A Novel Electrochemical Sensing Strategy Based on Poly (3, 4-ethylenedioxythiophene): Polystyrene Sulfonate, AuNPs, and Ag+ for Highly Sensitive Detection of Alkaline Phosphatase
Abstract
:1. Introduction
2. Experiment
2.1. Apparatus
2.2. Reagents
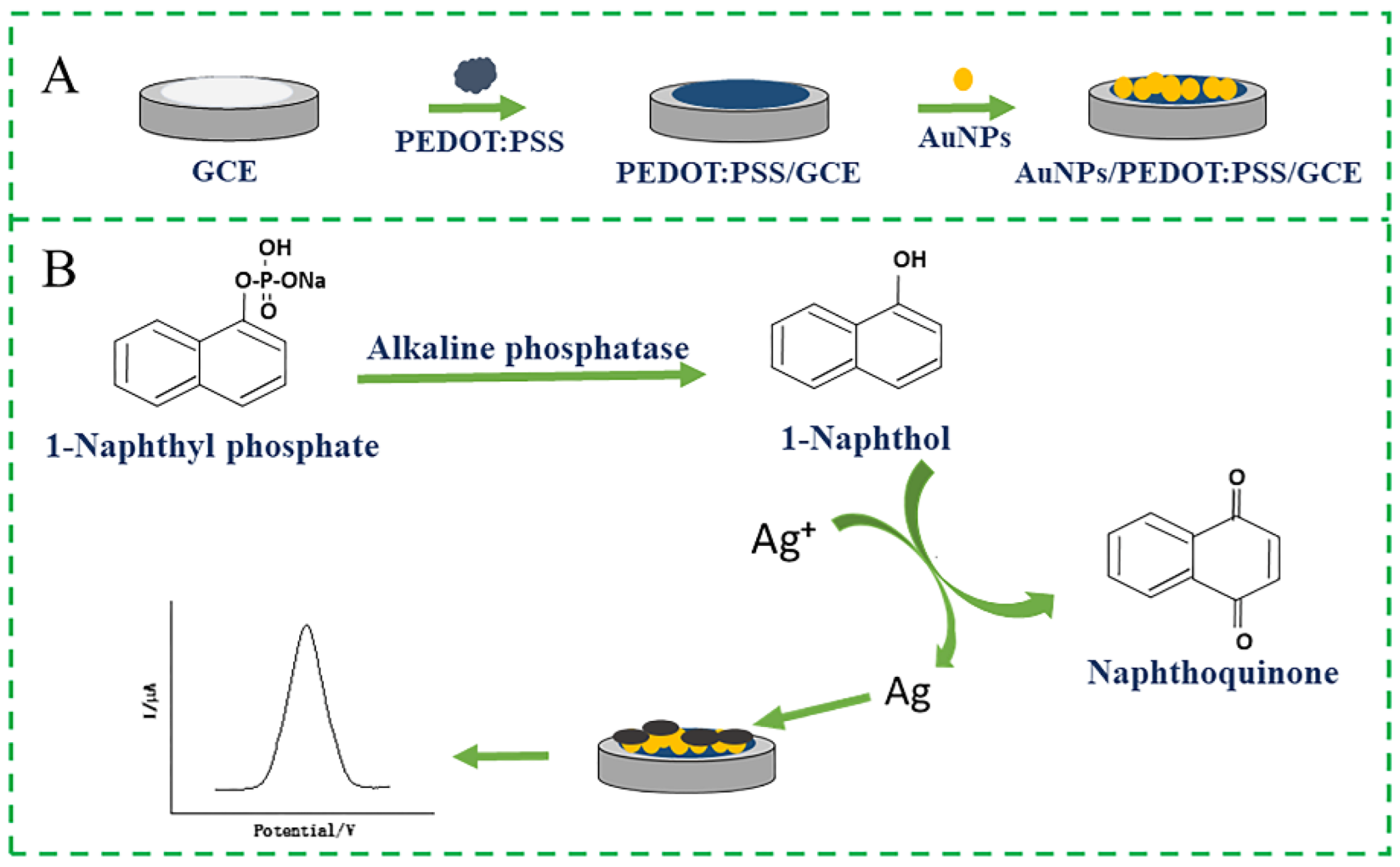
2.3. Construction of the Electrochemical Sensor
2.4. Electrochemical Detection of ALP
3. Results and Discussion
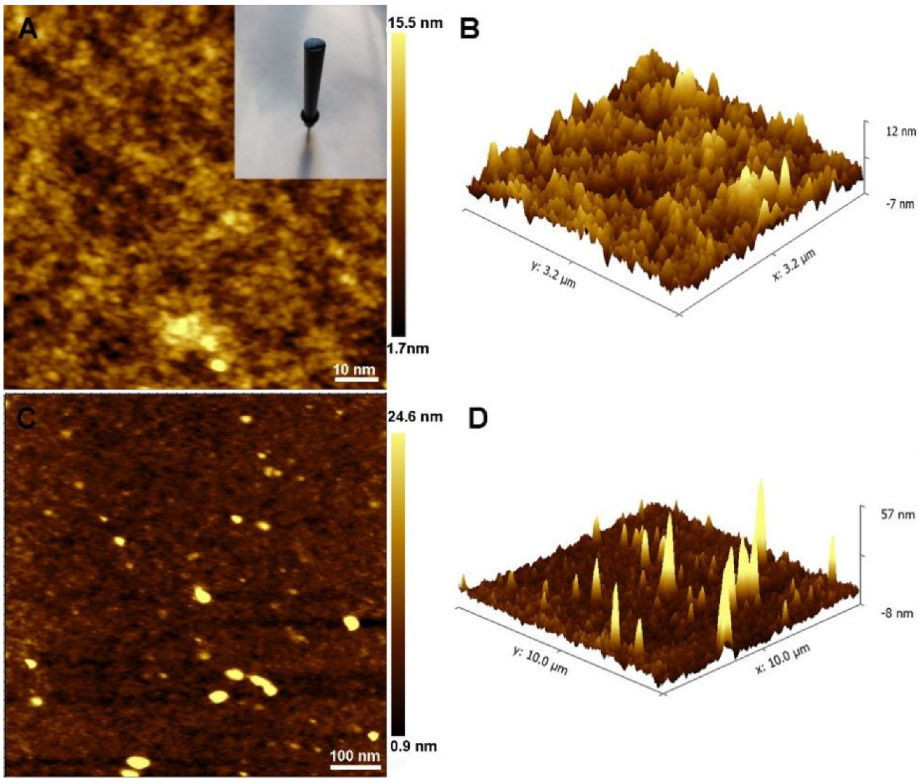
3.1. Morphology Characterization of the PEDOT:PSS and AuNPs/PEDOT:PSS
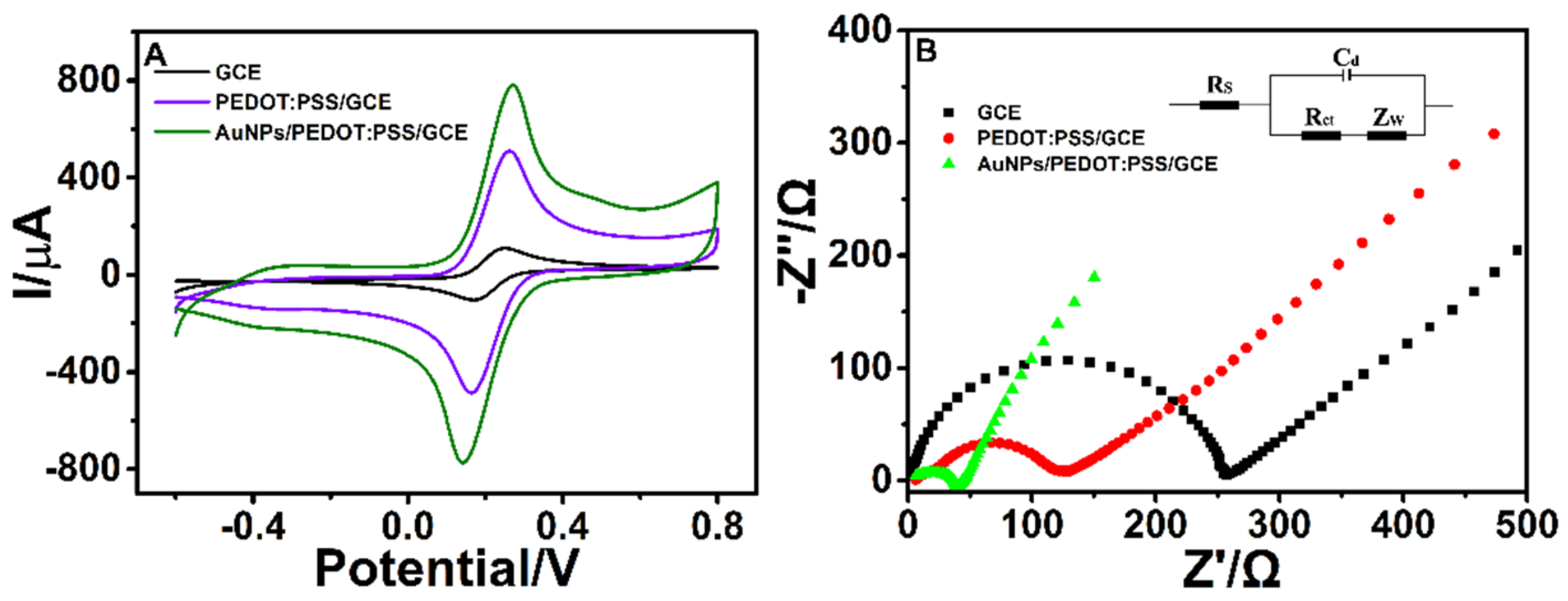
3.2. Electrochemical Analytical Performance of AuNPs/PEDOT:PSS
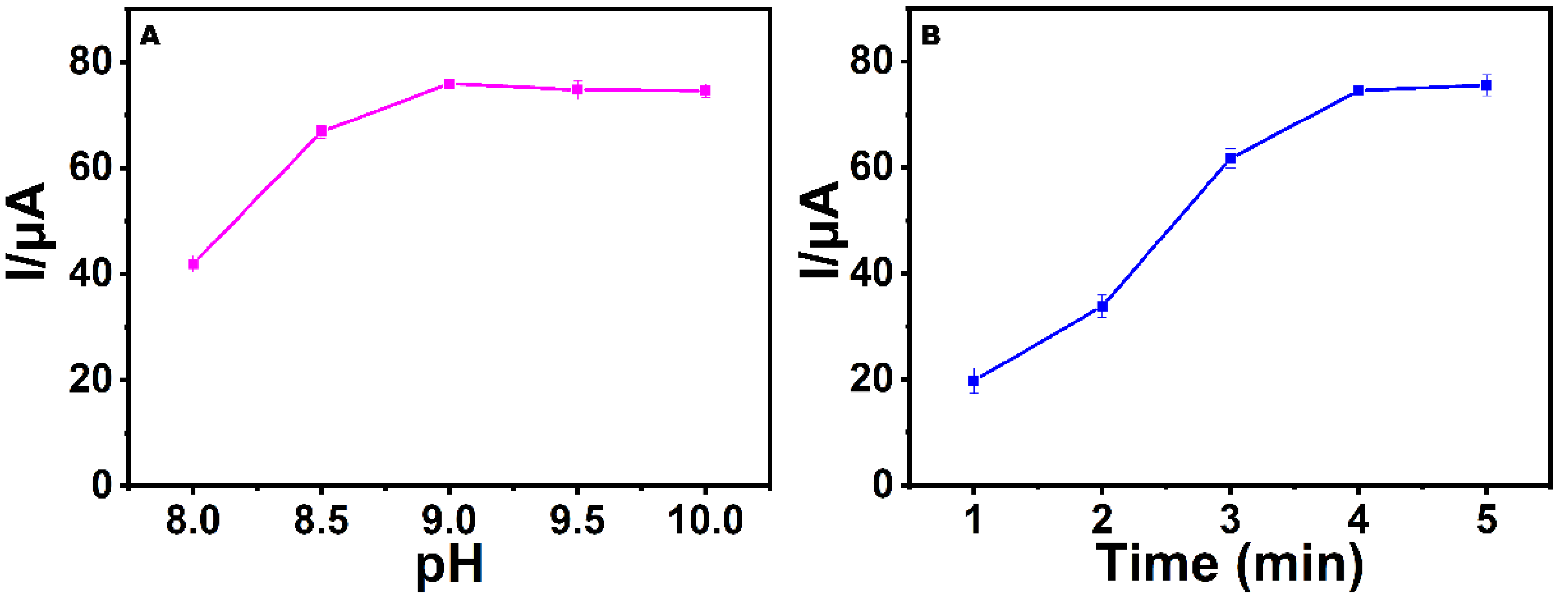
3.3. Optimization of pH and the Incubation Time
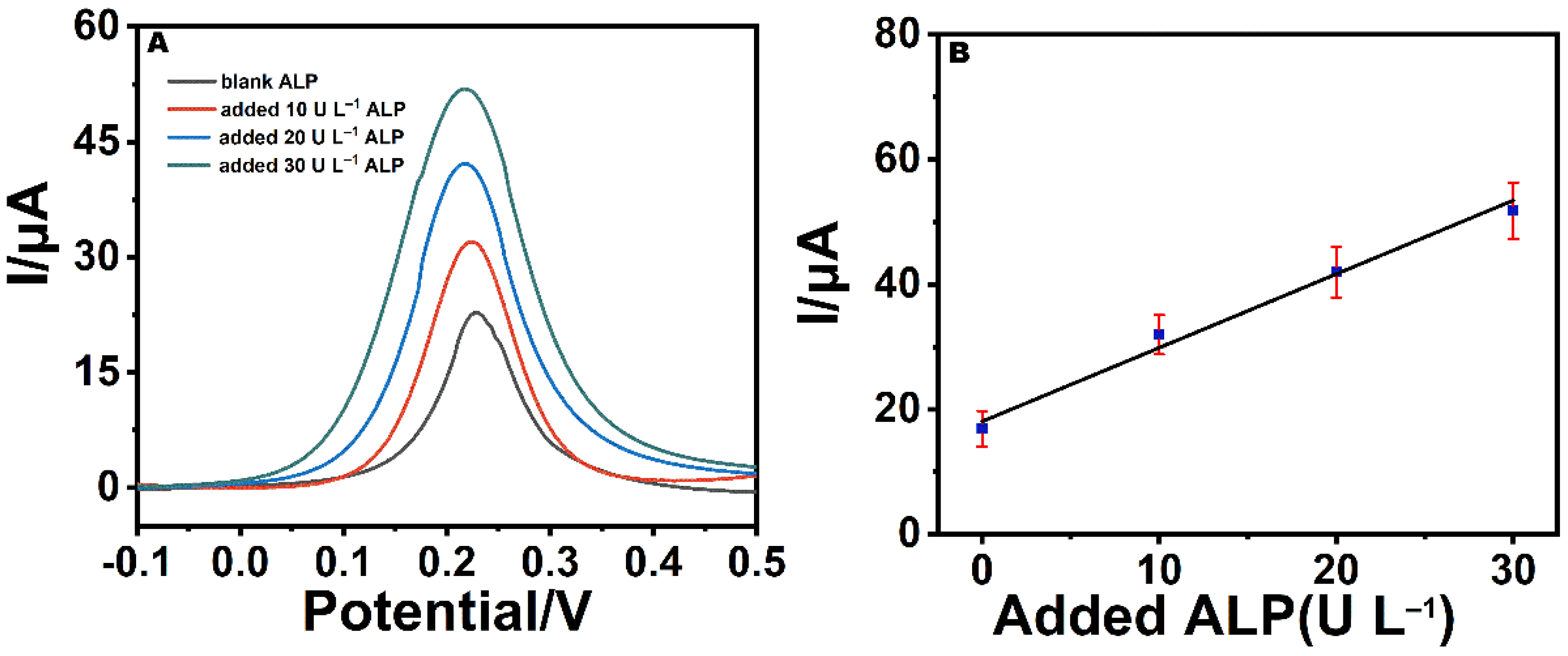
3.4. Electrochemical Determination of ALP
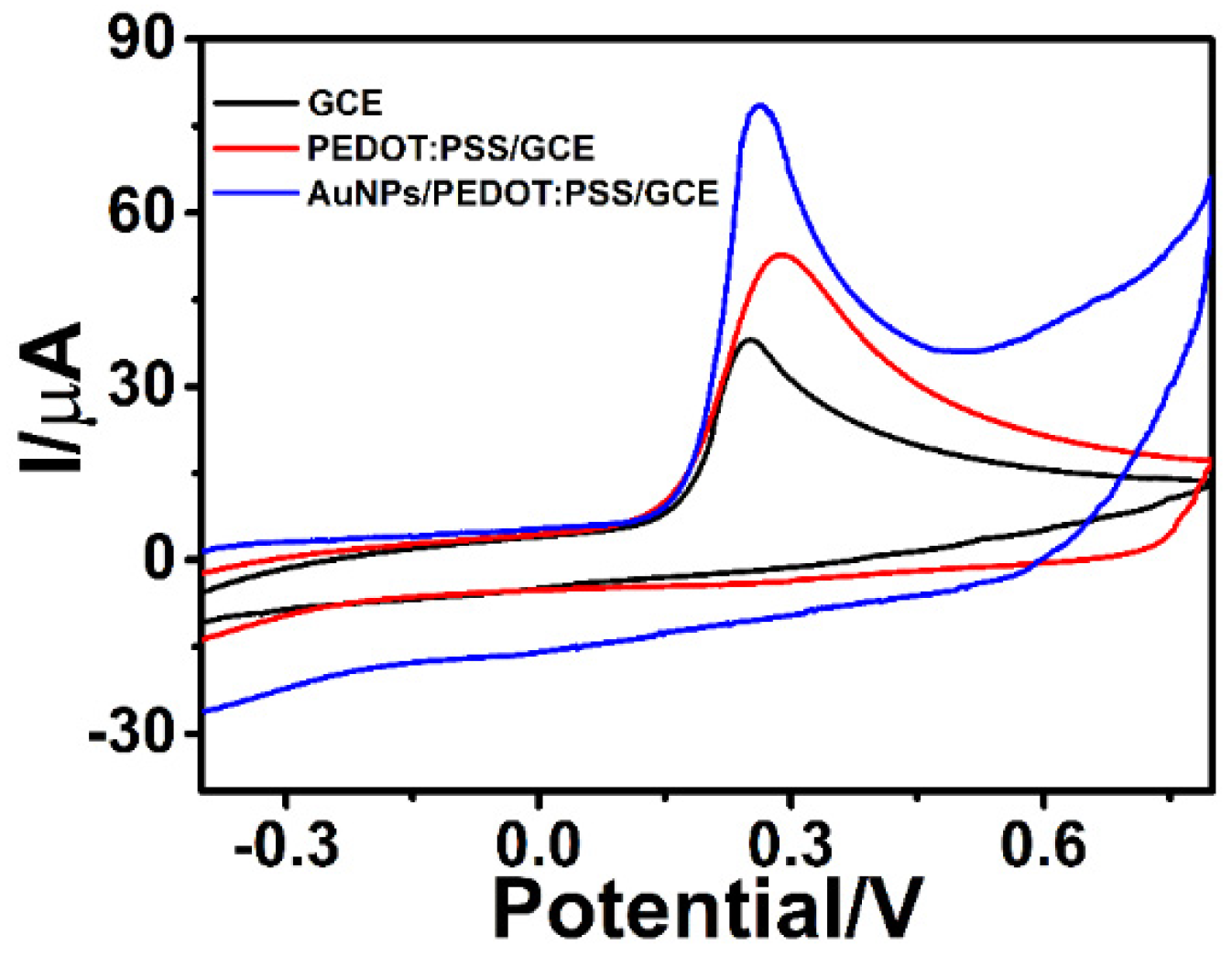
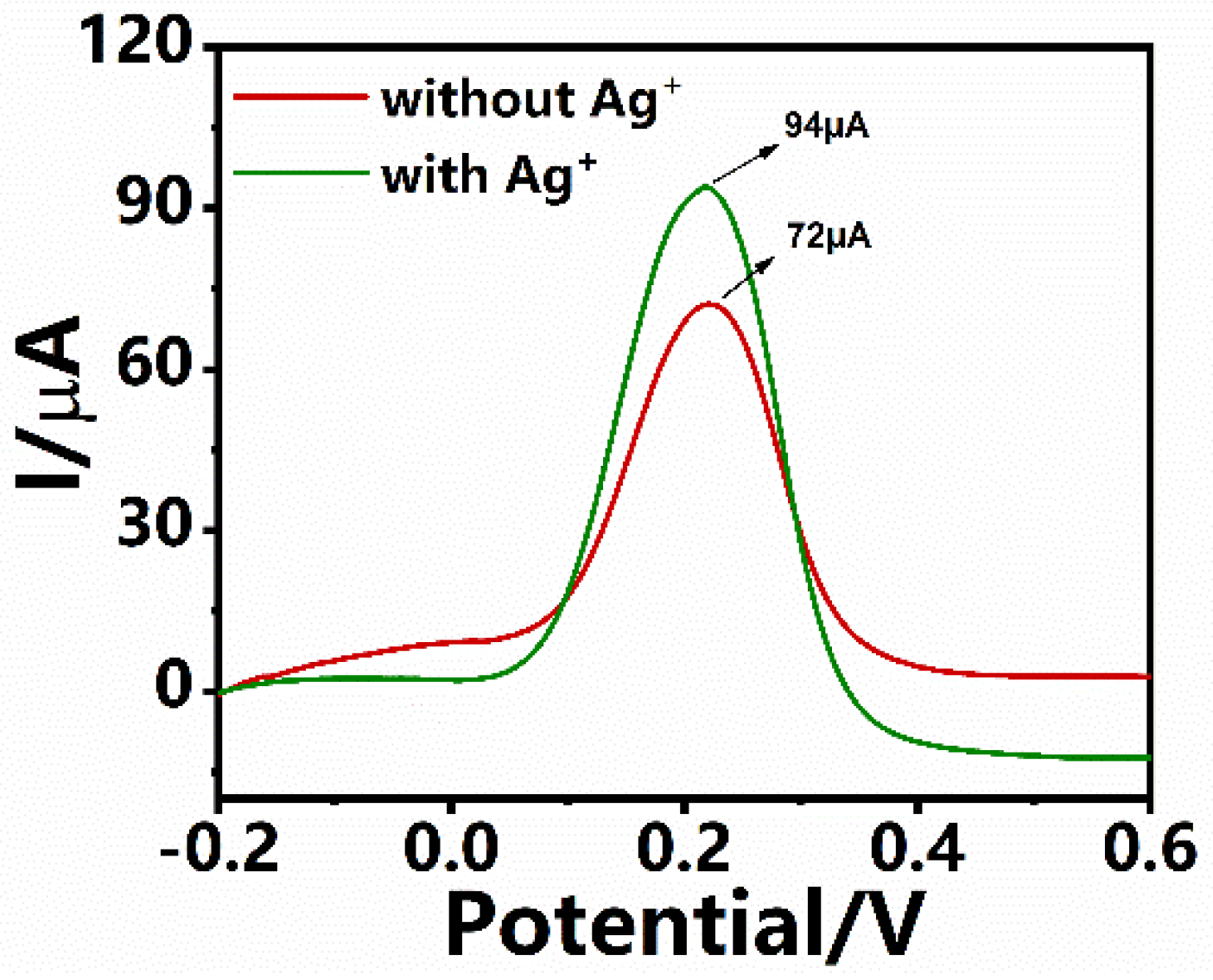
3.5. Signal Amplification with Ag
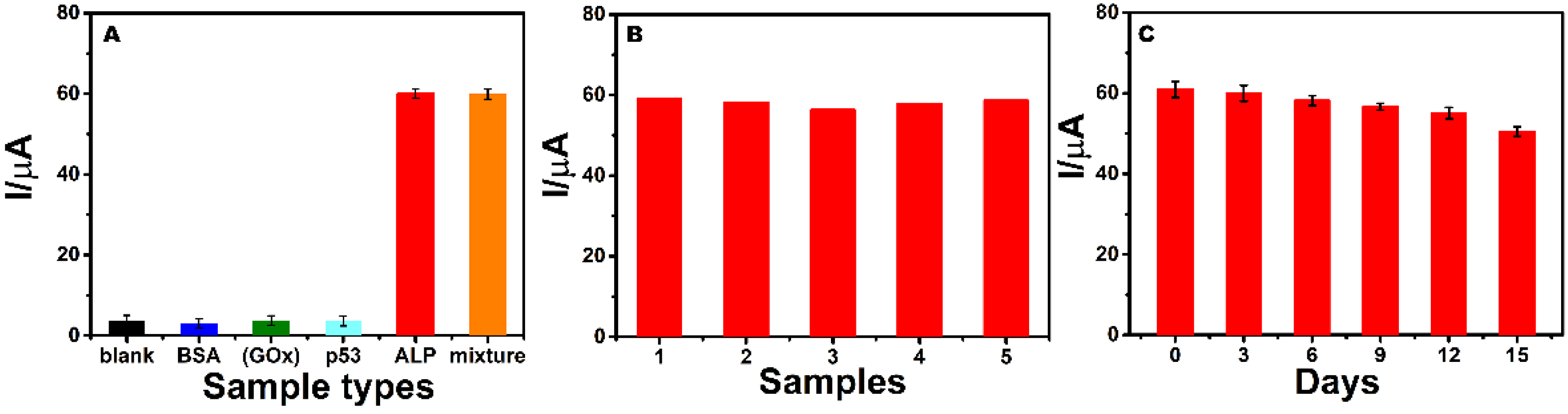
3.6. Selectivity, Repeatability, and Stability of the Detection
3.7. Analytical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, W.; Fu, Y.; Zhao, M.; He, H.; Tian, X.; Hu, L.; Zhang, Y. Electrochemiluminescence resonance energy transfer immunoassay for alkaline phosphatase using p-nitrophenyl phosphate as substrate. Anal. Chim. Acta 2020, 1097, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Purohit, B.; Kumar, A.; Chandra, P. Clinically comparable impedimetric immunosensor for serum alkaline phosphatase detection based on electrochemically engineered Au-nano-Dendroids and graphene oxide nanocomposite. Biosens. Bioelectron. 2020, 148, 111815. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Han, X.X.; Zhang, Q.C.; Yao, H.Q.; Gao, Z.N. Copper sulfide nanoparticle-decorated graphene as a catalytic amplification platform for electrochemical detection of alkaline phosphatase activity. Anal. Chim. Acta 2015, 878, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Couttenye, M.M.; D’Haese, P.C.; Van Hoof, V.O.; Lemoniatou, E.; Goodman, W.; Verpooten, G.A.; De Broe, M.E. Low serum levels of alkaline phosphatase of bone origin: A good marker of adynamic bone disease in haemodialysis patients. Nephrol. Dial. Transplant. 1996, 11, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Colombatto, P.; Randone, G.A.; Civitico, G.; Monti Gorin, L.; Dolcl, N.; Medaina, G.; Calleri, F.; Oliveri, M.; Baldi, G.; Tapper, R.; et al. A new hepatitis c virus-like flavivirus in patients with cryptogenic liver disease associated with elevated GGT and alkaline phosphatase serum levels. J. Viral Hepat. 2010, 4, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, S.; Yu, X.; Hao, L. Responsive methylene blue release from lanthanide coordination polymer for label-free, immobilization-free and sensitive electrochemical alkaline phosphatase activity assay. Analyst 2019, 144, 5971–5979. [Google Scholar] [CrossRef]
- Fan, H.Y.; Dukenbayev, K.; Sun, Q.L.; Khamijan, M.; Turdaliyev, A.; Ysmaiyl, A.; Tassanbiyeva, A.; Ma, C.P.; Xie, Y.Q. A carbon dot-based co-nanozyme with alkaline phosphatase—Mechanism and application. RSC Adv. 2021, 11, 33253–33259. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pandey, S.; Khan, M.S.; Wu, H.F. Protein stabilized fluorescent gold nanocubes as selective probe foralkaline phosphatase via inner filter effect. Sens. Actuators B Chem. 2018, 259, 83–89. [Google Scholar] [CrossRef]
- Shaban, S.M.; Moon, B.S.; Pyun, D.G.; Kim, D.H. A colorimetric alkaline phosphatase biosensor based on p-aminophenol-mediated growth of silver nanoparticles. Colloids Surf. B 2021, 205, 111835. [Google Scholar] [CrossRef]
- Ruan, C.; Wang, W.; Gu, B. Detection of alkaline phosphatase using surface-enhanced raman spectroscopy. Anal. Chem. 2006, 78, 3379–3384. [Google Scholar] [CrossRef]
- Iftikhar, T.; Asif, M.; Aziz, A.; Ashraf, G.; Jun, S.; Li, G.; Liu, H. Topical advances in nanomaterials based electrochemical sensors for resorcinol detection. Trends Environ. Anal. Chem. 2021, 31, e00138. [Google Scholar] [CrossRef]
- Xia, X.; Shuyu, L.; Hua, L.; Kang, R.; Yunxian, J.; Zixiang, W.S.; Zarnaz, K.; Zhihao, C.; Airong, Q.; Lifang, H. Piezo channels: Awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int. J. Mol. Sci. 2021, 22, 6429. [Google Scholar]
- Falahi, S.; Rafiee-Pour, H.A.; Zarejousheghani, M.; Rahimi, P.; Joseph, Y. Non-Coding RNA-Based biosensors for early detection of liver cancer. Biomedicines 2021, 9, 964. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Mengmeng, W.; Luyan, W.; Ning, X. Recent progress in electrochemical biosensors for detection of prostate-specific antigen. Int. J. Electrochem. Sci. 2018, 13, 4071–4084. [Google Scholar]
- Freitas, M.; Nouws, H.; Keating, E.; Fernandes, V.C.; Delerue-Matos, C. Immunomagnetic bead-based bioassay for the voltammetric analysis of the breast cancer biomarker HER2-ECD and tumour cells using quantum dots as detection labels. Microchim. Acta 2020, 187, 18. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and analysis of cancer biomarkers based on electrochemical biosensors. J. Electrochem. Soc. 2020, 167, 037525. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, W.; Lu, J.; Hao, L.; Yang, H.; Liu, Y.; Si, F.; Kong, J. Grafting of polymers via ring-opening polymerization for electrochemical assay of alkaline phosphatase activity. Anal. Chim. Acta 2021, 1185, 339069. [Google Scholar] [CrossRef]
- Wang, W.; Jing, L.; Hao, L.; Yang, H.; Song, X.; Si, F. Electrochemical detection of alkaline phosphatase activity through enzyme-catalyzed reaction using aminoferrocene as an electroactive probe. Anal. Bioanal. Chem. 2021, 413, 1827–1836. [Google Scholar] [CrossRef]
- Chopade, P.; Dugasani, S.R.; Jeon, S.; Jeong, J.H.; Park, S.H. Enhanced functionalities of DNA thin films by facile conjugation with conducting polymers. Curr. Appl. Phys. 2020, 20, 161–166. [Google Scholar] [CrossRef]
- Li, W.; Cheng, N.; Cao, Y.; Zhao, Z.; Xiao, Z.; Wei, Z.; Sun, Z. Boost the performance of inverted perovskite solar cells with PEDOT:PSS/Graphene quantum dots composite hole transporting layer. Org. Electron. 2019, 78, 105575. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Liu, K.; Parit, M.; Jiang, Z.; Zhang, X.; Li, B.; Si, C. Conductive PEDOT:PSS/cellulose nanofibril paper electrodes for flexible supercapacitors with superior areal capacitance and cycling stability. Chem. Eng. J. 2022, 428, 131994. [Google Scholar] [CrossRef]
- Pang, D.; Ma, C.; Chen, D.; Shen, Y.l.; Zhu, W.; Gao, J.; Song, H.; Zhang, X.; Zhang, S. Silver nanoparticle-functionalized poly (3, 4-ethylenedioxythiophene): Polystyrene film on glass substrate for electrochemical determination of nitrite. Org. Electron. 2019, 75, 1053742. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Z.; Yang, H.; Li, P.; Yu, Z.; Zhang, W.; Shi, J.; Hu, J.; Chen, Y. Synthesis and electrochemical performance of gold nanoparticles deposited onto a reduced graphene oxide/nickel foam hybrid structure for hydrazine detection. J. Mater. Sci. 2020, 5, 55. [Google Scholar] [CrossRef]
- Ma, C.; Yang, C.; Zhang, M. A novel electrochemical hydrogen peroxide sensor based on AuNPs/ n -type GaN electrode. Chem. Lett. 2020, 6, 49. [Google Scholar] [CrossRef]
- Bai, L.; Chen, Y.; Liu, X.; Zhou, J.; Cao, J.; Hou, L.; Guo, S. Ultrasensitive electrochemical detection of mycobacterium tuberculosis IS6110 fragment using gold nanoparticles decorated fullerene nanoparticles/nitrogen-doped graphene nanosheet as signal tags. Anal. Chim. Acta 2019, 1080, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, H.; Ma, Z. Conductive hydrogel composed of 1,3,5-benzenetricarboxylic acid and Fe3+ used as enhanced electrochemical immunosensing substrate for tumor biomarker. Bioelectrochemistry 2017, 114, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Z.; Wang, G. A novel signal amplification strategy electrochemical immunosensor for ultra-sensitive determination of p53 protein. Bioelectrochemistry 2020, 137, 107647. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, C.J.; Feng, J.; Hou, Y.; Ruan, Y.; Weng, X.; Milcovich, G. Gold nanoparticles/electrochemically expanded graphite composite: A bifunctional platform toward glucose sensing and SERS applications. J. Electroanal. Chem. 2019, 851, 113471. [Google Scholar] [CrossRef]
- Rathera, J.A.; Abri, A.A.; Kannan, P. Electrochemical sensing of parabens in solubilized ionic liquid system at polyaniline decorated gold nanoparticles constructed interface. Microchem. J. 2020, 159, 105379. [Google Scholar] [CrossRef]
- Zhou, C.H.; Lia, X.; Zi, Q.J.; Wang, J.; Zhao, W.Y.; Cao, Q.E. An enzyme-induced metallization-based electrochemical signal amplification strategy for ultrahigh sensitive alkaline phosphatase detection at attomolar concentrations. Anal. Chem. J. 2020, 75, 812–819. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F.A. Fabrication and characterization of an ammonia gas sensor based on PEDOT-PSS with N-Doped graphene quantum dots dopant. IEEE Sens. J. 2016, 16, 6149–6154. [Google Scholar] [CrossRef]
- Roberts, J.G.; Sombers, L.A. Fast scan cyclic voltammetry: Chemical sensing in the brain and beyond. Anal. Chem. 2017, 7b04732. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, N.; Khan, I. Plasmonic gold nanoparticles (AuNPs): Properties, synthesis and their advanced energy, environmental and biomedical applications. Chem. Asian. J. 2021, 16, 720–742. [Google Scholar] [CrossRef] [PubMed]
- Thiago, R.L.C.P. Measuring electrochemical surface area of nanomaterials versus randles-Ševčík equation. Chem. Electro. Chem. 2020, 17, 3414–3415. [Google Scholar]
- Wu, L.; Lu, X.; Dhanjai, W.Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, H.; Huang, K.; Quan, J.; Yu, F.; Liu, X.; Jiang, H.; Wang, X. Target-triggered hybridization chain reaction for ultrasensitive dual-signal miRNA detection. Biosens. Bioelectron. 2022, 215, 0956–5663. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Feng, J.; Wang, P.; Dong, Y. The label-free immunosensor based on rhodium@palladium nanodendrites/sulfo group functionalized multi-walled carbon nanotubes for the sensitive analysis of carcino embryonic antigen. Anal. Chim. Acta 2018, 1007, 61–70. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Z.; Li, H.; Xiong, Y.; Cai, S.; Wang, C.; Chen, Y.; Han, N.; Yang, R. Magnet-assisted electrochemical immunosensor based on surface-clean Pd-Au nanosheets for sensitive detection of SARS-CoV-2 spike protein. Electrochim. Acta 2022, 404, 139766. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Zheng, J. Selective determination of cholesterol based on cholesterol oxidase-alkaline phosphatase bienzyme electrode. Analyst 2012, 137, 5363–5367. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Gu, S.; Liang, J. Effects of wnt/b-catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Prolif. 2012, 45, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, X. Alkaline phosphatase-responsive anodic electrochemiluminescence of CdSe nanoparticles. Anal. Chem. 2012, 84, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, J.; Duan, M.; Zhang, H.; Jiang, J.; Yu, R. Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal. Chem. 2013, 85, 3797–3801. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sun, Y.; Lin, L.; Shi, C.; Zhang, X.; Wang, G. Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu(ii). Analyst 2016, 141, 5549–5554. [Google Scholar] [CrossRef]
- Feng, Y.A.; Xu, H.; Zhou, Y.; Wang, B.J.; Xiao, J.; Wang, Y.W.; Peng, Y. Ratiometric detection and bioimaging of endogenous alkaline phosphatase by a NIR fluorescence probe. Sens. Actuators B Chem. 2022, 358, 131505. [Google Scholar] [CrossRef]
- Xiang, M.H.; Liu, J.W.; Li, N.; Tang, H.; Yu, R.Q.; Jiang, J.H. Fluorescent graphitic carbon nitride nanosheet biosensor for highly sensitive, label-free detection of alkaline phosphatase. Nanoscale 2016, 8, 4727–4732. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Zhou, X.; Chen, J.; Wang, M.; Su, X. Fe–N–C single-atom nanozymes with peroxidase-like activity for the detection of alkaline phosphatase. Analyst 2021, 146, 896–903. [Google Scholar] [CrossRef]
- Li, Y.; Xie, R.; Pang, X.; Zhou, Z.; Zhang, Y. Aggregation-induced emission fluorescent probe for monitoring endogenous alkaline phosphatase in living cells. Talanta 2019, 205, 120143. [Google Scholar] [CrossRef]
- Song, H.; Niu, X.; Ye, K.; Wang, L.; Peng, Y. A novel alkaline phosphatase activity sensing strategy combining enhanced peroxidase-mimetic feature of sulfuration-engineered coox with electrostatic aggregation. Anal. Bioanal. Chem. 2020, 412, 5551–5561. [Google Scholar] [CrossRef]


| Method | Linear Range (U L–1) | Detection Limit (U L–1) | Reference |
|---|---|---|---|
| Electrochemiluminescence | 2–25 | 2 | [42] |
| Electrochemiluminescence | 5–50 | 0.8 | [1] |
| Fluorescence | 0.3–7.5 | 0.3 | [43] |
| Electrochemical immunoassay | 10–120 | 5.4 | [44] |
| Fluorescent technology | 0.0–1000 | 0.87 | [45] |
| Fluorescent technology | 0.1–1000 | 0.08 | [46] |
| colorimetric methodology | 0.1–1.5 | 0.05 | [47] |
| Fluorescent technology | 1–100 | 0.06 | [48] |
| colorimetric methodology | 0.8–320 | 0.38 | [49] |
| Electrochemical immunoassay | 0.1–120 | 0.03 | This work |
| Sample | Added (U L–1) | Total Found (U L–1) | Recovery % | RSD % |
|---|---|---|---|---|
| 1 | 10 | 26.02 ± 2.82 | 91.4 | 8.8 |
| 2 | 20 | 37.10 ± 3.64 | 101.1 | 8.6 |
| 3 | 30 | 47.93 ± 4.04 | 103.5 | 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Kang, J.; Liu, J.; Wang, G. A Novel Electrochemical Sensing Strategy Based on Poly (3, 4-ethylenedioxythiophene): Polystyrene Sulfonate, AuNPs, and Ag+ for Highly Sensitive Detection of Alkaline Phosphatase. Nanomaterials 2022, 12, 3392. https://doi.org/10.3390/nano12193392
Lei J, Kang J, Liu J, Wang G. A Novel Electrochemical Sensing Strategy Based on Poly (3, 4-ethylenedioxythiophene): Polystyrene Sulfonate, AuNPs, and Ag+ for Highly Sensitive Detection of Alkaline Phosphatase. Nanomaterials. 2022; 12(19):3392. https://doi.org/10.3390/nano12193392
Chicago/Turabian StyleLei, Jiangshan, Jian Kang, Jifa Liu, and Guannan Wang. 2022. "A Novel Electrochemical Sensing Strategy Based on Poly (3, 4-ethylenedioxythiophene): Polystyrene Sulfonate, AuNPs, and Ag+ for Highly Sensitive Detection of Alkaline Phosphatase" Nanomaterials 12, no. 19: 3392. https://doi.org/10.3390/nano12193392






