 38, 1173–1175. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Iosi, F.; Maranghi, F.; Tassinari, R.; Cubadda, F.; Aureli, F.; Raggi, A.; Superti, F.; Mantovani, A.; De Berardis, B. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells. Food Chem. Toxicol. 2017, 102, 63–75. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Domingo, M.G.; Steimetz, T.; Ramos, E.; Paparella, M.L.; Olmedo, D.G. Migration of titanium dioxide microparticles and nanoparticles through the body and deposition in the gingiva: An experimental study in rats. Eur. J. Oral Sci. 2015, 123, 242–248. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Winn, L.M.; Afrooz, A.R.; Saleh, N.B.; Walker, V.K. The effect of TiO2 and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxicol. Appl. Pharm. 2011, 257, 429–436. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Wang, Y.; Zhao, L.; Hu, G.; Liu, J.; Feng, H.; Long, C.; Yan, T.; Zhou, S.; et al. Combined effect of titanium dioxide nanoparticles and glucose on the cardiovascular system in young rats after oral administration. J. Appl. Toxicol. 2019, 39, 590–602. [Google Scholar] [CrossRef]
- Heringa, M.B.; Peters, R.J.B.; Bleys, R.; van der Lee, M.K.; Tromp, P.C.; van Kesteren, P.C.E.; van Eijkeren, J.C.H.; Undas, A.K.; Oomen, A.G.; Bouwmeester, H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018, 15, 15. [Google Scholar] [CrossRef]
- Proquin, H.; Jonkhout, M.C.M.; Jetten, M.J.; van Loveren, H.; de Kok, T.M.; Briedé, J.J. Transcriptome changes in undifferentiated Caco-2 cells exposed to food-grade titanium dioxide (E171): Contribution of the nano- and micro- sized particles. Sci. Rep. 2019, 9, 18287. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Sayed, H.A. Hazards of nanotechnology: Effect of titanium dioxide nanoparticles on the liver and renal cortex of albino rats. An electron microscopic study. Egypt. J. Histol. 2013, 36, 389–399. [Google Scholar] [CrossRef]
- Faheem, N.M.; Elsaid, A.G. Amelioration of Titanium Dioxide nanoparticles induced injury on the cerebellum of the adult mice by a hydromethanolic root extract of Withania Somnifera. Egypt. J. Histol. 2018, 41, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Han, Y.; Li, F.; Li, Z.; McClements, D.J.; He, L.; Decker, E.A.; Xing, B.; Xiao, H. Impact of protein-nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: Not just simple protein corona effects. NanoImpact 2019, 13, 37–43. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, J.A.; Jo, M.R.; Choi, S.J. Bioavailability of Silica, Titanium Dioxide, and Zinc Oxide Nanoparticles in Rats. J. Nanosci. Nanotechnol. 2016, 16, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Bartel, L.K.; Hunter, D.A.; Anderson, K.B.; Yau, W.; Wu, J.; Gato, W.E. Short-term evaluation of hepatic toxicity of titanium dioxide nanofiber (TDNF). Drug Chem. Toxicol. 2019, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 1–44. [Google Scholar] [CrossRef]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010, 26, 225–238. [Google Scholar] [CrossRef]
- Farcal, L.; Torres Andón, F.; Di Cristo, L.; Rotoli, B.M.; Bussolati, O.; Bergamaschi, E.; Mech, A.; Hartmann, N.B.; Rasmussen, K.; Riego-Sintes, J.; et al. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials: First Steps towards an Intelligent Testing Strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar] [CrossRef]
- Jones, K.; Morton, J.; Smith, I.; Jurkschat, K.; Harding, A.H.; Evans, G. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2015, 233, 95–101. [Google Scholar] [CrossRef]
- Sohal, I.S.; DeLoid, G.M.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Effects of ingested food-grade titanium dioxide, silicon dioxide, iron (III) oxide and zinc oxide nanoparticles on an in vitro model of intestinal epithelium: Comparison between monoculture vs. a mucus-secreting coculture model. NanoImpact 2020, 17, 100209. [Google Scholar] [CrossRef]
- Cabellos, J.; Delpivo, C.; Fernández-Rosas, E.; Vázquez-Campos, S.; Janer, G. Contribution of M-cells and other experimental variables in the translocation of TiO2 nanoparticles across in vitro intestinal models. NanoImpact 2017, 5, 51–60. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedata, P.; Ricci, G.; Malorni, L.; Venezia, A.; Cammarota, M.; Volpe, M.G.; Iannaccone, N.; Guida, V.; Schiraldi, C.; Romano, M.; et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res. Int. 2019, 119, 634–642. [Google Scholar] [CrossRef]
- Faust, J.J.; Doudrick, K.; Yang, Y.; Westerhoff, P.; Capco, D.G. Food grade titanium dioxide disrupts intestinal brush border microvilli in vitro independent of sedimentation. Cell Biol. Toxicol. 2014, 30, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.W.; Shull, G.M.; Fountain, J.H.; Guo, Z.; Musselman, L.P.; Fiumera, A.C.; Mahler, G.J. Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and Drosophila melanogaster. Nanotoxicology 2018, 12, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Yan, G.; Deng, P.; Peng, F.; Lin, H.; Xu, Y.; Cao, Z.; Zhou, T.; Xue, A.; Wang, Y. NMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administration. Nanotechnology 2010, 21, 125105. [Google Scholar] [CrossRef]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef]
- Talbot, P.; Radziwill-Bienkowska, J.M.; Kamphuis, J.B.J.; Steenkeste, K.; Bettini, S.; Robert, V.; Noordine, M.L.; Mayeur, C.; Gaultier, E.; Langella, P.; et al. Food-grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: Implications for gut barrier protection. J. Nanobiotechnol. 2018, 16, 53. [Google Scholar] [CrossRef]
- Meyer, T.; Venus, T.; Sieg, H.; Böhmert, L.; Kunz, B.M.; Krause, B.; Jalili, P.; Hogeveen, K.; Chevance, S.; Gauffre, F. Simultaneous quantification and visualization of titanium dioxide nanomaterial uptake at the single cell level in an in vitro model of the human small intestine. Small Methods 2019, 3, 1800540. [Google Scholar] [CrossRef]
- Song, Z.M.; Chen, N.; Liu, J.H.; Tang, H.; Deng, X.; Xi, W.S.; Han, K.; Cao, A.; Liu, Y.; Wang, H. Biological effect of food additive titanium dioxide nanoparticles on intestine: An in vitro study. J. Appl. Toxicol. 2015, 35, 1169–1178. [Google Scholar] [CrossRef]
- Jo, M.R.; Yu, J.; Kim, H.J.; Song, J.H.; Kim, K.M.; Oh, J.M.; Choi, S.J. Titanium Dioxide Nanoparticle-Biomolecule Interactions Influence Oral Absorption. Nanomaterials 2016, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; Mas del Molino, E.; Fernández-Rosas, E.; Fernández, A.; Vázquez-Campos, S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef] [PubMed]
- MacNicoll, A.; Kelly, M.; Aksoy, H.; Kramer, E.; Bouwmeester, H.; Chaudhry, Q. A study of the uptake and biodistribution of nano-titanium dioxide using in vitro and in vivo models of oral intake. J. Nanopart. Res. 2015, 17, 66. [Google Scholar] [CrossRef]
- Veronesi, G.; Brun, E.; Fayard, B.; Cotte, M.; Carriere, M. Structural properties of rutile TiO2 nanoparticles accumulated in a model of gastrointestinal epithelium elucidated by micro-beam x-ray absorption fine structure spectroscopy. Appl. Phys. Lett. 2012, 100, 214101. [Google Scholar] [CrossRef]
- Zhou, H.; Pandya, J.K.; Tan, Y.; Liu, J.; Peng, S.; Muriel Mundo, J.L.; He, L.; Xiao, H.; McClements, D.J. Role of Mucin in Behavior of Food-Grade TiO2 Nanoparticles under Simulated Oral Conditions. J. Agric. Food Chem. 2019, 67, 5882–5890. [Google Scholar] [CrossRef]
- Tay, C.Y.; Fang, W.; Setyawati, M.I.; Chia, S.L.; Tan, K.S.; Hong, C.H.; Leong, D.T. Nano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epithelium. ACS Appl. Mater. Interfaces 2014, 6, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Modrzynska, J.; Berthing, T.; Ravn-Haren, G.; Kling, K.; Mortensen, A.; Rasmussen, R.R.; Larsen, E.H.; Saber, A.T.; Vogel, U.; Loeschner, K. In vivo-induced size transformation of cerium oxide nanoparticles in both lung and liver does not affect long-term hepatic accumulation following pulmonary exposure. PLoS ONE 2018, 13, e0202477. [Google Scholar] [CrossRef] [PubMed]
- Auttachoat, W.; McLoughlin, C.E.; White, K.L., Jr.; Smith, M.J. Route-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nanoparticles. J. Immunotoxicol. 2014, 11, 273–282. [Google Scholar] [CrossRef]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef]
- Farrell, T.P.; Magnuson, B. Absorption, Distribution and Excretion of Four Forms of Titanium Dioxide Pigment in the Rat. J. Food Sci. 2017, 82, 1985–1993. [Google Scholar] [CrossRef]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Luo, M.; Tan, Z.; Dai, M.Y.; Xie, M.Z.; Lin, J.; Hua, H.Y.; Ma, Q.; Zhao, J.S.; Liu, A.M. Oral administration of nano-titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environ. Toxicol. Pharmacol. 2017, 49, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.M.; Ibrahim, M.A.; Noshy, P.A. Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of Tiron in adult male rats. Biochem. Biophys. Res. Commun. 2017, 486, 595–600. [Google Scholar] [CrossRef]
- Talamini, L.; Gimondi, S.; Violatto, M.B.; Fiordaliso, F.; Pedica, F.; Tran, N.L.; Sitia, G.; Aureli, F.; Raggi, A.; Nelissen, I.; et al. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology 2019, 13, 1087–1101. [Google Scholar] [CrossRef]
- Martins, A.D.C., Jr.; Azevedo, L.F.; de Souza Rocha, C.C.; Carneiro, M.F.H.; Venancio, V.P.; de Almeida, M.R.; Antunes, L.M.G.; de Carvalho Hott, R.; Rodrigues, J.L.; Ogunjimi, A.T.; et al. Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co-exposed to silver and titanium dioxide nanoparticles. J. Toxicol. Environ. Health A 2017, 80, 1156–1165. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Guo, Q.; Jin, S.; Zhou, Y.; Oh, Y.; Feng, Y.; Wu, Q.; Gu, N. A mechanistic study to increase understanding of titanium dioxide nanoparticles-increased plasma glucose in mice. Food Chem. Toxicol. 2016, 95, 175–187. [Google Scholar] [CrossRef]
- Onishchenko, G.E.; Erokhina, M.V.; Abramchuk, S.S.; Shaitan, K.V.; Raspopov, R.V.; Smirnova, V.V.; Vasilevskaya, L.S.; Gmoshinski, I.V.; Kirpichnikov, M.P.; Tutelyan, V.A. Effects of titanium dioxide nanoparticles on small intestinal mucosa in rats. Bull. Exp. Biol. Med. 2012, 154, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Canli, E.G.; Gumus, C.; Canli, M.; Ila, H.B. The effects of titanium nanoparticles on enzymatic and non-enzymatic biomarkers in female Wistar rats. Drug Chem. Toxicol. 2022, 45, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Pridvorova, S.M.; Zherdev, A.V.; Klochkov, S.G.; Novikova, O.V.; Shevtsova, E.F.; Bachurin, S.O.; Dzantiev, B.B. Size-Dependent Differences in Biodistribution of Titanium Dioxide Nanoparticles After Sub-Acute Intragastric Administrations to Rats. Curr. Nanosci. 2016, 12, 228–236. [Google Scholar] [CrossRef]
- Canli, E.G.; Ila, H.B.; Canli, M. Response of the antioxidant enzymes of rats following oral administration of metal-oxide nanoparticles (Al2O3, CuO, TiO2). Environ. Sci. Pollut. Res. Int. 2019, 26, 938–945. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Zhao, X.; Sheng, L.; Wang, L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomed. 2017, 12, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Mohammadipour, A.; Haeri, P.; Ebrahimzadeh-Bideskan, A. The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran. J. Basic Med. Sci. 2019, 22, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Basu, N.; George, S. Dietary nanoparticles compromise epithelial integrity and enhance translocation and antigenicity of milk proteins: An in vitro investigation. NanoImpact 2021, 24, 100369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, S.; Liu, Y.; Wang, Y. The combined effect of food additive titanium dioxide and lipopolysaccharide on mouse intestinal barrier function after chronic exposure of titanium dioxide-contained feedstuffs. Part. Fibre Toxicol. 2021, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Coméra, C.; Cartier, C.; Gaultier, E.; Catrice, O.; Panouillé, Q.; El Hamdi, S.; Tirez, K.; Nelissen, I.; Theodorou, V.; Houdeau, E. Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food-grade TiO2 particles: An in vivo and ex vivo study in mice. Part. Fibre Toxicol. 2020, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Liu, Y.; Tang, Y.; Xu, X.; Wang, M.; Tao, X.; Xu, H. Pre-exposure to TiO2-NPs aggravates alcohol-related liver injury by inducing intestinal barrier damage in mice. Toxicol. Sci. 2022, 185, 28–37. [Google Scholar] [CrossRef]
- Yao, L.; Tang, Y.; Chen, B.; Hong, W.; Xu, X.; Liu, Y.; Aguilar, Z.P.; Xu, H. Oral exposure of titanium oxide nanoparticles induce ileum physical barrier dysfunction via Th1/Th2 imbalance. Environ. Toxicol. 2020, 35, 982–990. [Google Scholar] [CrossRef]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: In vivo and in vitro study. Int. J. Nanomed. 2019, 14, 1919–1936. [Google Scholar] [CrossRef]
- Jensen, D.M.; Løhr, M.; Sheykhzade, M.; Lykkesfeldt, J.; Wils, R.S.; Loft, S.; Møller, P. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis 2019, 34, 203–214. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; Yin, Y.; Guo, Q.; Yang, D.; Wei, X.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; et al. Mechanisms of titanium dioxide nanoparticle-induced oxidative stress and modulation of plasma glucose in mice. Environ. Toxicol. 2019, 34, 1221–1235. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brassinne, F.; Sebaihi, N.; Petry, J.; Cokic, S.M.; Van Landuyt, K.L.; Godderis, L.; Mast, J.; Lison, D.; Hoet, P.H. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part. Fibre Toxicol. 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Kumar, A.; Vallabani, N.V.; Pandey, A.K.; Dhawan, A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine 2014, 9, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.A.; Rizk, M.Z.; Hamed, M.A.; Aboul-Ela, E.I.; El-Rigal, N.S.; Aly, H.F.; Abdel-Hamid, A.Z. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: Effect of dose and particle size. Biomarkers 2019, 24, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Azim, S.A.A.; Darwish, H.A.; Rizk, M.Z.; Ali, S.A.; Kadry, M.O. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: Possible role of some antioxidants. Exp. Toxicol. Pathol. 2015, 67, 305–314. [Google Scholar] [CrossRef]
- Orazizadeh, M.; Fakhredini, F.; Mansouri, E.; Khorsandi, L. Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chem. Biol. Interact. 2014, 220, 214–221. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J.; et al. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef]
- Dorier, M.; Beal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef]
- Gandamalla, D.; Lingabathula, H.; Yellu, N. Nano titanium exposure induces dose- and size-dependent cytotoxicity on human epithelial lung and colon cells. Drug Chem. Toxicol. 2019, 42, 24–34. [Google Scholar] [CrossRef]
- Tada-Oikawa, S.; Ichihara, G.; Fukatsu, H.; Shimanuki, Y.; Tanaka, N.; Watanabe, E.; Suzuki, Y.; Murakami, M.; Izuoka, K.; Chang, J.; et al. Titanium Dioxide Particle Type and Concentration Influence the Inflammatory Response in Caco-2 Cells. Int. J. Mol. Sci. 2016, 17, 576. [Google Scholar] [CrossRef]
- Chalew, T.E.A.; Ajmani, G.S.; Huang, H.O.; Schwab, K.J. Evaluating Nanoparticle Breakthrough during Drinking Water Treatment. Environ. Health Perspect. 2013, 121, 1161–1166. [Google Scholar] [CrossRef]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jalili, P.; Gueniche, N.; Lanceleur, R.; Burel, A.; Lavault, M.T.; Sieg, H.; Bohmert, L.; Meyer, T.; Krause, B.C.; Lampen, A.; et al. Investigation of the in vitro genotoxicity of two rutile TiO2 nanomaterials in human intestinal and hepatic cells and evaluation of their interference with toxicity assays. NanoImpact 2018, 11, 69–81. [Google Scholar] [CrossRef]
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.W.; Förster, I.; Schins, R.P. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Danielsen, P.H.; Derr, R.; Moelijker, N.; Fowler, P.; Stone, V.; Hendriks, G.; Møller, P.; Kermanizadeh, A. The mechanism-based toxicity screening of particles with use in the food and nutrition sector via the ToxTracker reporter system. Toxicol. Vitr. 2019, 61, 104594. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Vital, N.; Rolo, D.; Roque, R.; Gonçalves, L.M.; Bettencourt, A.; Silva, M.J.; Louro, H. Investigation of the genotoxicity of digested titanium dioxide nanomaterials in human intestinal cells. Food Chem. Toxicol. 2022, 161, 112841. [Google Scholar] [CrossRef]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact 2017, 5, 70–82. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, T.; De Loid, G.M.; Gaffrey, M.J.; Weitz, K.K.; Thrall, B.D.; Qian, W.J.; Demokritou, P. Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model. NanoImpact 2020, 17, 1–27. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, P.; Han, S.; Zhang, J.; Li, Z.; Zhou, S.; Jia, G. Tissue-specific oxidative stress and element distribution after oral exposure to titanium dioxide nanoparticles in rats. Nanoscale 2020, 12, 20033–20046. [Google Scholar] [CrossRef]
- Waseem, M.; Kaushik, P.; Dutta, S.; Chakraborty, R.; Hassan, M.I.; Parvez, S. Modulatory Role of Quercetin in Mitochondrial Dysfunction in Titanium Dioxide Nanoparticle-Induced Hepatotoxicity. ACS Omega 2022, 7, 3192–3202. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef]
- Pereira, L.C.; Pazin, M.; Franco-Bernardes, M.F.; Martins, A.D.C., Jr.; Barcelos, G.R.M.; Pereira, M.C.; Mesquita, J.P.; Rodrigues, J.L.; Barbosa, F., Jr.; Dorta, D.J. A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs). J. Trace Elem. Med. Biol. 2018, 47, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Teubl, B.J.; Schimpel, C.; Leitinger, G.; Bauer, B.; Fröhlich, E.; Zimmer, A.; Roblegg, E. Interactions between nano-TiO2 and the oral cavity: Impact of nanomaterial surface hydrophilicity/hydrophobicity. J. Hazard. Mater. 2015, 286, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aker, W.G.; Hwang, H.M.; Yedjou, C.G.; Yu, H.; Tchounwou, P.B. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci. Total Environ. 2011, 409, 4753–4762. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice. Nanoscale Res. Lett. 2017, 12, 478. [Google Scholar] [CrossRef]
- McCracken, C.; Zane, A.; Knight, D.A.; Dutta, P.K.; Waldman, W.J. Minimal Intestinal Epithelial Cell Toxicity in Response to Short- and Long-Term Food-Relevant Inorganic Nanoparticle Exposure. Chem. Res. Toxicol. 2013, 26, 1514–1525. [Google Scholar] [CrossRef]
- Hassanein, K.M.A.; El-Amir, Y.O. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int. J. Vet. Sci. Med. 2018, 6, 16–21. [Google Scholar] [CrossRef]
- Canli, E.G.; Canli, M. Effects of aluminum, copper, and titanium nanoparticles on some blood parameters in Wistar rats. Turk. J. Zool. 2017, 41, 259–266. [Google Scholar] [CrossRef]
- Jensen, D.M.; Christophersen, D.V.; Sheykhzade, M.; Skovsted, G.F.; Lykkesfeldt, J.; Münter, R.; Roursgaard, M.; Loft, S.; Møller, P. Vasomotor function in rat arteries after ex vivo and intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Part. Fibre Toxicol. 2018, 15, 12. [Google Scholar] [CrossRef]
- Chen, X.X.; Cheng, B.; Yang, Y.X.; Cao, A.; Liu, J.H.; Du, L.J.; Liu, Y.; Zhao, Y.; Wang, H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small 2013, 9, 1765–1774. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Goyary, D.; Karmakar, S.; Chattopadhyay, P. Exploration of cytotoxic and genotoxic endpoints following sub-chronic oral exposure to titanium dioxide nanoparticles. Toxicol. Ind. Health 2019, 35, 577–592. [Google Scholar] [CrossRef]
- Giovanni, M.; Tay, C.Y.; Setyawati, M.I.; Xie, J.P.; Ong, C.N.; Fan, R.L.; Yue, J.Q.; Zhang, L.F.; Leong, D.T. Toxicity Profiling of Water Contextual Zinc Oxide, Silver, and Titanium Dioxide Nanoparticles in Human Oral and Gastrointestinal Cell Systems. Environ. Toxicol. 2015, 30, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Beer, H.D.; Hornung, V.; Krämer, U.; Schins, R.P.; Förster, I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology 2011, 5, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Westermann, M.; Glei, M. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch. Toxicol. 2017, 91, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Mechanistic Investigation of the Biological Effects of SiO2, TiO2, and ZnO Nanoparticles on Intestinal Cells. Small 2015, 11, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Cancer Therapeutic Effects of Titanium Dioxide Nanoparticies Are Associated with Oxidative Stress and Cytokine induction. Pathobiology 2015, 82, 243–251. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Guo, Q.; Zong, H.; Yan, Y.; Yin, Y.; Wang, Y.; Oh, Y.; Feng, Y.; Wu, Q.; et al. RNA sequencing analysis shows that titanium dioxide nanoparticles induce endoplasmic reticulum stress, which has a central role in mediating plasma glucose in mice. Nanotoxicology 2018, 12, 341–356. [Google Scholar] [CrossRef]
- Cai, J.; Zang, X.; Wu, Z.; Liu, J.; Wang, D. Translocation of transition metal oxide nanoparticles to breast milk and offspring: The necessity of bridging mother-offspring-integration toxicological assessments. Environ. Int. 2019, 133, 105153. [Google Scholar] [CrossRef]
- Ibrahim, R.; Salem, M.Y.; Helal, O.K.; El-Monem, A.; Sahar, N. Effect of titanium dioxide nanoparticles on the spleen of adult male albino rats: Histological and immunohistochemical study. Egypt. J. Histol. 2018, 41, 311–328. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, X.; Zhou, Y.; Yu, D.; Deng, Y.; Ouyang, J.; Yang, B.; Luo, D.; Zhang, D.; Kuang, H. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int. J. Nanomed. 2018, 13, 777–789. [Google Scholar] [CrossRef]
- Shahin, N.N.; Mohamed, M.M. Nano-sized titanium dioxide toxicity in rat prostate and testis: Possible ameliorative effect of morin. Toxicol. Appl. Pharm. 2017, 334, 129–141. [Google Scholar] [CrossRef]
- Susewind, J.; Carvalho-Wodarz, C.D.; Repnik, U.; Collnot, E.M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2016, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, K.; Albrecht, C.; Boots, A.W.; Forster, I.; Schins, R.P.F. Cytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco-2 cells. Nanotoxicology 2009, 3, 355–364. [Google Scholar] [CrossRef]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’Amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef]
- Nejad, S.M.; Takahashi, H.; Hosseini, H.; Watanabe, A.; Endo, H.; Narihira, K.; Kikuta, T.; Tachibana, K. Acute effects of sono-activated photocatalytic titanium dioxide nanoparticles on oral squamous cell carcinoma. Ultrason. Sonochem. 2016, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Wilson, C.L.; Hayward, S.L.; Kidambi, S. Titanium Dioxide Nanoparticles Trigger Loss of Function and Perturbation of Mitochondrial Dynamics in Primary Hepatocytes. PLoS ONE 2015, 10, e0134541. [Google Scholar] [CrossRef]
- Shrestha, S.; Mao, Z.W.; Fedutik, Y.; Gao, C.Y. Influence of titanium dioxide nanorods with different surface chemistry on the differentiation of rat bone marrow mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 6955–6966. [Google Scholar] [CrossRef]
- Kim, D.H.; Kundu, J.; Chae, I.G.; Lee, J.K.; Heo, J.S.; Chun, K.S. Titanium dioxide nanoparticles induce COX-2 expression through ROS generation in human periodontal ligament cells. J. Toxicol. Sci. 2019, 44, 335–345. [Google Scholar] [CrossRef]
- Agnihothram, S.; Mullis, L.; Townsend, T.A.; Watanabe, F.; Mustafa, T.; Biris, A.; Manjanatha, M.G.; Azevedo, M.P. Titanium Dioxide Nanoparticles Evoke Proinflammatory Response during Murine Norovirus Infection Despite Having Minimal Effects on Virus Replication. Int. J. Nanotechnol. Eng. Med. 2016, 1, 63–73. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Kanda, Y.; Nakajima, H.; Sakagami, H. Induction of prostaglandin E2 production by TiO2 nanoparticles in human gingival fibroblast. In Vivo 2014, 28, 217–222. [Google Scholar]
- Teubl, B.J.; Leitinger, G.; Schneider, M.; Lehr, C.M.; Frohlich, E.; Zimmer, A.; Roblegg, E. The buccal mucosa as a route for TiO2 nanoparticle uptake. Nanotoxicology 2015, 9, 253–261. [Google Scholar] [CrossRef]
- Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the characteristics and cytotoxicity of titanium dioxide nanomaterials following simulated In Vitro digestion. Nanomaterials 2020, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, F.; Zhang, H. In vitro response of immune cells on metal oxide nanoparticles with different solubility. J. Nanosci. Nanotechnol. 2016, 16, 5546–5552. [Google Scholar] [CrossRef] [PubMed]
- Jalili, P.; Krause, B.-C.; Lanceleur, R.; Burel, A.; Jungnickel, H.; Lampen, A.; Laux, P.; Luch, A.; Fessard, V.; Hogeveen, K. Chronic effects of two rutile TiO2 nanomaterials in human intestinal and hepatic cell lines. Part. Fibre Toxicol. 2022, 19, 37. [Google Scholar] [CrossRef]
- Park, S.B.; Jung, W.H.; Kim, K.Y.; Koh, B. Toxicity assessment of SiO2 and TiO2 in normal colon cells, in vivo and in human colon organoids. Molecules 2020, 25, 3594. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Jetten, M.J.; Jonkhout, M.C.M.; Garduno-Balderas, L.G.; Briede, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Transcriptomics analysis reveals new insights in E171-induced molecular alterations in a mouse model of colon cancer. Sci. Rep. 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipour, A.; Fazel, A.; Haghir, H.; Motejaded, F.; Rafatpanah, H.; Zabihi, H.; Hosseini, M.; Bideskan, A.E. Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ. Toxicol. Pharm. 2014, 37, 617–625. [Google Scholar] [CrossRef]
- Chen, C.W.; Huang, J.H.; Lai, T.C.; Jan, Y.H.; Hsiao, M.; Chen, C.H.; Hwu, Y.K.; Liu, R.S. Evaluation of the intracellular uptake and cytotoxicity effect of TiO2 nanostructures for various human oral and lung cells under dark conditions. Toxicol. Res. 2016, 5, 303–311. [Google Scholar] [CrossRef]
- Ebrahimzadeh Bideskan, A.; Mohammadipour, A.; Fazel, A.; Haghir, H.; Rafatpanah, H.; Hosseini, M.; Rajabzadeh, A. Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis. Exp. Toxicol. Pathol. 2017, 69, 329–337. [Google Scholar] [CrossRef]
- Evans, S.M.; Ashwood, P.; Warley, A.; Berisha, F.; Thompson, R.P.; Powell, J.J. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology 2002, 123, 1543–1553. [Google Scholar] [CrossRef]
- Huang, C.; Sun, M.; Yang, Y.; Wang, F.; Ma, X.; Li, J.; Wang, Y.; Ding, Q.; Ying, H.; Song, H.; et al. Titanium dioxide nanoparticles prime a specific activation state of macrophages. Nanotoxicology 2017, 11, 737–750. [Google Scholar] [CrossRef]
- Watari, F.; Abe, S.; Koyama, C.; Yokoyama, A.; Akasaka, T.; Uo, M.; Matsuoka, M.; Totsuka, Y.; Esaki, M.; Morita, M. Behavior of in vitro, in vivo and internal motion of micro/nano particles of titanium, titanium oxides and others. J. Ceram. Soc. Jpn. 2008, 116, 1–5. [Google Scholar] [CrossRef]
- Powell, J.J.; Harvey, R.S.; Ashwood, P.; Wolstencroft, R.; Gershwin, M.E.; Thompson, R.P. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J. Autoimmun. 2000, 14, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Jarvis, K.; Coffman, S.S. Association of Type 2 Diabetes with Submicron Titanium Dioxide Crystals in the Pancreas. Chem. Res. Toxicol. 2018, 31, 506–509. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef]
- Blevins, L.K.; Crawford, R.B.; Bach, A.; Rizzo, M.D.; Zhou, J.; Henriquez, J.E.; Khan, D.; Sermet, S.; Arnold, L.L.; Pennington, K.L.; et al. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2). Food. Chem. Toxicol. 2019, 133, 110793. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, D.; Zhou, S.; Jia, G. Gender difference in hepatic toxicity of titanium dioxide nanoparticles after subchronic oral exposure in Sprague-Dawley rats. J. Appl. Toxicol. 2019, 39, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Grissa, I.; Ezzi, L.; Chakroun, S.; Mabrouk, A.; Ben Saleh, A.; Braham, H.; Haouas, Z.; Ben Cheikh, H. Rosmarinus officinalis L. ameliorates titanium dioxide nanoparticles and induced some toxic effects in rats’ blood. Environ. Sci. Pollut. Res. 2017, 24, 12474–12483. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guo, Q.; Wang, C.; Ma, X.; He, H.; Oh, Y.; Feng, Y.; Wu, Q.; Gu, N. Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. J. Appl. Toxicol. 2015, 35, 1122–1132. [Google Scholar] [CrossRef]
- Moradi, A.; Ziamajidi, N.; Ghafourikhosroshahi, A.; Abbasalipourkabir, R. Effects of vitamin A and vitamin E on attenuation of titanium dioxide nanoparticles-induced toxicity in the liver of male Wistar rats. Mol. Biol. Rep. 2019, 46, 2919–2932. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Zhuo, L.; Chen, S.; Zhao, L.; Luan, X.; Wang, H.; Jia, G. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicol. Lett. 2015, 239, 123–130. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Faddah, L.M.; Mohamed, A.M.; Abdel Baky, N.A.; Mohammad, R.A. Potential impact of quercetin and idebenone against immuno- inflammatory and oxidative renal damage induced in rats by titanium dioxide nanoparticles toxicity. J. Oleo Sci. 2013, 62, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wu, N.; Zhou, Y.; Ji, L.; Chen, T.; Wang, L. Gastric toxicity involving alterations of gastritis-related protein expression in mice following long-term exposure to nano TiO2. Food Res. Int. 2017, 95, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, S.A.; Darwish, H.A.; Ali, S.A.; Rizk, M.Z.; Kadry, M.O. Potential antifibrotic and angiostatic impact of idebenone, carnosine and vitamin E in nano-sized titanium dioxide-induced liver injury. Cell Physiol. Biochem. 2015, 35, 2402–2411. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, B.; Li, L.; Guo, Q.; Yang, D.; Wei, X.; Fan, X.; Liu, J.; Wu, Q.; Oh, Y.; et al. The toxic effects of titanium dioxide nanoparticles on plasma glucose metabolism are more severe in developing mice than in adult mice. Environ. Toxicol. 2020, 35, 443–456. [Google Scholar] [CrossRef]
- Elnagar, A.M.B.; Ibrahim, A.; Soliman, A.M. Histopathological Effects of Titanium Dioxide Nanoparticles and the Possible Protective Role of N-Acetylcysteine on The Testes of Male Albino Rats. Int. J. Fertil. Steril. 2018, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Grissa, I.; ElGhoul, J.; Mrimi, R.; Mir, L.E.; Cheikh, H.B.; Horcajada, P. In deep evaluation of the neurotoxicity of orally administered TiO2 nanoparticles. Brain Res. Bull. 2020, 155, 119–128. [Google Scholar] [CrossRef]
- Morgan, A.; Galal, M.K.; Ogaly, H.A.; Ibrahim, M.A.; Abd-Elsalam, R.M.; Noshy, P. Tiron ameliorates oxidative stress and inflammation in titanium dioxide nanoparticles induced nephrotoxicity of male rats. Biomed. Pharmacother. 2017, 93, 779–787. [Google Scholar] [CrossRef]
- Kaiser, J.P.; Roesslein, M.; Diener, L.; Wick, P. Human health risk of ingested nanoparticles that are added as multifunctional agents to paints: An in vitro study. PLoS ONE 2013, 8, e83215. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Tisseyre, C.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Rabilloud, T.; Carrière, M. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ. Sci. Nano 2019, 6, 1549–1561. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.M.; de Azevedo, W.M.; Dagli, M.L.; Toma, S.H.; Leite, A.Z.; Lordello, M.L.; Nishitokukado, I.; Ortiz-Agostinho, C.L.; Duarte, M.I.; Ferreira, M.A.; et al. Titanium dioxide induced inflammation in the small intestine. World J. Gastroenterol. 2012, 18, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Donner, E.M.; Myhre, A.; Brown, S.C.; Boatman, R.; Warheit, D.B. In vivo micronucleus studies with 6 titanium dioxide materials (3 pigment-grade & 3 nanoscale) in orally-exposed rats. Regul. Toxicol. Pharmacol. 2016, 74, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.T.; Safwat, G.M. Grape Seed Proanthocyanidin Extract Mitigates Titanium Dioxide Nanoparticle (TiO2-NPs)-Induced Hepatotoxicity through TLR-4/NF-κB Signaling Pathway. Biol. Trace Elem. Res. 2020, 196, 579–589. [Google Scholar] [CrossRef]
- Hong, J.; Wang, L.; Zhao, X.; Yu, X.; Sheng, L.; Xu, B.; Liu, D.; Zhu, Y.; Long, Y.; Hong, F. Th2 factors may be involved in TiO2 NP-induced hepatic inflammation. J. Agric. Food Chem. 2014, 62, 6871–6878. [Google Scholar] [CrossRef]
- Elbastawisy, Y.M.; Almasry, S.M. Histomorphological evaluation of maternal and neonatal distal airspaces after maternal intake of nanoparticulate titanium dioxide: An experimental study in Wistar rats. J. Mol. Histol. 2014, 45, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Gato, W.E.; Hunter, D.A.; Byrd, I.C.; Mays, C.A.; Yau, W.; Wu, J. Assessment of the short-term toxicity of TiO2 nanofiber in Sprague Dawley rats. Environ. Toxicol. 2017, 32, 1775–1783. [Google Scholar] [CrossRef]
- Mohamed, H.R.H. Estimation of TiO2 nanoparticle-induced genotoxicity persistence and possible chronic gastritis-induction in mice. Food Chem. Toxicol. 2015, 83, 76–83. [Google Scholar] [CrossRef]
- Vasantharaja, D.; Ramalingam, V.; Reddy, G.A. Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Nanomed. J. 2015, 2, 46–53. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Marcus, I.M.; Guysi, R.L.; Walker, S.L. Metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota. Environ. Eng. Sci. 2015, 32, 602–612. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Scarcello, E.; Ibouraadaten, S.; Yakoub, Y.; Leinardi, R.; Ambroise, J.; Bearzatto, B.; Gala, J.-L.; Paquot, A.; Muccioli, G.G. Dietary nanoparticles alter the composition and function of the gut microbiota in mice at dose levels relevant for human exposure. Food Chem. Toxicol. 2021, 154, 112352. [Google Scholar] [CrossRef]
- Kurtz, C.C.; Mitchell, S.; Nielsen, K.; Crawford, K.D.; Mueller-Spitz, S.R. Acute high-dose titanium dioxide nanoparticle exposure alters gastrointestinal homeostasis in mice. J. Appl. Toxicol. 2020, 40, 1384–1395. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Cheng, S.; Fan, J.; Qin, X.; Wang, T.; Zhang, Y.; Zhang, J.; Qiu, Y.; Qiu, J. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Arch. Toxicol. 2020, 94, 1173–1190. [Google Scholar] [CrossRef]
- Bredeck, G.; Kämpfer, A.A.M.; Sofranko, A.; Wahle, T.; Lison, D.; Ambroise, J.; Stahlmecke, B.; Albrecht, C.; Schins, R.P.F. Effects of dietary exposure to the engineered nanomaterials CeO2, SiO2, Ag, and TiO2 on the murine gut microbiome. Nanotoxicology 2021, 15, 934–950. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wang, Y.; Ba, T.; Li, Y.; Pu, J.; Chen, T.; Song, Y.S.; Gu, Y.G.; Qian, Q.; Yang, J.L.; et al. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicol. Lett. 2014, 226, 314–319. [Google Scholar] [CrossRef]
- Louro, H.; Tavares, A.; Vital, N.; Costa, P.M.; Alverca, E.; Zwart, E.; de Jong, W.H.; Fessard, V.; Lavinha, J.; Silva, M.J. Integrated Approach to the In Vivo Genotoxic Effects of a Titanium Dioxide Nanomaterial Using LacZ Plasmid-Based Transgenic Mice. Environ. Mol. Mutagenes. 2014, 55, 500–509. [Google Scholar] [CrossRef]
- Grissa, I.; Elghoul, J.; Ezzi, L.; Chakroun, S.; Kerkeni, E.; Hassine, M.; El Mir, L.; Mehdi, M.; Ben Cheikh, H.; Haouas, Z. Anemia and genotoxicity induced by sub-chronic intragastric treatment of rats with titanium dioxide nanoparticles. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2015, 794, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sycheva, L.P.; Zhurkov, V.S.; Iurchenko, V.V.; Daugel-Dauge, N.O.; Kovalenko, M.A.; Krivtsova, E.K.; Durnev, A.D. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2011, 726, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, J.; Banerjee, R.; Mukherjee, A. Genotoxicity analysis of rutile titanium dioxide nanoparticles in mice after 28 days of repeated oral administration. Nucleus 2020, 63, 17–24. [Google Scholar] [CrossRef]
- Botelho, M.C.; Costa, C.; Silva, S.; Costa, S.; Dhawan, A.; Oliveira, P.A.; Teixeira, J.P. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro. Biomed. Pharmacother. 2014, 68, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kurzawa-Zegota, M.; Sharma, V.; Najafzadeh, M.; Reynolds, P.D.; Davies, J.P.; Shukla, R.K.; Dhawan, A.; Anderson, D. Titanium Dioxide Nanoparticles Induce DNA Damage in Peripheral Blood Lymphocytes from Polyposis coli, Colon Cancer Patients and Healthy Individuals: An Ex Vivo/In Vitro Study. J. Nanosci. Nanotechnol. 2017, 17, 9274–9285. [Google Scholar] [CrossRef]
- Bischoff, N.S.; Proquin, H.; Jetten, M.J.; Schrooders, Y.; Jonkhout, M.C.M.; Briedé, J.J.; van Breda, S.G.; Jennen, D.G.J.; Medina-Reyes, E.I.; Delgado-Buenrostro, N.L. The Effects of the Food Additive Titanium Dioxide (E171) on Tumor Formation and Gene Expression in the Colon of a Transgenic Mouse Model for Colorectal Cancer. Nanomaterials 2022, 12, 1256. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Díaz-Urbina, D.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; González, M.I.; Reyes, J.L.; Villamar-Duque, T.E.; Flores-Sánchez, M.L.O.; Hernández-Pando, R. Food-grade titanium dioxide (E171) induces anxiety, adenomas in colon and goblet cells hyperplasia in a regular diet model and microvesicular steatosis in a high fat diet model. Food Chem. Toxicol. 2020, 146, 111786. [Google Scholar] [CrossRef]
- Heo, M.B.; Kwak, M.; An, K.S.; Kim, H.J.; Ryu, H.Y.; Lee, S.M.; Song, K.S.; Kim, I.Y.; Kwon, J.-H.; Lee, T.G. Oral toxicity of titanium dioxide P25 at repeated dose 28-day and 90-day in rats. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef]
- Han, H.Y.; Yang, M.J.; Yoon, C.; Lee, G.H.; Kim, D.W.; Kim, T.W.; Kwak, M.; Heo, M.B.; Lee, T.G.; Kim, S. Toxicity of orally administered food-grade titanium dioxide nanoparticles. J. Appl. Toxicol. 2021, 41, 1127–1147. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Zhuo, L.; Chen, S.; Zhao, L.; Chen, T.; Li, Y.; Zhang, W.; Gao, X.; Li, P.; et al. Interaction of titanium dioxide nanoparticles with glucose on young rats after oral administration. Nanomedicine 2015, 11, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.F.; Soliman, M.M.; Abdel-Rahman, G.H.; Nassan, M.A.; Ismail, S.A.; Farouk, M.; Solcan, C. Hepatoprotective effect of N-acetylcystiene on the toxic hazards of titanium dioxide nanoparticles. Am. J. Pharmacol. Toxicol. 2013, 8, 141. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Díaz-Urbina, D.; Déciga-Alcaraz, A.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Pedraza-Chaverri, J. Food-grade titanium dioxide decreases hematocrit and hemoglobin and increases compulsive-like behavior in male mice. J. Appl. Toxicol. 2022, 42, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zheng, P.; Zhou, D.; Zhou, S.; Jia, G. Effect of oral exposure to titanium dioxide nanoparticles on lipid metabolism in Sprague-Dawley rats. Nanoscale 2020, 12, 5973–5986. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Wang, X.; Zhuo, L.; Chen, S.; Tang, S.; Zhao, L.; Luan, X.; Jia, G. Effect of titanium dioxide nanoparticles on glucose homeostasis after oral administration. J. Appl. Toxicol. 2018, 38, 810–823. [Google Scholar] [CrossRef]
- Rodríguez-Escamilla, J.C.; Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Flores-Flores, J.O.; Ganem-Rondero, A.; Rodríguez-Sosa, M.; Terrazas, L.I.; Delgado-Buenrostro, N.L.; Chirino, Y.I. Food-grade titanium dioxide (E171) by solid or liquid matrix administration induces inflammation, germ cells sloughing in seminiferous tubules and blood-testis barrier disruption in mice. J. Appl. Toxicol. 2019, 39, 1586–1605. [Google Scholar] [CrossRef]
- Abu Zeid, E.H.; Alam, R.T.M.; Abd El-Hameed, N.E. Impact of titanium dioxide on androgen receptors, seminal vesicles and thyroid hormones of male rats: Possible protective trial with aged garlic extract. Andrologia 2017, 49, 5. [Google Scholar] [CrossRef]
- Sharafutdinova, L.A.; Fedorova, A.M.; Bashkatov, S.A.; Sinel’nikov, K.N.; Valiullin, V.V. Structural and Functional Analysis of the Spermatogenic Epithelium in Rats Exposed to Titanium Dioxide Nanoparticles. Bull. Exp. Biol. Med. 2018, 166, 279–282. [Google Scholar] [CrossRef]
- Hong, F.; Wang, L. Nanosized titanium dioxide-induced premature ovarian failure is associated with abnormalities in serum parameters in female mice. Int. J. Nanomed. 2018, 13, 2543–2549. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Pathmasiri, W.; Snyder, R.W.; Caffaro, M.M.; Watson, S.L.; Patel, P.R.; Beeravalli, L.; Prattipati, S.; Aravamudhan, S.; Sumner, S.J. Oral administration of TiO2 nanoparticles during early life impacts cardiac and neurobehavioral performance and metabolite profile in an age-and sex-related manner. Part. Fibre Toxicol. 2022, 19, 3. [Google Scholar] [CrossRef]
- Halawa, A.; Elshopakey, G.; El-Adl, M.; Lashen, S.; Shalaby, N.; Eldomany, E.; Farghali, A.; Rezk, S. Chitosan attenuated the neurotoxicity-induced titanium dioxide nanoparticles in brain of adult rats. Environ. Toxicol. 2022, 37, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Karimipour, M.; Javanmard, M.Z.; Ahmadi, A.; Jafari, A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 2018, 16, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipour, A.; Hosseini, M.; Fazel, A.; Haghir, H.; Rafatpanah, H.; Pourganji, M.; Bideskan, A.E. The effects of exposure to titanium dioxide nanoparticles during lactation period on learning and memory of rat offspring. Toxicol. Ind. Health 2016, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, J.S.; Kim, S.Y.; Park, M.K.; Choi, S.D.; Kim, U.J.; Park, K.; Jeong, E.J.; Nam, S.Y.; Yu, W.J. Titanium dioxide nanoparticles oral exposure to pregnant rats and its distribution. Part. Fibre Toxicol. 2019, 16, 31. [Google Scholar] [CrossRef]
- Warheit, D.B.; Boatman, R.; Brown, S.C. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Regul. Toxicol. Pharm. 2015, 73, 887–896. [Google Scholar] [CrossRef]
- El Dine, F.; Hussein, H.; Ghazala, R.A. Evaluation of epigenetic changes of liver tissue induced by oral administration of Titanium dioxide nanoparticles and possible protective role of Nigella Sativa oil, in adult male albino rats. Nanomed. J. 2018, 5, 192–198. [Google Scholar] [CrossRef]
- Dorier, M.; Tisseyre, C.; Dussert, F.; Béal, D.; Arnal, M.E.; Douki, T.; Valdiglesias, V.; Laffon, B.; Fraga, S.; Brandão, F.; et al. Toxicological impact of acute exposure to E171 food additive and TiO2 nanoparticles on a co-culture of Caco-2 and HT29-MTX intestinal cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2019, 845, 402980. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Q.; Tian, L.; Li, K.; Lai, W.; Bian, L.; Han, J.; Jia, R.; Liu, X.; Xi, Z. Intestinal toxicity of micro-and nano-particles of foodborne titanium dioxide in juvenile mice: Disorders of gut microbiota–host co-metabolites and intestinal barrier damage. Sci. Total Environ. 2022, 821, 153279. [Google Scholar] [CrossRef]
- Mohamadzadeh, N.; Javanmard, M.Z.; Karimipour, M.; Farjah, G. Developmental toxicity of the neural tube induced by titanium dioxide nanoparticles in mouse embryos. Avicenna J. Med. Biotechnol. 2021, 13, 74. [Google Scholar] [CrossRef]
- Kulthong, K.; Hooiveld, G.J.E.J.; Duivenvoorde, L.P.M.; Miro Estruch, I.; Bouwmeester, H.; van der Zande, M. Comparative study of the transcriptomes of Caco-2 cells cultured under dynamic vs. static conditions following exposure to titanium dioxide and zinc oxide nanomaterials. Nanotoxicology 2021, 15, 1233–1252. [Google Scholar] [CrossRef]
- Barreau, F.; Tisseyre, C.; Ménard, S.; Ferrand, A.; Carriere, M. Titanium dioxide particles from the diet: Involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part. Fibre Toxicol. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-M.; Zhang, Y.-L.; Gao, Y.-J.; Lyu, L.-Z.; Wang, Y. The influence of long-term dietary intake of titanium dioxide particles on elemental homeostasis and tissue structure of mouse organs. J. Nanosci. Nanotechnol. 2021, 21, 5014–5025. [Google Scholar] [CrossRef] [PubMed]
- Heringa, M.B.; Geraets, L.; van Eijkeren, J.C.; Vandebriel, R.J.; de Jong, W.H.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef] [Green Version]
- Sallam, M.F.; Ahmed, H.; El-Nekeety, A.A.; Diab, K.A.; Abdel-Aziem, S.H.; Sharaf, H.A.; Abdel-Wahhab, M.A. Assessment of the Oxidative Damage and Genotoxicity of Titanium Dioxide Nanoparticles and Exploring the Protective Role of Holy Basil Oil Nanoemulsions in Rats. Biol. Trace Elem. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Chen, L.; Nie, P.; Yao, L.; Tang, Y.; Hong, W.; Liu, W.; Fu, F.; Xu, H. TiO2 NPs induce the reproductive toxicity in mice with gestational diabetes mellitus through the effects on the endoplasmic reticulum stress signaling pathway. Ecotoxicol. Environ. Saf. 2021, 226, 112814. [Google Scholar] [CrossRef]
- Danafar, A.; Khoradmehr, A.; Bondarabadi, M.H.; Mazaheri, F.; Tamadon, A.; Pourmasoumi, S.; Gholizadeh, L.; Moshrefi, M.; Halvaei, I.; Hosseini, A. Impairment of sperm efficiency in mice following short-term nano-titanium dioxide exposure: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 1045. [Google Scholar] [CrossRef] [PubMed]
- Keramati Khiarak, B.; Karimipour, M.; Ahmadi, A.; Farjah, G.H. Effects of oral administration of titanium dioxide particles on sperm parameters and in vitro fertilization potential in mice: A comparison between nano-and fine-sized particles. Vet. Res. Forum 2020, 11, 401–408. [Google Scholar]
- Su, J.; Duan, X.; Qiu, Y.; Zhou, L.; Zhang, H.; Gao, M.; Liu, Y.; Zou, Z.; Qiu, J.; Chen, C. Pregnancy exposure of titanium dioxide nanoparticles causes intestinal dysbiosis and neurobehavioral impairments that are not significant postnatally but emerge in adulthood of offspring. J. Nanobiotechnol. 2021, 19, 234. [Google Scholar] [CrossRef]
- El-Bestawy, E.M.; Tolba, A.M. Effects of titanium dioxide nanoparticles on the myocardium of the adult albino rats and the protective role of β-carotene (histological, immunohistochemical and ultrastructural study). J. Mol. Histol. 2020, 51, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, N.S.; de Kok, T.M.; Sijm, D.T.H.M.; van Breda, S.G.; Briedé, J.J.; Castenmiller, J.J.M.; Opperhuizen, A.; Chirino, Y.I.; Dirven, H.; Gott, D. Possible adverse effects of food additive E171 (titanium dioxide) related to particle specific human toxicity, including the immune system. Int. J. Mol. Sci. 2020, 22, 207. [Google Scholar] [CrossRef]
- Hong, J.; Hong, F.; Ze, Y.; Zhang, Y.-Q. The nano-TiO2 exposure can induce hepatic inflammation involving in a JAK–STAT signalling pathway. J. Nanopart. Res. 2016, 18, 162. [Google Scholar] [CrossRef]
- Konstantinova, V.; Ibrahim, M.; Lie, S.A.; Birkeland, E.S.; Neppelberg, E.; Marthinussen, M.C.; Costea, D.E.; Cimpan, M.R. Nano-TiO2 penetration of oral mucosa: In vitro analysis using 3D organotypic human buccal mucosa models. J. Oral Pathol. Med. 2017, 46, 214–222. [Google Scholar] [CrossRef]
- Gitrowski, C.; Al-Jubory, A.R.; Handy, R.D. Uptake of different crystal structures of TiO2 nanoparticles by Caco-2 intestinal cells. Toxicol. Lett. 2014, 226, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Pele, L.C.; Thoree, V.; Bruggraber, S.F.; Koller, D.; Thompson, R.P.; Lomer, M.C.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fibre Toxicol. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, Z.J.; Ba, T.; Pu, J.; Chen, T.; Song, Y.S.; Gu, Y.G.; Qian, Q.; Xu, Y.Y.; Xiang, K.; et al. Susceptibility of Young and Adult Rats to the Oral Toxicity of Titanium Dioxide Nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Gaiser, B.K.; Hutchison, G.R.; Stone, V. An in vitro liver model—Assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part. Fibre Toxicol. 2012, 9, 13. [Google Scholar] [CrossRef]
- Jafari, A.; Karimipour, M.; Khaksar, M.R.; Ghasemnejad-Berenji, M. Protective effects of orally administered thymol against titanium dioxide nanoparticle-induced testicular damage. Environ. Sci Pollut. Res. Int. 2020, 27, 2353–2360. [Google Scholar] [CrossRef]
- Shrivastava, R.; Raza, S.; Yadav, A.; Kushwaha, P.; Flora, S.J. Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem. Toxicol. 2014, 37, 336–347. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Sevencan, C.; Bay, B.H.; Xie, J.; Zhang, Y.; Demokritou, P.; Leong, D.T. Nano-TiO2 Drives Epithelial-Mesenchymal Transition in Intestinal Epithelial Cancer Cells. Small 2018, 14, e1800922. [Google Scholar] [CrossRef]
- Khorsandi, L.; Orazizadeh, M.; Mansori, E.; Fakhredini, F. Glycyrrhizic acid attenuated lipid peroxidation induced by titanium dioxide nanoparticles in rat liver. Bratisl. Lek. Listy 2015, 116, 383–388. [Google Scholar] [CrossRef]
- Cao, X.; DeLoid, G.M.; Bitounis, D.; De La Torre-Roche, R.; White, J.C.; Zhang, Z.; Ho, C.G.; Ng, K.W.; Eitzer, B.D.; Demokritou, P. Co-exposure to the food additives SiO2 (E551) or TiO2 (E171) and the pesticide boscalid increases cytotoxicity and bioavailability of the pesticide in a tri-culture small intestinal epithelium model: Potential health implications. Environ. Sci. Nano 2019, 6, 2786–2800. [Google Scholar] [CrossRef]
- Ansari, M.; Kurian, G.A. Differential effect of aqueous Desmodium gangeticum root extract mediated TiO2 nanoparticles on isolated mitochondria, cells and Wistar rats. Asian Pac. J. Trop. Biomed. 2017, 7, 1031–1035. [Google Scholar] [CrossRef]
- Canli, E.G.; Atli, G.; Canli, M. Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ. Toxicol. Pharm. 2017, 50, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Filippi, C.; Pryde, A.; Cowan, P.; Lee, T.; Hayes, P.; Donaldson, K.; Plevris, J.; Stone, V. Toxicology of ZnO and TiO2 nanoparticles on hepatocytes: Impact on metabolism and bioenergetics. Nanotoxicology 2015, 9, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Niu, Y.J.; Li, Y.W.; Zhao, C.F.; Song, B.; Li, Y.; Zhou, Y.K. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ. Toxicol. Pharmacol. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Gu, N.; Hu, H.; Guo, Q.; Jin, S.; Wang, C.; Oh, Y.; Feng, Y.; Wu, Q. Effects of oral administration of titanium dioxide fine-sized particles on plasma glucose in mice. Food Chem. Toxicol. 2015, 86, 124–131. [Google Scholar] [CrossRef]
- Morgan, A.; Ibrahim, M.A.; Galal, M.K.; Ogaly, H.A.; Abd-Elsalam, R.M. Innovative perception on using Tiron to modulate the hepatotoxicity induced by titanium dioxide nanoparticles in male rats. Biomed. Pharm. 2018, 103, 553–561. [Google Scholar] [CrossRef]
- Zhou, Y.; Hong, F.; Wu, N.; Ji, J.; Cui, Y.; Li, J.; Zhuang, J.; Wang, L. Suppression of ovarian follicle development by nano TiO2 is associated with TGF-β-mediated signaling pathways. J. Biomed. Mater. Res. A 2019, 107, 414–422. [Google Scholar] [CrossRef]
- Abu-Dief, E.E.; Abdel-Aziz, H.; Nor-Eldin, E.K.; Khalil, K.M.; Ragab, E.E. Ultrastructural, histochemical and biochemical effects of titanium dioxide nanoparticles on adult male albino rat liver and possible prophylactic effects of milk thistle seeds. Egypt. J. Histol. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- El-Din, E.A.A.; Mostafa, H.E.; Samak, M.A.; Mohamed, E.M.; El-Shafei, D.A. Could curcumin ameliorate titanium dioxide nanoparticles effect on the heart? A histopathological, immunohistochemical, and genotoxic study. Environ. Sci. Pollut. Res. Int. 2019, 26, 21556–21564. [Google Scholar] [CrossRef]
- Hashem, M.M.; Abo-El-Sooud, K.; Abd-Elhakim, Y.M.; Badr, Y.A.; El-Metwally, A.E.; Bahy-El-Dien, A. The long-term oral exposure to titanium dioxide impaired immune functions and triggered cytotoxic and genotoxic impacts in rats. J. Trace Elem. Med. Biol. 2020, 60, 126473. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Spremo-Potparević, B.; Bajić, V.; Živković, L.; Topalović, D.; Sredojević, D.N.; Lazić, V.; Nedeljković, J.M. Acute toxicity study in mice of orally administrated TiO2 nanoparticles functionalized with caffeic acid. Food Chem. Toxicol. 2018, 115, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.R.H.; Hussien, N.A. Genotoxicity Studies of Titanium Dioxide Nanoparticles (TiO2NPs) in the Brain of Mice. Scientifica 2016, 2016, 6710840. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| TiO2 NM Characteristics | Categories * | No. |
|---|---|---|
| Crystalline phase | Anatase | 81 |
| Mixture | 39 | |
| Rutile | 23 | |
| NA | 59 | |
| Size (nm) | <25 | 104 |
| 25–50 | 54 | |
| 50–100 | 45 | |
| >100 | 36 | |
| NA | 23 | |
| Hydrodynamic size (DLS size, nm) | 25–50 | 9 |
| >100 | 50 | |
| NA | 127 | |
| Specific Surface Area (SSA, m2/g) | <50 | 31 |
| 50–100 | 28 | |
| >100 | 20 | |
| NA | 122 | |
| Surface charge (mV) | Negative | 44 |
| Positive | 22 | |
| NA | 127 |
| Type of Study | Type of Cells/Model | Organ/Cell Target | No. |
|---|---|---|---|
| In vivo | |||
| Murine | |||
| Liver | 31 | ||
| Blood | 20 | ||
| Spleen | 16 | ||
| Kidney | 13 | ||
| Intestine | 12 | ||
| Other cell types | 11 | ||
| Nonmurine | |||
| 6 | |||
| In vitro | |||
| Human | |||
| GIT-related cells | 38 | ||
| Other cell types | 16 | ||
| Murine | 15 | ||
| Human Volunteers | |||
| GIT-related cells | 3 | ||
| Other cell types | 7 |
| Adverse Outcome | Supporting References |
|---|---|
| Colorectal cancer | [7,8,19,129,178,180,181,205] |
| Liver injury | [23,40,56,72,78,98,119,143,184,185,206,207,208] |
| Reproductive toxicity | [70,114,190,191,192,193,194,195,196,197,198,199,200,201,202,203,209,210,211,212] |
| Cardiac damage | [40,184,185,194,213] |
| Kidney damage | [72,145,151,184,185] |
| Haematological effects | [40,56,185,187] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolo, D.; Assunção, R.; Ventura, C.; Alvito, P.; Gonçalves, L.; Martins, C.; Bettencourt, A.; Jordan, P.; Vital, N.; Pereira, J.; et al. Adverse Outcome Pathways Associated with the Ingestion of Titanium Dioxide Nanoparticles—A Systematic Review. Nanomaterials 2022, 12, 3275. https://doi.org/10.3390/nano12193275
Rolo D, Assunção R, Ventura C, Alvito P, Gonçalves L, Martins C, Bettencourt A, Jordan P, Vital N, Pereira J, et al. Adverse Outcome Pathways Associated with the Ingestion of Titanium Dioxide Nanoparticles—A Systematic Review. Nanomaterials. 2022; 12(19):3275. https://doi.org/10.3390/nano12193275
Chicago/Turabian StyleRolo, Dora, Ricardo Assunção, Célia Ventura, Paula Alvito, Lídia Gonçalves, Carla Martins, Ana Bettencourt, Peter Jordan, Nádia Vital, Joana Pereira, and et al. 2022. "Adverse Outcome Pathways Associated with the Ingestion of Titanium Dioxide Nanoparticles—A Systematic Review" Nanomaterials 12, no. 19: 3275. https://doi.org/10.3390/nano12193275






