Avoidance Behaviour of Six Collembolan Species Shows Species-Specific Sensitivity—Impact of Ag NM300K
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Test Soil
2.3. Test Material and Spiking
2.4. Materials Characterization
2.5. Experimental Procedure
2.6. Data Analysis
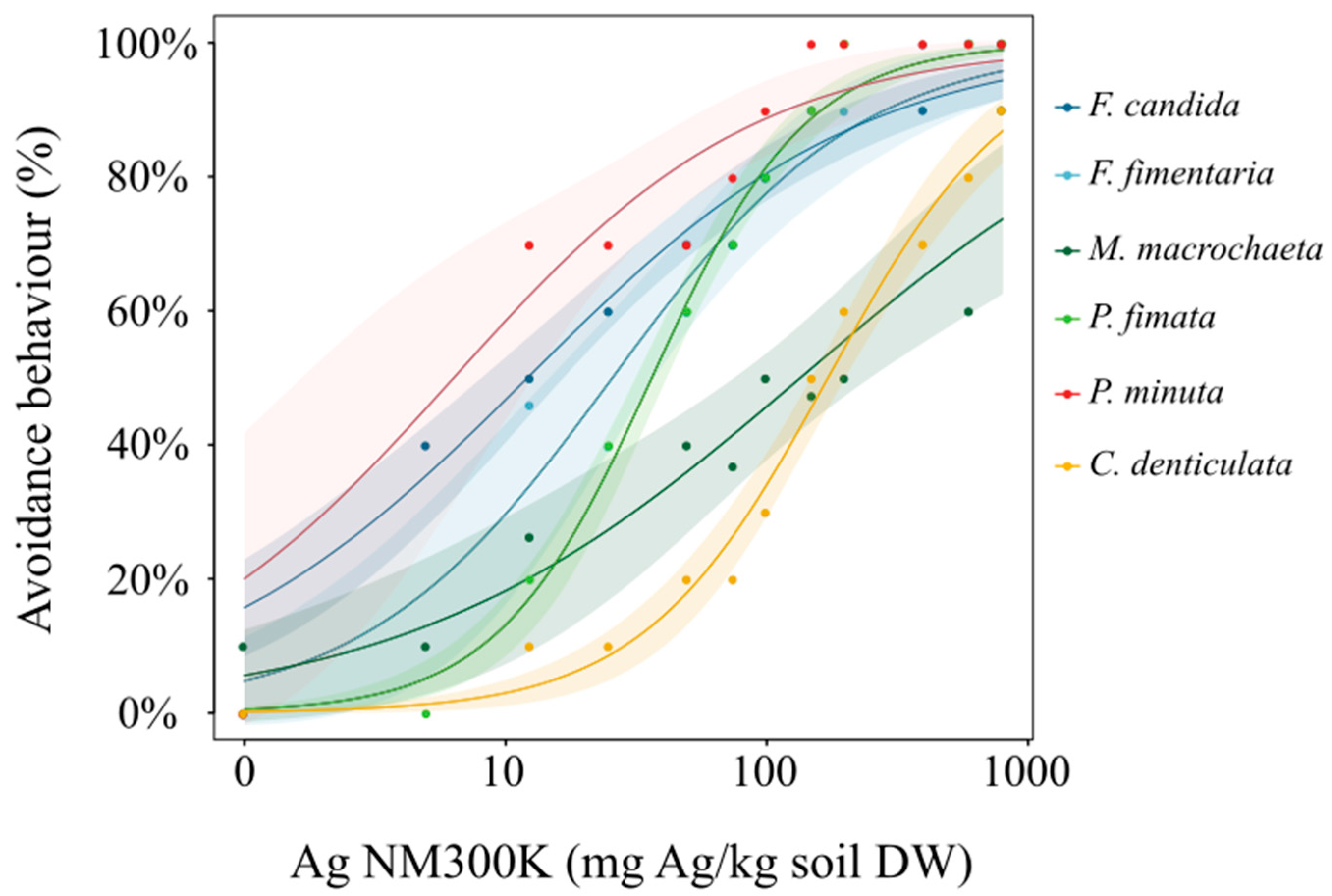
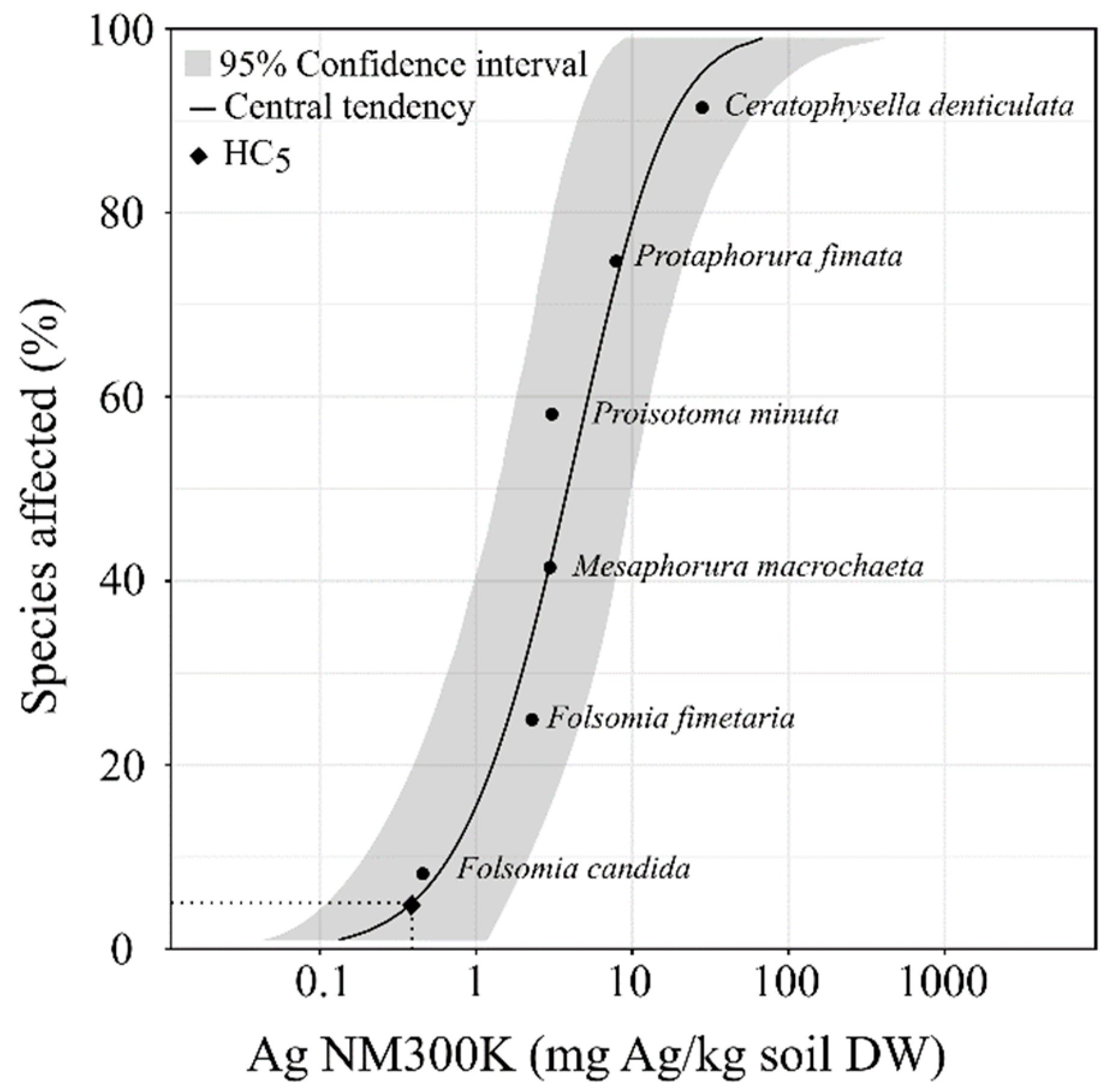
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: New York, NY, USA, 1997; p. 341. [Google Scholar]
- Potapov, A.M.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A.V. Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Potapov, A.; Bellini, B.; Chown, S.; Deharveng, L.; Janssens, F.; Kováč, Ľ.; Kuznetsova, N.; Ponge, J.-F.; Potapov, M.; Querner, P.; et al. Towards a global synthesis of Collembola knowledge: Challenges and potential solutions. Soil Org. 2020, 92, 161–188. [Google Scholar] [CrossRef]
- Heupel, K. Avoidance response of different collembolan species to Betanal. Eur. J. Soil Biol. 2002, 38, 273–276. [Google Scholar] [CrossRef]
- Ockleford, C.; Adriaanse, P.; Berny, P.; Brock, T.; Duquesne, S.; Grilli, S.; Hernandez-Jerez, A.F.; Bennekou, S.H.; Klein, M.; Kuhl, T.; et al. Scientific opinion addressing the state of the science on risk assessment of plant protection products for in-soil organisms. EFSA J. 2017, 15, e04690. [Google Scholar] [CrossRef]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Health Res. 2015, 12, 5581–5602. [Google Scholar] [CrossRef]
- Schlich, K.; Hoppe, M.; Kraas, M.; Fries, E.; Hund-Rinke, K. Ecotoxicity and fate of a silver nanomaterial in an outdoor lysimeter study. Ecotoxicology 2017, 26, 738–751. [Google Scholar] [CrossRef]
- Geranio, L.; Heuberger, M.; Nowack, B. The Behaviour of silver nanotextiles during washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef]
- Cleveland, D.; Long, S.E.; Pennington, P.L.; Cooper, E.; Fulton, M.H.; Scott, G.I.; Brewer, T.; Davis, J.; Petersen, E.J.; Wood, L. Pilot estuarine mesocosm study on the environmental fate of Silver nanomaterials leached from consumer products. Sci. Total Environ. 2012, 421, 267–272. [Google Scholar] [CrossRef]
- Mariyadas, J.; Amorim, M.J.; Jensen, J.; Scott-Fordsmand, J.J. Earthworm avoidance of silver nanomaterials over time. Environ. Pollut. 2018, 239, 751–756. [Google Scholar] [CrossRef]
- Gomes, S.I.; Scott-Fordsmand, J.J.; Amorim, M.J. Cellular energy allocation to assess the impact of nanomaterials on soil invertebrates (Enchytraeids): The effect of Cu and Ag. Int. J. Environ. Health Res. 2015, 12, 6858–6878. [Google Scholar] [CrossRef]
- Gomes, S.I.; Roca, C.P.; Scott-Fordsmand, J.J.; Amorim, M.J. High-throughput transcriptomics reveals uniquely affected pathways: AgNPs, PVP-coated AgNPs and Ag NM300K case studies. Environ. Sci. Nano 2017, 4, 929–937. [Google Scholar] [CrossRef]
- Bicho, R.C.; Santos, F.C.; Scott-Fordsmand, J.J.; Amorim, M.J. Multigenerational effects of copper nanomaterials (CuONMs) are different of those of CuCl2: Exposure in the soil invertebrate Enchytraeus crypticus. Sci. Rep. 2017, 7, 8457. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Alternative test methods for (nano)materials hazards assessment: Challenges and recommendations for regulatory preparedness. Nano Today 2021, 40, 101242. [Google Scholar] [CrossRef]
- Waalewijn-Kool, P.L.; Klein, K.; Forniés, R.M.; van Gestel, C.A. Bioaccumulation and toxicity of silver nanoparticles and silver nitrate to the soil arthropod Folsomia candida. Ecotoxicology 2014, 23, 1629–1637. [Google Scholar] [CrossRef]
- Mendes, L.A.; Maria, V.L.; Scott-Fordsmand, J.J.; Amorim, M.J. Ag nanoparticles (Ag NM300K) in the terrestrial environment: Effects at population and cellular level in Folsomia candida (Collembola). Int. J. Environ. Health Res. 2015, 12, 12530–12542. [Google Scholar] [CrossRef]
- Mendes, L.A.; Maria, V.L.; Scott-Fordsmand, J.J.; Amorim, M.J. Multigenerational exposure of Folsomia candida to silver: Effect of different contamination scenarios (continuous versus pulsed and recovery). Sci. Total Environ. 2018, 631, 326–333. [Google Scholar] [CrossRef]
- Hlavkova, D.; Beklova, M.; Kopel, P.; Havelkova, B. Effects of silver nanoparticles and ions exposure on the soil invertebrates Folsomia candida and Enchytraeus crypticus. Bull. Environ. Contam. Toxicol. 2020, 105, 244–249. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Maraldo, K.; van den Brink, P.J. The toxicity of copper contaminated soil using a gnotobiotic Soil Multi-species Test System (SMS). Environ. Int. 2008, 34, 524–530. [Google Scholar] [CrossRef]
- Jensen, J.; Scott-Fordsmand, J.J. Ecotoxicity of the veterinary pharmaceutical ivermectin tested in a soil multi-species (SMS) system. Environ. Pollut. 2012, 171, 133–139. [Google Scholar] [CrossRef]
- Schnug, L.; Jensen, J.; Scott-Fordsmand, J.J.; Leinaas, H.P. Toxicity of three biocides to springtails and earthworms in a soil multi-species (SMS) test system. Soil Biol. Biochem. 2014, 74, 115–126. [Google Scholar] [CrossRef]
- Sechi, V.; D’Annibale, A.; Maraldo, K.; Johansen, A.; Bossi, R.; Jensen, J.; Krogh, P.H. Species composition of a soil invertebrate multi-species test system determines the level of ecotoxicity. Environ. Pollut. 2014, 184, 586–596. [Google Scholar] [CrossRef]
- ISO 17512-2:2011; International Standard Determining the Quality of Soils and Effects of Chemicals on Behaviour. Part2: Test with Collembolans (Folsomia candida). International Organization for Standardization: Geneve, Switzerland, 2011.
- Erktan, A.; Or, D.; Scheu, S. The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biol. Biochem. 2020, 148, 107876. [Google Scholar] [CrossRef]
- Salmon, S.; Rebuffat, S.; Prado, S.; Sablier, M.; D’Haese, C.; Sun, J.-S.; Ponge, J.-F. Chemical communication in springtails: A review of facts and perspectives. Biol. Fertil. 2019, 55, 425–438. [Google Scholar] [CrossRef]
- Hagvar, S. Navigation and behaviour of four Collembola species migrating on the snow surface. Pedobiologia 2000, 44, 221–233. [Google Scholar] [CrossRef]
- Klein, C.L.; Comero, S.; Stahlmecke, B.; Romazanov, J.; Kuhlbusch, T.A.J.; Doren, E.; Van Mast, P.D.T.J.; Wick, P.; Krug, H.; Locoro, G.; et al. NM-Series of Representative Manufactured Nanomaterials nm-300 Silver Characterisation, Stability, Homogeneity; EUR—Scientific and Technical Research Reports, JRC Publication 2011. No. JRC60709, EUR; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Weeks, J.M.; Hopkin, S.P. Importance of contamination history for understanding toxicity of copper to earthworm Eisenia fetida (Oligochaeta: Annelida), using neutral-red retention assay. Environ. Toxicol. Chem. 2000, 19, 1774–1780. [Google Scholar] [CrossRef]
- Ritz, C.; Strebig, J. Package ‘drc’: Analysis of Dose-Response Curves, Version 2.5-12. 2015. Available online: https://cran.r-project.org/web/packages/drc/drc.pdf (accessed on 1 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 June 2022).
- R Studio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2022; Available online: http://www.rstudio.com (accessed on 1 June 2022).
- Fountain, M.T.; Hopkin, S.P. A comparative study of the effects of metal contamination on Collembola in the field and in the laboratory. Ecotoxicology 2004, 13, 573–587. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Yan, X.; Chang, L.; Wu, D. Euedaphic and hemiedaphic Collembola suffer larger damages than epedaphic species to nitrogen input. Environ. Pollut. 2016, 208, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Filser, J.; Wittmann, R.; Lang, A. Response types in Collembola towards copper in the microenvironment. Environ. Pollut. 2000, 107, 71–78. [Google Scholar] [CrossRef]
- Joseph, S.V.; Bettiga, C.; Ramirez, C.; Soto-Adames, F.N. Evidence of Protaphorura fimata (Collembola: Poduromorpha: Onychiuridae) feeding on germinating lettuce in the Salinas Valley of California. J. Econ. Entomol. 2015, 108, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Nickerl, J.; Helbig, R.; Schulz, H.J.; Werner, C.; Neinhuis, C. Diversity and potential correlations to the function of Collembola cuticle structures. Zoomorphology 2013, 132, 183–195. [Google Scholar] [CrossRef]
- Holmstrup, M.; Slotsbo, S.; Rozsypal, J.; Henriksen, P.G.; Bayley, M. Accumulation of free amino acids during exposure to drought in three springtail species. J. Insect Physiol. 2015, 82, 114–121. [Google Scholar] [CrossRef]
- Cole, L.J.; McCracken, D.I.; Foster, G.N.; Aitken, M.N. Using Collembola to assess the risks of applying metal-rich sewage sludge to agricultural land in western Scotland. Agric. Ecosyst. Environ. 2001, 83, 177–189. [Google Scholar] [CrossRef]
- Köser, J.; Engelke, M.; Hoppe, M.; Nogowski, A.; Filser, J.; Thöming, J. Predictability of silver nanoparticle speciation and toxicity in ecotoxicological media. Environ. Sci. Nano 2017, 4, 1470–1483. [Google Scholar] [CrossRef]
- Kleiven, M.; Rossbach, L.M.; Gallego-Urrea, J.A.; Brede, D.A.; Oughton, D.H.; Coutris, C. Characterizing the behavior, uptake, and toxicity of NM300K silver nanoparticles in Caenorhabditis elegans. Environ. Toxicol. Chem. 2018, 37, 1799–1810. [Google Scholar] [CrossRef]

| Species | Family | Length (mm) | Habitat | Furca |
|---|---|---|---|---|
| Folsomia candida | Isotomidae | 1.5–2.2 | euedaphic | well developed |
| Folsomia fimetaria | Isotomidae | 0.8–1.3 | euedaphic | well developed |
| Proisotoma minuta | Isotomidae | 1–1.2 | hemiedaphic | well developed |
| Mesaphorura macrochaeta | Onychiuridae | 0.6–0.8 | euedaphic | without |
| Protaphorura fimata | Onychiuridae | 0.7–1.4 | euedaphic | without |
| Ceratophysella denticulata | Hypogastruridae | 0.9–2.1 | epiedaphic | well developed |
| Species | EC10 | EC50 | S | p | Habitat Preference |
|---|---|---|---|---|---|
| Folsomia candida | 0.46 (0.1–0.9) | 12 (8–16) | 0.67 (0.74–0.59) | <0.0000 | euedaphic |
| Folsomia fimentaria | 2.3 (0.5–5) | 25 (14–36) | 1.06 (1.11–1.05) | 0.0014 | euedaphic |
| Proisotoma minuta | 3 (3–6) | 15 (7–22) | 0.75 (0.84–0.63) | 0.0012 | hemiedaphic |
| Mesaprophorura macrochaeta | 3 (2–8) | 135 (67–202) | 0.57 (0.66–0.45) | 0.0009 | euedaphic |
| Protaphorura fimata | 8 (5.5–10) | 36 (31–41) | 1.46 (1.57–1.34) | 0.0000 | euedaphic |
| Ceratophysella denticulate | 28 (18–38) | 171 (146–196) | 1.22 (1.34–1.11) | <0.0000 | epiedaphic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, M.; Amorim, M.J.B.; Hackenberger, B.K.; Scott-Fordsmand, J.J. Avoidance Behaviour of Six Collembolan Species Shows Species-Specific Sensitivity—Impact of Ag NM300K. Nanomaterials 2022, 12, 3276. https://doi.org/10.3390/nano12193276
Kovačević M, Amorim MJB, Hackenberger BK, Scott-Fordsmand JJ. Avoidance Behaviour of Six Collembolan Species Shows Species-Specific Sensitivity—Impact of Ag NM300K. Nanomaterials. 2022; 12(19):3276. https://doi.org/10.3390/nano12193276
Chicago/Turabian StyleKovačević, Marija, Mónica J. B. Amorim, Branimir K. Hackenberger, and Janeck J. Scott-Fordsmand. 2022. "Avoidance Behaviour of Six Collembolan Species Shows Species-Specific Sensitivity—Impact of Ag NM300K" Nanomaterials 12, no. 19: 3276. https://doi.org/10.3390/nano12193276
APA StyleKovačević, M., Amorim, M. J. B., Hackenberger, B. K., & Scott-Fordsmand, J. J. (2022). Avoidance Behaviour of Six Collembolan Species Shows Species-Specific Sensitivity—Impact of Ag NM300K. Nanomaterials, 12(19), 3276. https://doi.org/10.3390/nano12193276







