Temperature Control of Yellow Photoluminescence from SiO2-Coated ZnO Nanocrystals
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis of Zinc Oxide Nanocrystals
2.2. Coating of ZnO Nanocrystals with SiO2
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. High-Resolution Transmission Microscopy (HR-TEM)
2.5. Energy-Dispersive X-ray Spectroscopy (EDX)
2.6. Powder X-ray Diffraction Analysis (XRD)
2.7. Size Distribution and Zeta Potential Analysis
2.8. Diffuse Reflectance Spectroscopy (DRS)
2.9. Photoluminescence (PL) and Photoluminescence-Excitation (PLE) Measurements
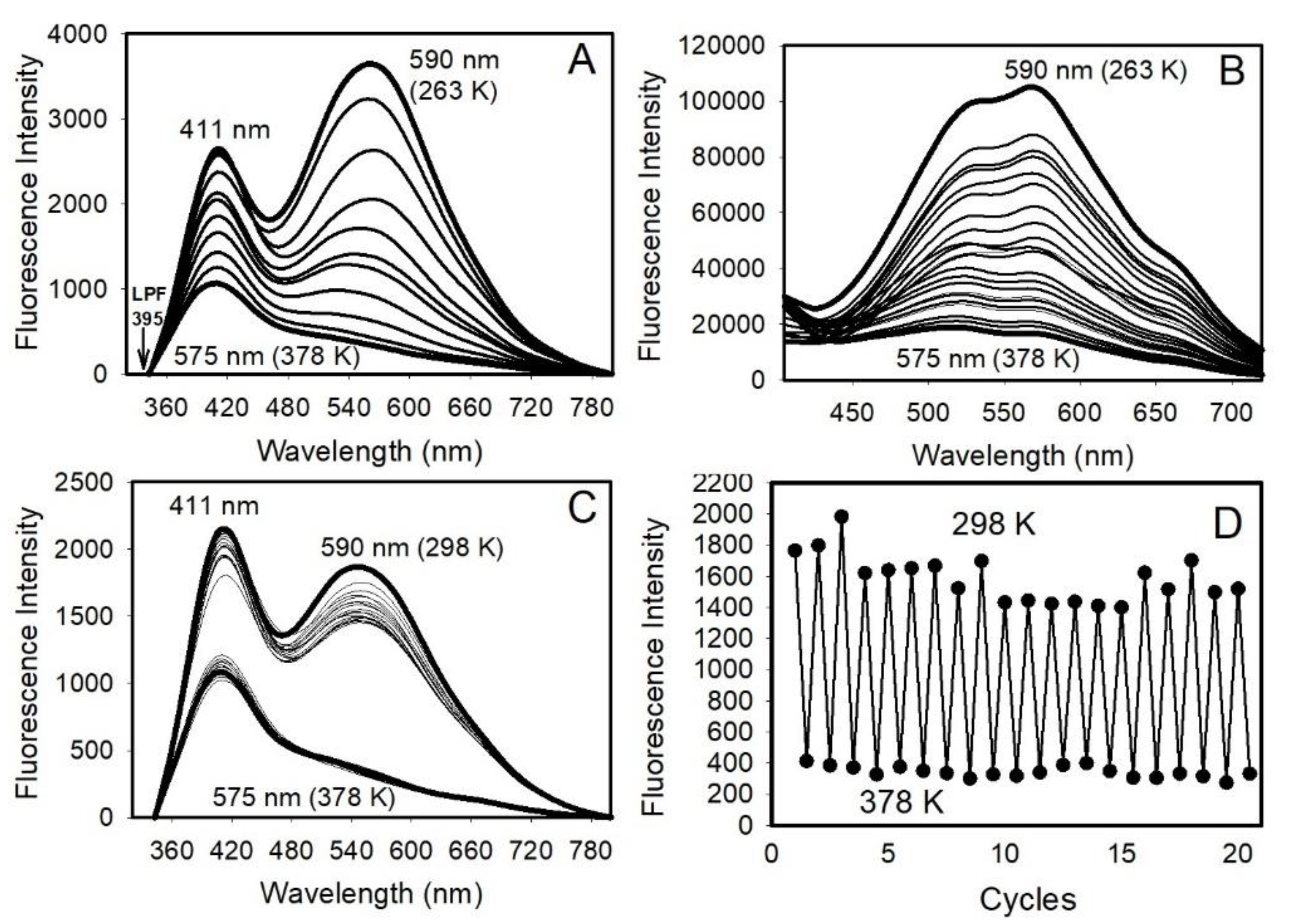
2.10. Variable-Temperature (VT) Measurements
2.11. Excited-State PL Lifetime Measurements and Time-Resolved Photoluminescence (TRPL) Measurements
2.12. DAS Measurements
3. Results and Discussion
3.1. Material Characterizations of SiO2-Coated ZnO Nanocrystals (Elements, Shape, Crystallography, Size Distribution, and Charges)
3.2. Optical Properties of the SiO2-Coated ZnO Nanocrystals
3.3. The Origin of Temperature-Dependent PL of ZnO/SiO2 Nanomaterials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ajmal, H.M.S.; Khan, F.; Nam, K.; Kim, H.Y.; Kim, S.D. Ultraviolet Photodetection Based on High-Performance Co-Plus-Ni Doped ZnO Nanorods Grown by Hydrothermal Method on Transparent Plastic Substrate. Nanomaterials 2020, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Pearton, S.J.; Norton, D.P.; Heo, Y.W.; Tien, L.C.; Ivill, M.P.; Li, Y.; Kang, B.S.; Ren, F.; Kelly, J.; Hebard, A.F. ZnO Spintronics and Nanowire Devices. J. Electron. Mater. 2006, 35, 862–868. [Google Scholar] [CrossRef]
- Rahman, F. Zinc oxide light-emitting diodes: A review. Opt. Eng. 2019, 58, 010901. [Google Scholar] [CrossRef]
- Shih, C.C.; Lee, W.Y.; Chiu, Y.C.; Hsu, H.W.; Chang, H.C.; Liu, C.L.; Chen, W.C. High-Performance Transparent Transistor Memory Devices Using Nano-Floating Gate of Polymer/ZnO Nanocomposites. Sci. Rep. 2016, 6, 20129. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO Nanostructured Materials for Emerging Solar Cell Applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar]
- Miccoli, B.; Cauda, V.; Bonanno, A.; Sanginario, A.; Bejtka, K.; Bella, F.; Demarchi, D. One-Dimensional ZnO/Gold Junction for Simultaneous and Versatile Multisensing Measurements. Sci. Rep. 2016, 6, 29763. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Rahman, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of Zinc Oxide Nanoparticles in Countering Negative Effects Generated by Cadmium in Lycopersicon esculentum. J. Plant. Growth Regul. 2021, 40, 101–115. [Google Scholar] [CrossRef]
- Sulciute, A.; Nishimura, K.; Gilshtein, E.; Cesano, F.; Viscardi, G.; Albert, G.N.; Ohno, Y.; Rackauskas, S. ZnO Nanostructures Application in Electrochemistry: Influence of Morphology. J. Phys. Chem. C 2021, 125, 1932–7447. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef]
- Zhao, S.; Ji, Z.; Yang, Y.; Huo, D.; Lv, Y. Nano-ZnO Embedded SiO2 Glass with Intense Ultraviolet Emission. Mater. Lett. 2007, 61, 2547–2550. [Google Scholar] [CrossRef]
- Xia, H.L.; Tang, F.Q. Surface Synthesis of Zinc Oxide Nanoparticles on Silica Spheres: Preparation and Characterization. J. Phys. Chem. B 2003, 107, 9175–9178. [Google Scholar]
- Abdullah, M.; Shibamoto, S.; Okuyama, K. Synthesis of ZnO/SiO2 Nanocomposites Emitting Specific Luminescence Colors. Opt. Mater. 2004, 26, 95–100. [Google Scholar]
- Xiao, F.; Chen, R.; Shen, Y.Q.; Liu, B.; Gurzadyan, G.G.; Dong, Z.L.; Zhang, Q.Y.; Sun, H.D. Infrared Emission Properties and Energy Transfer in ZnO–SiO2:Yb3+ Composites. J. Alloys Compd. 2011, 509, 7794–7797. [Google Scholar]
- Hong, J.H.; Wang, Y.F.; He, G.; Wang, J.X. The Effect of Calcination Temperature on the Photoluminescence from Sol–Gel Derived Amorphous ZnO/Silica Composites. J. Non-Cryst. Solids. 2010, 356, 2778–2780. [Google Scholar]
- An, S.S.A.; Yi, D.K.; Ramasamy, M.; Das, M. Role of Surface Modification in Zinc Oxide Nanoparticles and Its Toxicity Assessment toward Human Dermal Fibroblast Cells. Int. J. Nanomed. 2014, 9, 3707. [Google Scholar] [CrossRef]
- Shimada, T.; Yasui, T.; Yonese, A.; Yanagida, T.; Kaji, N.; Kanai, M.; Nagashima, K.; Kawai, T.; Baba, Y. Mechanical Rupture-Based Antibacterial and Cell-Compatible ZnO/SiO2 Nanowire Structures Formed by Bottom-Up Approaches. Micromachines 2020, 11, 610. [Google Scholar]
- Ali, A.M.; Ismail, A.A.; Najmy, R.; Al-Hajry, A. Preparation and Characterization of ZnO–SiO2 Thin Films as Highly Efficient Photocatalyst. J. Photochem. Photobiol. A 2014, 275, 37–46. [Google Scholar]
- Meng, F.; Liu, Y.; Wang, J.; Tan, X.; Sun, H.; Liu, S.; Wang, S. Temperature dependent photocatalysis of g-C3N4, TiO2 and ZnO: Differences in photoactive mechanism. J. Colloid Interface Sci. 2018, 532, 321–330. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Wang, X.; Ying, A.P.; Li, C. Time-Resolved Photoluminescence Characteristics of Subnanometer ZnO Clusters Confined in the Micropores of Zeolites. J. Phys. Chem. B 2006, 110, 25612–25618. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor Clusters in the Sol-Gel Process: Quantized Aggregation, Gelation, and Crystal Growth in Concentrated Zinc Oxide Colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Hoyer, P.; Eichberger, R.; Weller, H. Spectroelectrochemical Investigations of Nanocrystalline ZnO Films, Berichte Bunsenges. Für. Phys. Chem. 1993, 97, 630–635. [Google Scholar]
- Peng, D.; Chen, X.; Wang, Y.; Hu, Z.; Yu, K.; Zhu, Z. Temperature-dependent photoluminescence properties of needle-like ZnO nanostructures deposited on carbon nanotubes. Appl. Phys. A 2011, 105, 463–468. [Google Scholar]
- Li, C.P.; Zhang, L.; Liu, C.J.; Gao, G. Exciton Localization and Stimulated Emission of ZnO Nanorods. Key Eng. Mater. 2013, 538, 161–164. [Google Scholar]
- Mhlongo, G.H.; Motaung, D.E.; Nkosi, S.S.; Swart, H.C.; Malgas, G.F.; Hillie, K.T.; Mwakikunga, B.W. Temperature-Dependence on the Structural, Optical, and Paramagnetic Properties of ZnO Nanostructures. Appl. Surf. Sci. 2014, 293, 62–70. [Google Scholar]
- Bouzouraa, M.B.; Battie, Y.; Dalmasso, S.; Zaïbi, M.A.; Oueslati, M.; En Naciri, A. Temperature Dependent Optical Properties of ZnO Thin Film Using Ellipsometry and Photoluminescence. Superlattices Microstruct. 2018, 117, 457–468. [Google Scholar]
- Shakti, N.; Prakash, A.T.; Katiyar, M. Processing Temperature Dependent Morphological and Optical Properties of ZnO Nanorods. Mater. Sci. Semicond. Process. 2014, 20, 55–60. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, Y.; Zeng, X.; Chen, X.; Chu, Y. Oxygen Vacancy Mediated Temperature Dependent Emission Behavior of Localized Bound Excitons in ZnO Nanorods. J. Lumin. 2018, 195, 201–208. [Google Scholar]
- Godlewski, M.; Zakrzewski, A. Nonradiative Recombination Processes in II-VI Luminophores; Jain, M., Ed.; University of Western Australia: Crawley, Australia, 2015. [Google Scholar]
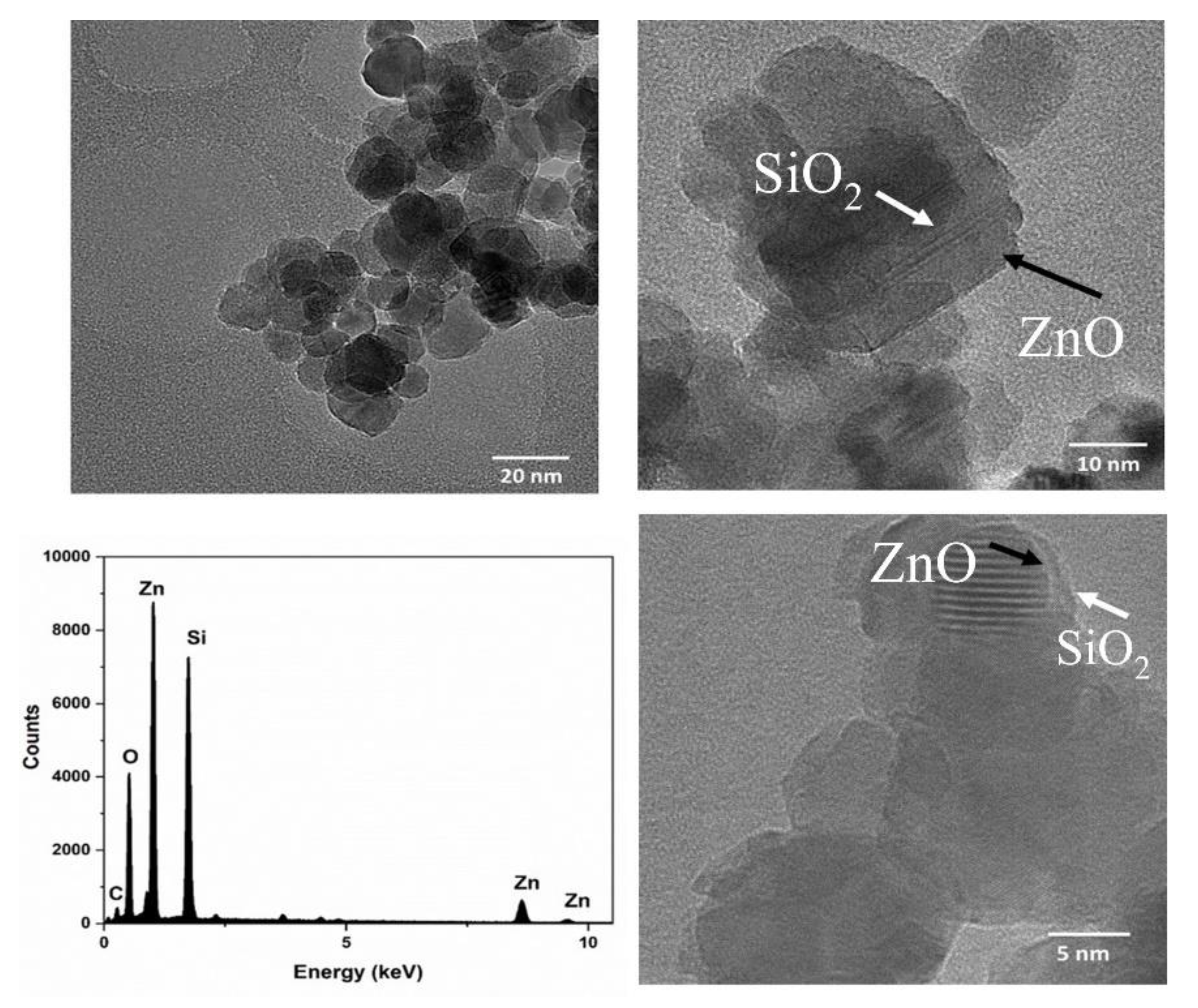


| Element | Weight % | Atomic % |
|---|---|---|
| Zn | 20.57 | 9.07 |
| Si | 61.17 | 62.73 |
| O | 15.42 | 27.77 |
| Samples | λobs (nm) | τ1 (ns) | f1% | τ2 (ns) | f2% | τ3 (ns) | f3% | τaverage (ns) | Chi-Square |
|---|---|---|---|---|---|---|---|---|---|
| 298 K | 411 | 2.7 | 21 | 20 | 22 | 49.6 | 57 | 33 | 1.091 |
| 298 K | 541 | 1.4 | 28 | 12 | 15 | 111.5 | 57 | 66 | 1.041 |
| 378 K | 411 | 2.5 | 9 | 16.4 | 27 | 46.8 | 64 | 35 | 1.002 |
| 378 K | 541 | 0.81 | 52 | 7.03 | 14 | 67.55 | 34 | 24 | 1.061 |
| Temperature | τ/ns Excited-State Lifetimes | DAS Maximum/nm | DAS Amplitudes | % of 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| 263 K | 0.5 | 4 | 90 | <500 | <550 | 600 | 0.59 | 0.01 | 0.40 | 40 |
| 298 K | 0.8 | 6 | 92 | <500 | <550 | 600 | 0.50 | 0.20 | 0.30 | 30 |
| 378 K | 0.7 | 7 | 68 | <500 | <550 | 600 | 0.90 | 0.04 | 0.06 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Poulose, V.; Laz, Y.T.; Chandra, F.; Abubakar, S.; Abdelhamid, A.S.; Alzamly, A.; Saleh, N. Temperature Control of Yellow Photoluminescence from SiO2-Coated ZnO Nanocrystals. Nanomaterials 2022, 12, 3368. https://doi.org/10.3390/nano12193368
Kumar N, Poulose V, Laz YT, Chandra F, Abubakar S, Abdelhamid AS, Alzamly A, Saleh N. Temperature Control of Yellow Photoluminescence from SiO2-Coated ZnO Nanocrystals. Nanomaterials. 2022; 12(19):3368. https://doi.org/10.3390/nano12193368
Chicago/Turabian StyleKumar, Narender, Vijo Poulose, Youssef Taiser Laz, Falguni Chandra, Salma Abubakar, Abdalla S. Abdelhamid, Ahmed Alzamly, and Na’il Saleh. 2022. "Temperature Control of Yellow Photoluminescence from SiO2-Coated ZnO Nanocrystals" Nanomaterials 12, no. 19: 3368. https://doi.org/10.3390/nano12193368
APA StyleKumar, N., Poulose, V., Laz, Y. T., Chandra, F., Abubakar, S., Abdelhamid, A. S., Alzamly, A., & Saleh, N. (2022). Temperature Control of Yellow Photoluminescence from SiO2-Coated ZnO Nanocrystals. Nanomaterials, 12(19), 3368. https://doi.org/10.3390/nano12193368







