Effect of Hydrophobic Nano-SiO2 Particle Concentration on Wetting Properties of Superhydrophobic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
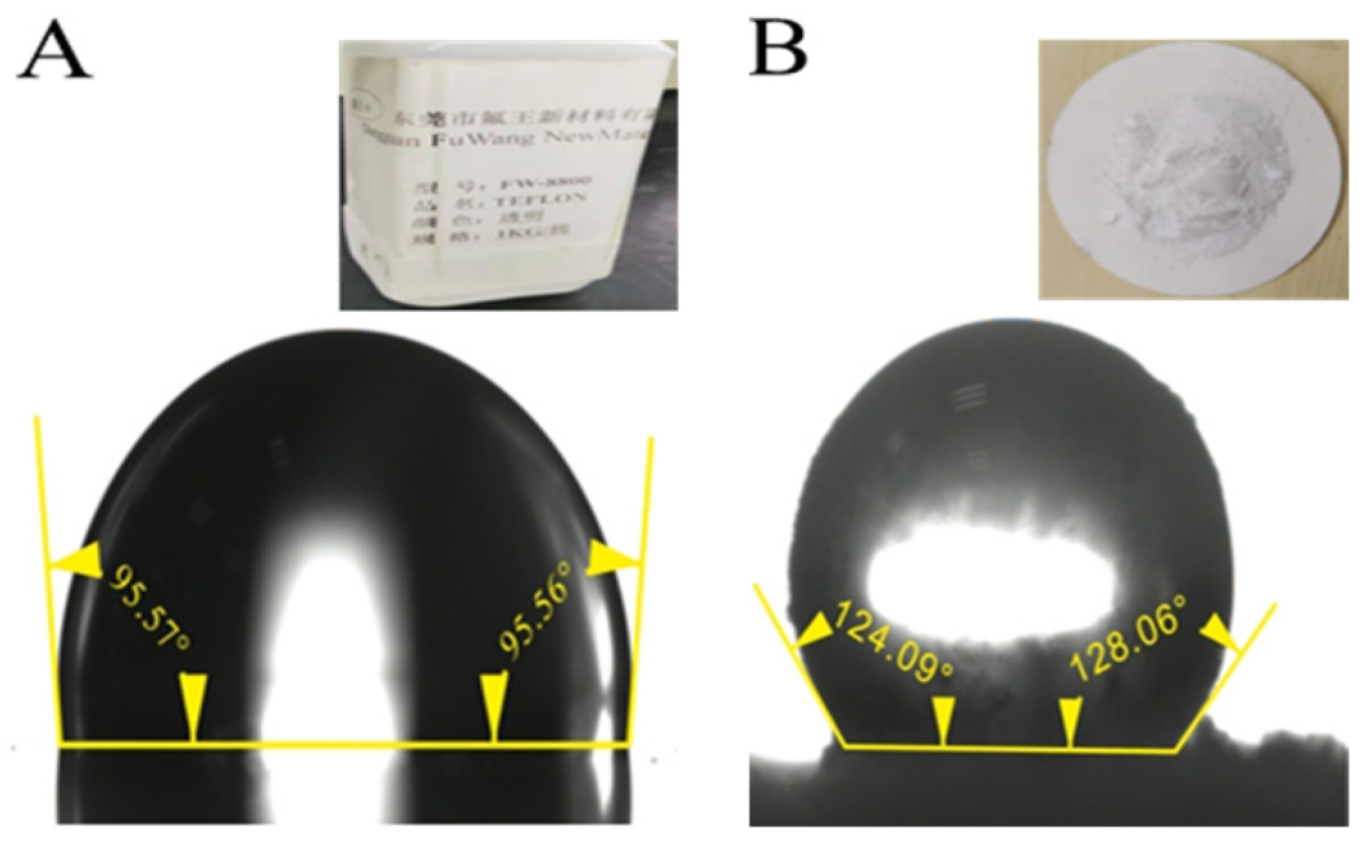
2.1. Materials
2.2. Methods
2.2.1. Preparation before Spraying
2.2.2. Preparation of Superhydrophobic Surfaces
2.3. Characterization
3. Results and Discussion
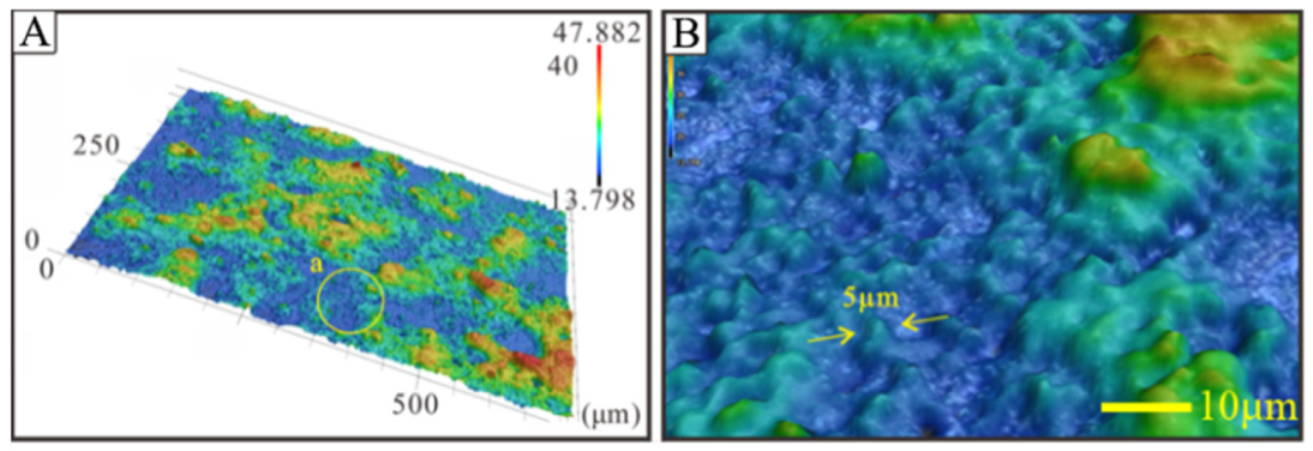
3.1. Surface Topography
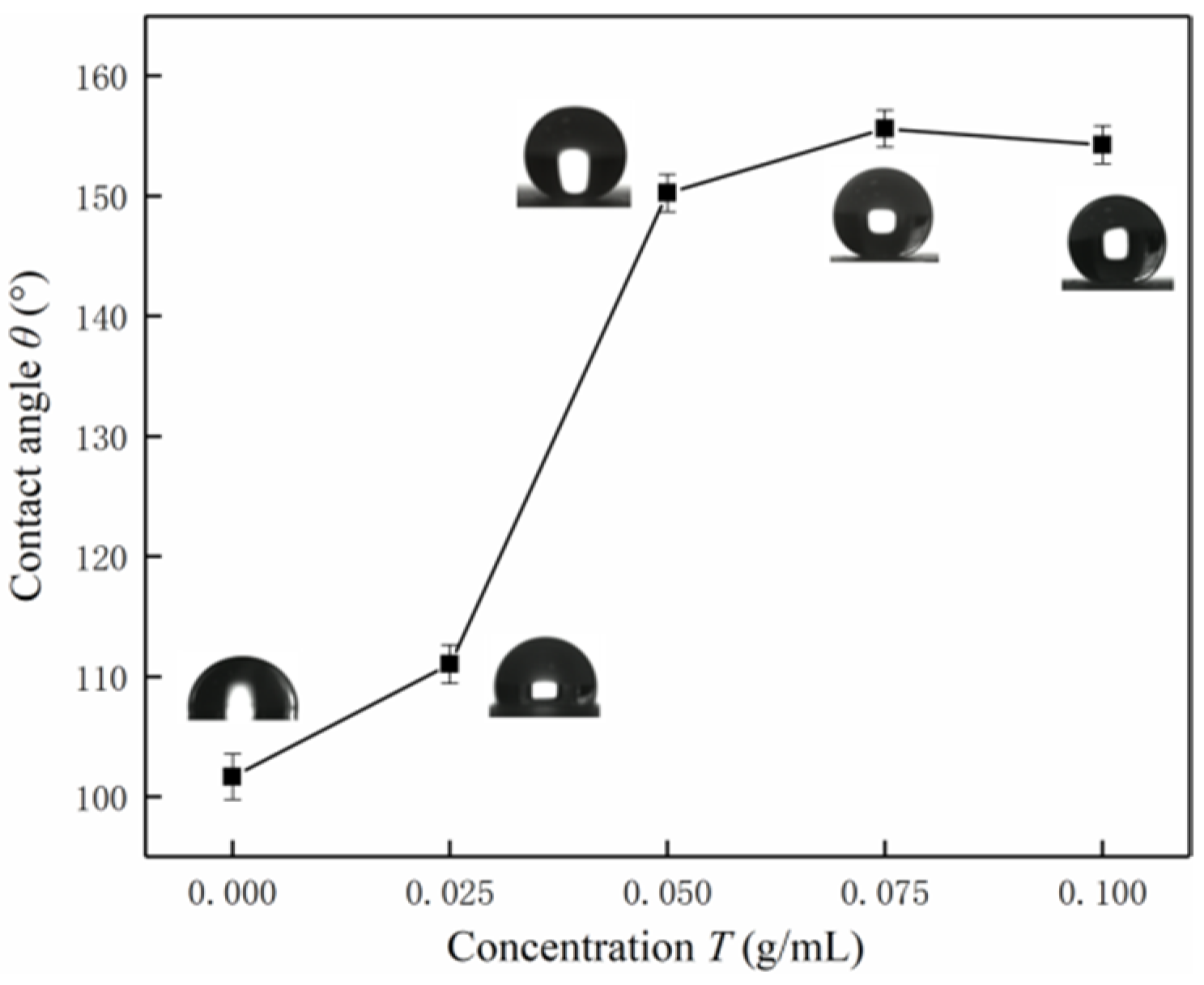
3.2. Surface Wettability
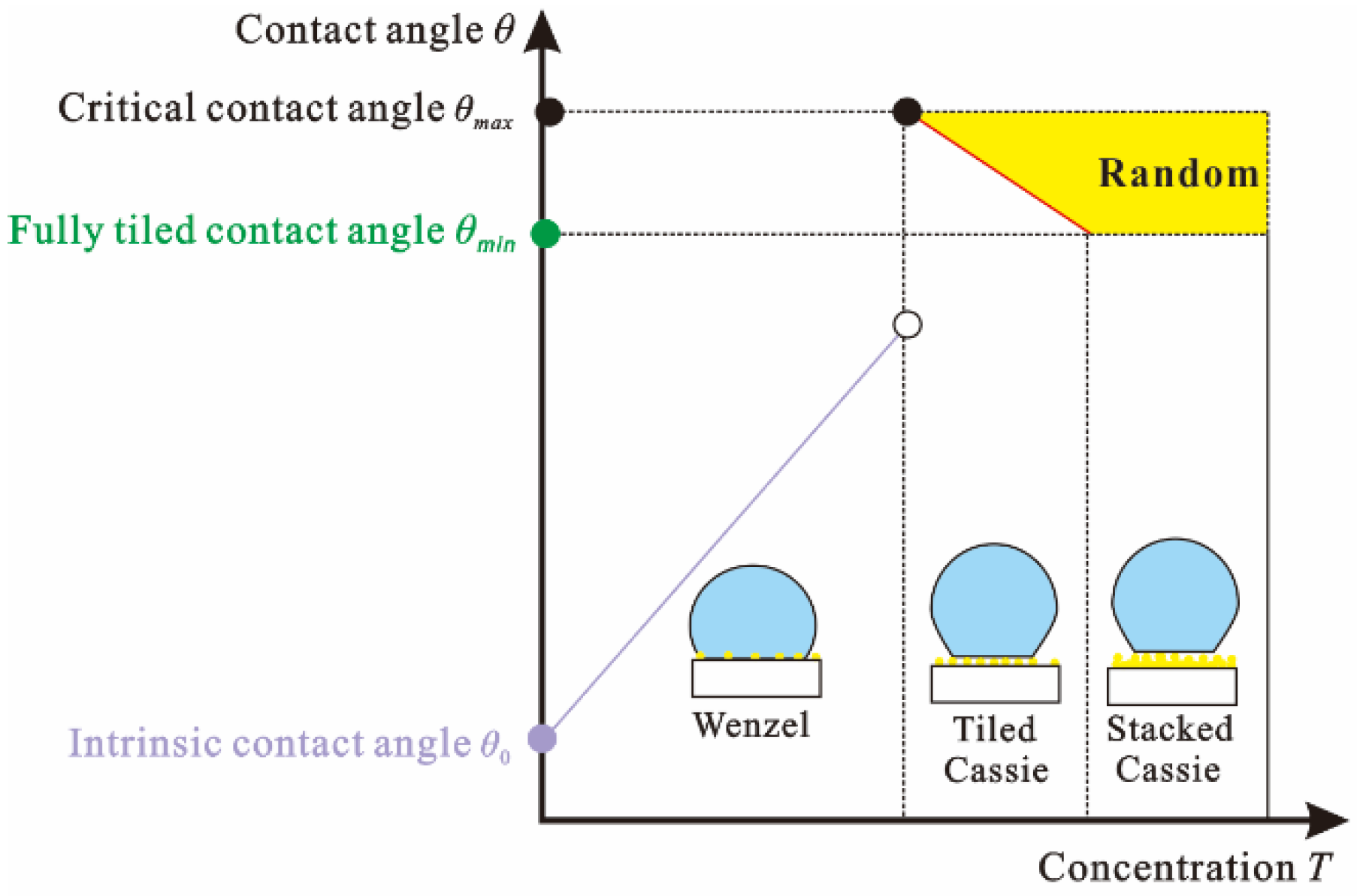
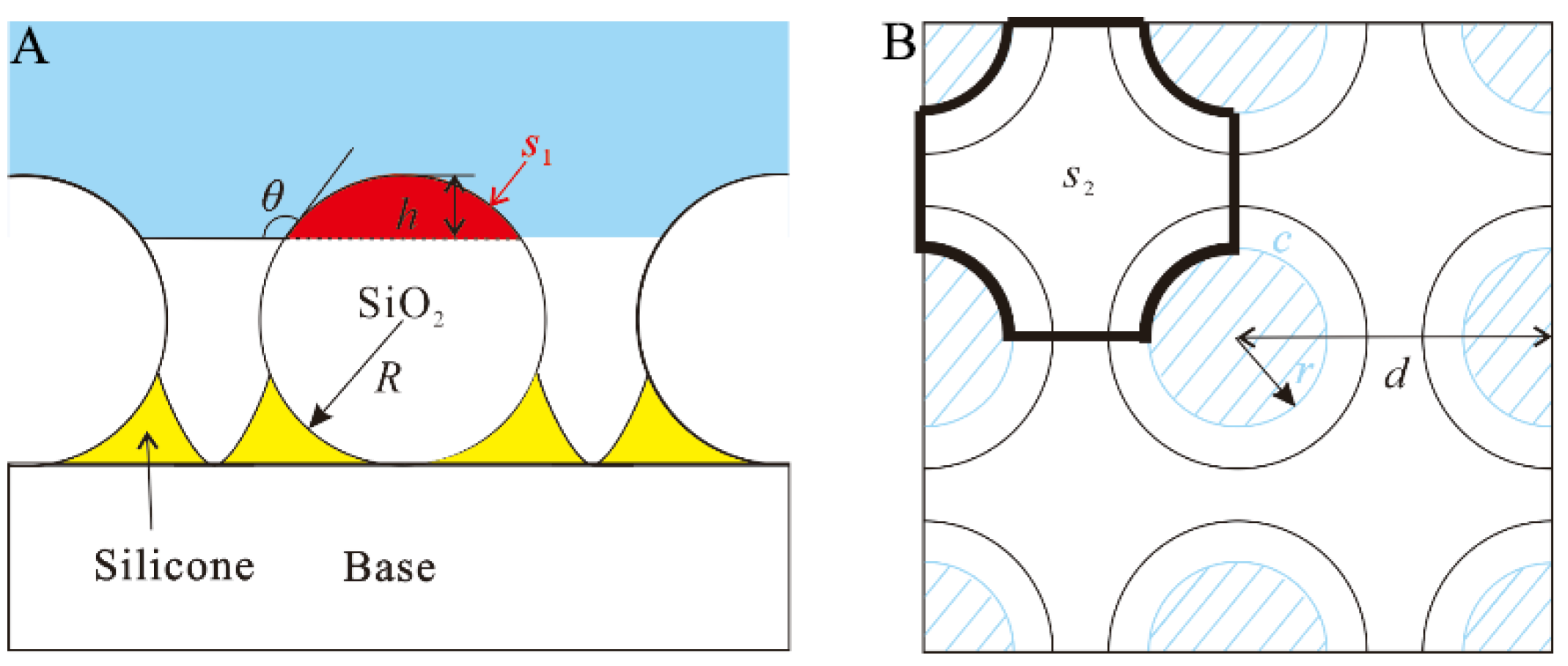
3.3. Wetting Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malobi, S.; Sunirmal, J. Development of superhydrophobic coating from biowaste and natural wax. Mater. Today 2022, 52, 1422–1428. [Google Scholar]
- Liu, G.; Ji, C.; Li, J.; Pan, X. Facile preparation and properties of superhydrophobic nanocellulose membrane. Arab. J. Chem. 2022, 15, 103964. [Google Scholar] [CrossRef]
- Qin, S.; Fang, H.; Sun, S.; Wang, X.; Cao, L.; Wu, D. Fabrication of air-permeable superhydrophobic surfaces with excellent non-wetting property. Mater. Lett. 2022, 313, 131783. [Google Scholar] [CrossRef]
- Hu, L.; Xue, C.; Liu, B.; Guo, X.; Wang, J.; Deng, F. Scalable superhydrophobic flexible nanofiber film for passive daytime radiative cooling. ACS Appl. Polym. Mater. 2022, 4, 3343–3351. [Google Scholar] [CrossRef]
- Ahmed, A.; Peichun, A.T. Fabrication of transparent and microstructured superhydrophobic substrates using additive manufacturing. Langmuir 2021, 37, 348–356. [Google Scholar]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. R. Soc. 2009, 367, 1631–1672. [Google Scholar] [CrossRef]
- Jin, M.R.; Xing, Q.L.; Chen, Z.K. A Review: Natural superhydrophobic surfaces and applications. J. Bio. Nanobiotechnol. 2020, 11, 110–149. [Google Scholar] [CrossRef]
- Tong, W.; Xiong, D.S. Bioinspired superhydrophobic materials: Progress and functional application. J. Inorg. Mater. 2019, 34, 1133–1144. [Google Scholar]
- Erbil, H.Y. Practical applications of superhydrophobic materials and coatings: Problems and perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, Y.; Yang, W.; Ni, M.; Zhang, X.; Liang, C. Anti-frosting performance of sprayable superhydrophobic coating suitable for outdoor coil of air source heat pump. Appl. Therm. Eng. 2020, 169, 114967. [Google Scholar] [CrossRef]
- Wu, Y.L.; She, W.; Shi, D.A.; Jiang, T.; Hao, T.H.; Liu, J.; Zhang, Q.C.; You, J.; Robert, K.Y.L. An extremely chemical and mechanically durable siloxane bearing copolymer coating with self-crosslinkable and anti icing properties. Compos. Pt. B-Eng. 2020, 195, 108031. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, A.; Tan, X.; Tu, Y.; Sydney, S.; Xiang, P.; Wang, M.; Guo, R.; Chen, X. A vei-over-sprout micro-nano PMMA/SiO2 superhydrophobic coating with impressive abrasion, icing, and corrosion resistance. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 601, 124998. [Google Scholar] [CrossRef]
- Hou, W.; Shen, Y.; Tao, J.; Xu, Y.; Jiang, J.; Chen, H.; Jia, Z. Anti-icing performance of the superhydrophobic surface with micro-cubic array structures fabricated by plasma etching. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 586, 124180. [Google Scholar] [CrossRef]
- Tuo, Y.; Zhang, H.; Rong, W.; Jiang, S.; Chen, W.; Liu, X. Drag reduction of anisotropic superhydrophobic surface prepared by laser etching. Langmuir 2019, 35, 11016–11022. [Google Scholar] [CrossRef]
- Heidari, G.; Hosseini, S.I. Electrochemical fabrication of superhydrophobic and superoleophilic coating: Applications in corrosion-resistant surfaces and oil cleanup. Bull. Mat. Sci. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Büra, I.; Mustafa, K. Initiated chemical vapor deposition of poly (hexafluorobutyl acrylate) thin films for superhydrophobic surface modification of nanostructured textile surfaces. J. Coat. Technol. Res. 2020, 17, 381–391. [Google Scholar]
- Oh, J.H.; Moon, M.W.; Park, C.H. Effect of crystallinity on the recovery rate of superhydrophobicity in plasma-nanostructured polymers. RSC Adv. 2020, 10, 10939–10948. [Google Scholar] [CrossRef]
- Lan, L.; Wang, H.; Zhu, L.; Di, Y.; Kang, J.; Qiu, J. Preparation and wetting mechanism of laser-etched composite self-assembled 1H,1H,2H,2H-perfluorodecyltriethoxysilane superhydrophobic surface coating. Phys. Status Solidi 2022, 219, 2100568. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Liu, Q.; Xiao, H.; Li, X.; Zhou, Y.; Wang, H.; Zheng, A.; Zhao, J.; Ren, L.; et al. Electrochemical polishing assisted selective laser melting of biomimetic superhydrophobic metallic parts. Appl. Surf. Sci. 2022, 596, 153601. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, Y.; Liu, N.; Li, Y.; Wang, P.; Dai, C.; Wei, Y. Multi-applicable, durable superhydrophobic anti-icing coating through template-method and chemical vapor deposition. Surf. Interfaces 2022, 32, 102100. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, X.; Zhang, H.; Huang, Z.; Zhang, Z. Superhydrophobic surface with excellent mechanical robustness, water impact resistance and hydrostatic pressure resistance by two-step spray-coating technique. Compos. Pt. A Appl. Sci. Manuf. 2021, 146, 106405. [Google Scholar] [CrossRef]
- Swain, B.; Pati, A.R.; Mallick, P.; Mohapatra, S.S.; Behera, A. Development of highly durable superhydrophobic coatings by one-step plasma spray methodology. J. Therm. Spray Technol. 2021, 30, 1–2. [Google Scholar] [CrossRef]
- Soni, R.; Joshi, S.R.; Karmacharya, M.; Min, H.; Kim, S.K.; Kumar, S.; Kim, G.H.; Cho, Y.K.; Lee, C.Y. Superhydrophobic and self-sterilizing surgical masks spray-coated with carbon nanotubes. ACS Appl. Nano Mater. 2021, 4, 8491–8499. [Google Scholar] [CrossRef]
- Gong, A.; Zheng, Y.; Yang, Z.; Guo, X.; Gao, Y.; Li, X. Spray fabrication of superhydrophobic coating on aluminum alloy for corrosion mitigation. Mater. Today Commun. 2020, 26, 101828. [Google Scholar] [CrossRef]
- Kim, H.; Nam, K.; Dong, Y.L. fabrication of robust superhydrophobic surfaces with dual-curing siloxane resin and controlled dispersion of nanoparticles. Polymers 2020, 12, 1420. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.; Yu, H.; Li, X. Preparation of a wear-resistant, superhydrophobic SiO2/silicone-modified polyurethane composite coating through a two-step spraying method. Prog. Org. Coat. 2020, 146, 105710. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Extrand, C.W. Criteria for ultralyophobic surfaces. Langmuir 2004, 20, 5013–5018. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Xia, T.; Zhang, Q. Effect of Hydrophobic Nano-SiO2 Particle Concentration on Wetting Properties of Superhydrophobic Surfaces. Nanomaterials 2022, 12, 3370. https://doi.org/10.3390/nano12193370
Xing L, Xia T, Zhang Q. Effect of Hydrophobic Nano-SiO2 Particle Concentration on Wetting Properties of Superhydrophobic Surfaces. Nanomaterials. 2022; 12(19):3370. https://doi.org/10.3390/nano12193370
Chicago/Turabian StyleXing, Lei, Tian Xia, and Qiaoxin Zhang. 2022. "Effect of Hydrophobic Nano-SiO2 Particle Concentration on Wetting Properties of Superhydrophobic Surfaces" Nanomaterials 12, no. 19: 3370. https://doi.org/10.3390/nano12193370
APA StyleXing, L., Xia, T., & Zhang, Q. (2022). Effect of Hydrophobic Nano-SiO2 Particle Concentration on Wetting Properties of Superhydrophobic Surfaces. Nanomaterials, 12(19), 3370. https://doi.org/10.3390/nano12193370




