Template-Free Hydrothermal Synthesis of Octahedron-, Diamond-, and Plate-like ZrO2 Mono-Dispersions
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faber, K.T. Superelastic and shape-memory ceramics may find application in high-temperature actuators and energy-harvesting devices. Science 2013, 341, 1464. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Luo, Z.; Liu, S.; Zeng, T.; Liu, L.; Xu, J.; Bai, Z.; Xua, W. Photoluminescence properties and photocatalytic activities of zirconia nanotube arrays fabricated by anodization. Opt. Mater. 2013, 35, 1461–1466. [Google Scholar] [CrossRef]
- Cao, H.Q.; Qiu, X.Q.; Luo, B.; Liang, Y.; Zhang, Y.H.; Tan, R.Q.; Zhao, M.J.; Zhu, Q.M. Synthsis and room-temperature ultraviolet photoluminescence properties of zirconia nanowires. Adv. Funct. Mater. 2004, 14, 243–246. [Google Scholar] [CrossRef]
- Zheng, L.; Li, K.; Ning, C.; Sun, J. Study on antibacterial and fluoride-releasing properties of a novel composite resin with fluorine-doped nano-zirconia fillers. J. Dent. 2021, 113, 103772. [Google Scholar] [CrossRef]
- Lu, Y.; Bangsaruntip, S.; Wang, X.; Zhang, L.; Nishi, Y.; Dai, H. DNA Functionalization of Carbon Nanotubes for Ultrathin Atomic Layer Deposition of High Dielectrics for Nanotube Transistors with 60 mV/Decade Switching. J. Am. Chem. Soc. 2006, 128, 3518–3519. [Google Scholar] [CrossRef]
- Gao, L.; Guan, R.; Zhang, S.; Zhi, H.; Jin, C.; Jin, L.; Wei, Y.; Wang, J. As-sintered manganese-stabilized zirconia ceramics with excellent electrical conductivity. Crystals 2022, 12, 620. [Google Scholar] [CrossRef]
- Dong, S.; Liu, W.; Liu, S.; Li, F.; Hou, J.; Hao, R.; Bai, X.; Zhao, H.; Liu, J.; Guo, L. Single atomic Pt on amorphous ZrO2 nanowires for advanced photocatalytic CO2 reduction. Mater. Today Nano 2022, 17, 100157. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, F.; Wu, N.Q.; Liu, X.G. Up- and down-conversion cubic zirconia and hafnia nanobelts. Adv. Mater. 2008, 20, 4826–4829. [Google Scholar] [CrossRef]
- Wang, A.; Lia, C.; Jiang, L.; Chen, B.; Zhang, S.; Xua, X.; Zhu, X. Preparation and formation mechanism of fast-growing ZrO2 nanotubes and slow-growing TiO2 nanotubes. Ceram. Int. 2022, 48, 27703–27711. [Google Scholar] [CrossRef]
- Espinoza-González, R.A.; Diaz-Droguett, D.E.; Avila, J.I.; Gonzalez-Fuentes, C.A.; Fuenzalida, V.M. Hydrothermal growth of zirconia nanobars on zirconium oxide. Mater. Lett. 2011, 65, 2121–2123. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Dawoud, T.; Ameen, F.; Ravi, G.; Yuvakkumar, R.; Kumar, P.; Hong, S.I.; Velauthapillai, D.; Saravanakumar, B. Synthesis of highly active biocompatible ZrO2 nanorods using a bioextract. Ceram. Int. 2020, 46, 25915–25920. [Google Scholar] [CrossRef]
- Kato, E.; Hirano, M.; Nagai, A. Growth of monoclinic ZrO2, thin, flaky crystals by hydrothermal decomposition of zirconium oxide sulfate crystals. J. Am. Ceram. Soc. 1995, 78, 2259–2262. [Google Scholar] [CrossRef]
- Dedov, N.V.; Dorda, F.A.; Goloshchapov, R.G.; Skotnova, V.N.; Korobtsev, V.P.; Khasanov, O.L.; Sokolov, V.M.; Dvilis, Ṡ. Finely disperse powders of stabilized zirconia with flake particles. Glass Ceram. 1995, 52, 335–337. [Google Scholar] [CrossRef]
- Nayak, B.B.; Mohanty, S.K.; Takmeel, M.Q.B.; Pradhan, D.; Mondal, A. Borohydride synthesis and stabilization of flake-like tetragonal zirconia nanocrystallites. Mater. Lett. 2010, 64, 1909–1911. [Google Scholar] [CrossRef]
- Poungchan, G.; Ksapabutr, B.; Panapoy, M. One-step synthesis of flower-like carbon-doped ZrO2 for visible-light-responsive photocatalyst. Mater. Des. 2016, 89, 137–145. [Google Scholar] [CrossRef]
- Aflaki, M.; Davar, F. Synthesis, luminescence and photocatalyst properties of zirconia nanosheets by modified Pechini method. J. Mol. Liq. 2016, 221, 1071–1079. [Google Scholar] [CrossRef]
- Dong, W.S.; Lin, F.Q.; Liu, C.L.; Li, M.Y. Synthesis of ZrO2 nanowires by ionic-liquid route. J. Colloid. Interf. Sci. 2009, 333, 734–740. [Google Scholar] [CrossRef]
- Ji, H.; Liu, X.; Wang, X.; Yao, X. Influence of counter-ions on the self-assembly of ZrO2 nanodisks. J. Colloid. Interf. Sci. 2011, 353, 356–362. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yuan, S.; Shi, L.; Zhao, Y.; Zhang, M.; Deng, W. Hydrothermal synthesis and humidity sensing properties of size-controlled zirconium oxide (ZrO2) nanorods. J. Colld. Inter. Sci. 2013, 396, 9–15. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Liu, J.; Jiang, F.; Tang, H.; Feng, G.; Jiang, W. Preparation, characterization and growth mechanism of ZrO2 nanosheets. Ceram. Int. 2020, 46, 4864–4869. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.J.; Yu, S.H. Recent advances in oriented attachment growth and synthesis of functional materials: Concept, evidence, mechanism, and future. J. Mater. Chem. 2009, 19, 191–207. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-Assembly of ZnO: From Nanodots to Nanorods. Angew. Chem. Int. Edit. 2002, 41, 1188. [Google Scholar] [CrossRef]
- Rahaman, M.A.; Rout, S.; Thomas, J.P.; McGillivary, D.; Leung, K.T. Defect-rich dopant-free ZrO2 nanostructures with superior dilute ferromagnetic semiconductor properties. J. Am. Chem. Soc. 2016, 138, 11896–11906. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Yu, J.; Ho, W.; Jlang, Z.; Zhang, L. Effects of F− doping on the photocatalytie activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic anions. 1. Hydroxyl-Mediated and direct electron-transfer reactions of phenol on a titanium dioxide-fluoride system. Langmuir 2000, 16, 2632–2641. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.S.; Sharma, S.C.; Nagaswarupa, H.P.; Prashantha, S.C.; Nagabhushana, H.; Vidya, Y.S. Hollow microspheres Mg-doped ZrO2 nanoparticles: Green assisted synthesis and applications in photo-catalysis and photoluminescence. J. Alloy. Comp. 2016, 672, 609–622. [Google Scholar] [CrossRef]
- Li, W.J.; Shi, E.W.; Xia, C.T.; Wang, B.G.; Hua, S.K.; Zhong, W.Z. Epitaxy of ZrO2 powders prepared by hydrothermal salt solution hydrolysis method. J. Synthetic. Crystal. 1998, 27, 65–69. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large Percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Ratnawati, J.; Gunlazuardi, E.L. Dewi, Slame, Effect of NaBF4 addition on the anodic synthesis of TiO2 nanotube arrays photocatalyst for production of hydrogen from glycerolewater solution. Int. J. Hydrog. Energ. 2014, 39, 16927–16935. [Google Scholar] [CrossRef]
- Zhou, G.B.; Pei, Y.; Jiang, Z.; Fan, K.N.; Qiao, M.H.; Sun, B.; Zong, B.N. Doping effects of B in ZrO2 on structural and catalytic properties of Ru/B-ZrO2 catalysts for benzene partial hydrogenation. J. Catal. 2014, 311, 393–403. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, B.; Jiang, G.; Yang, J.; Li, Y.; Liu, W.; Wang, J.; Kong, W. From modification to mechanism: Supercritical hydrothermal synthesis of nano-zirconia. Ceram. Int. 2022, 48, 4401–4423. [Google Scholar] [CrossRef]
- Ahmad, S.; Kharkwal, M.; Govind; Nagarajan, R. Application of KZnF3 as a single source precursor for the synthesis of nanocrystals of ZnO2:F and ZnO:F; synthesis, characterization, optical, and photocatalytic properties. J. Phys. Chem. C 2011, 115, 10131–10139. [Google Scholar] [CrossRef]
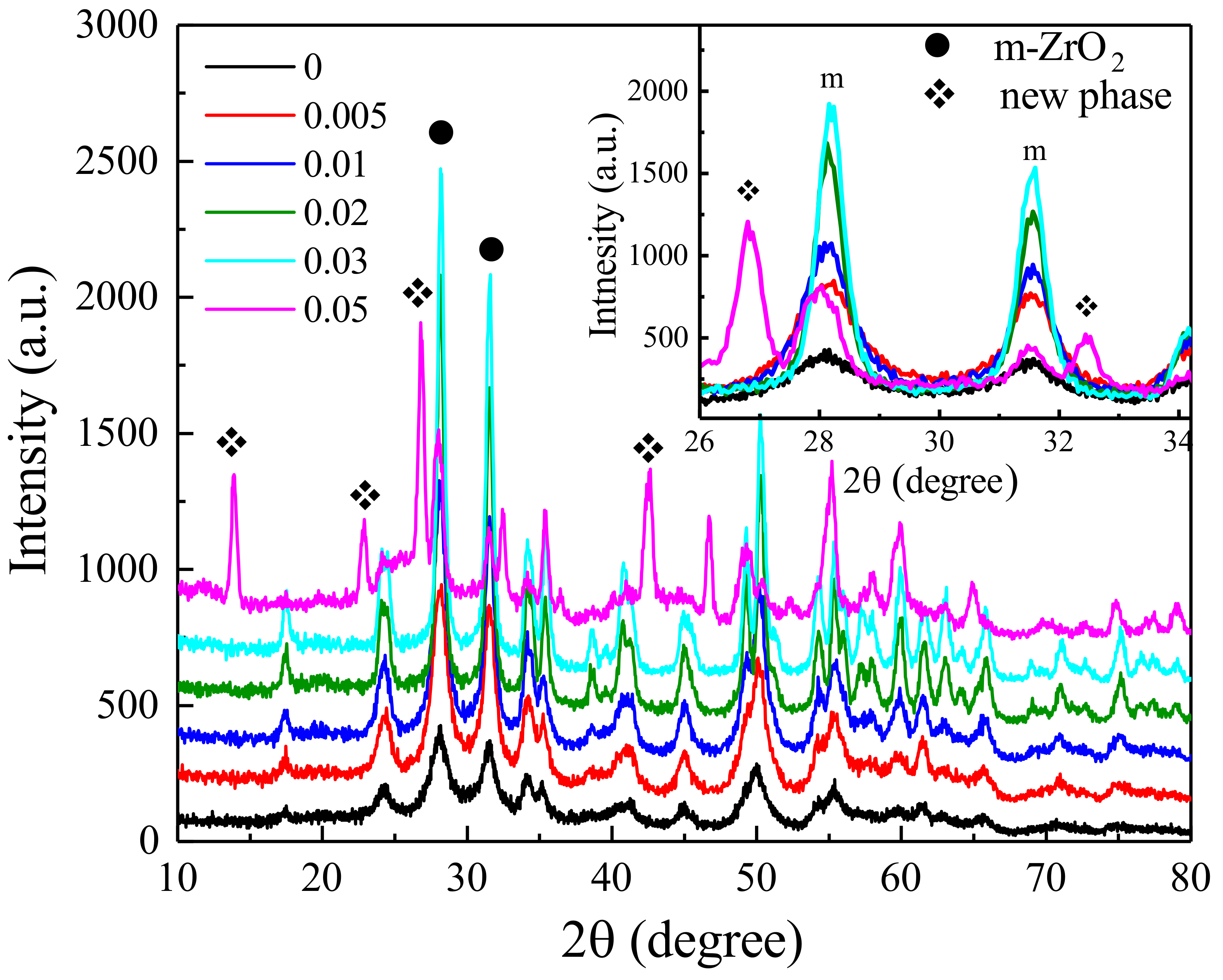



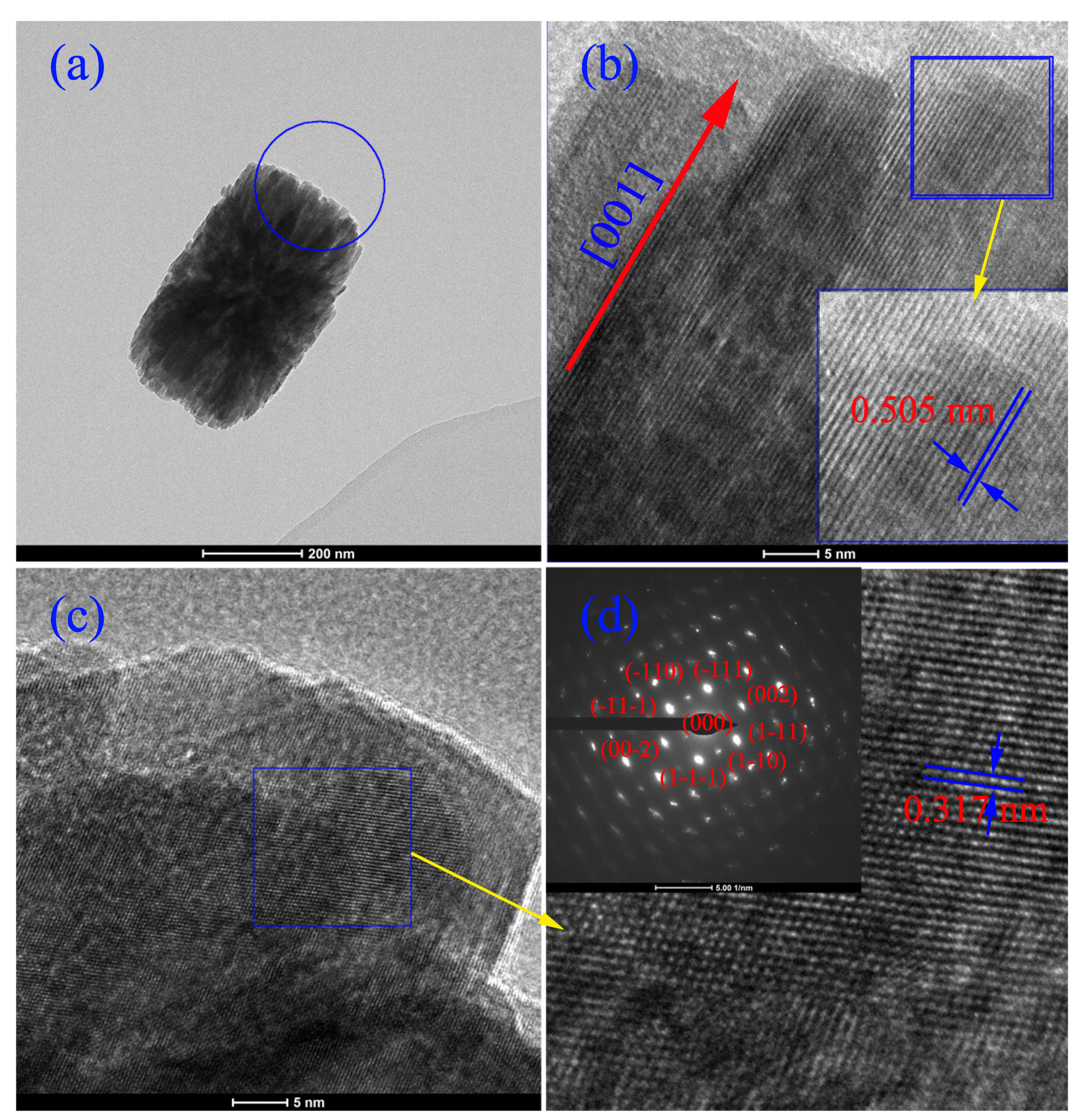
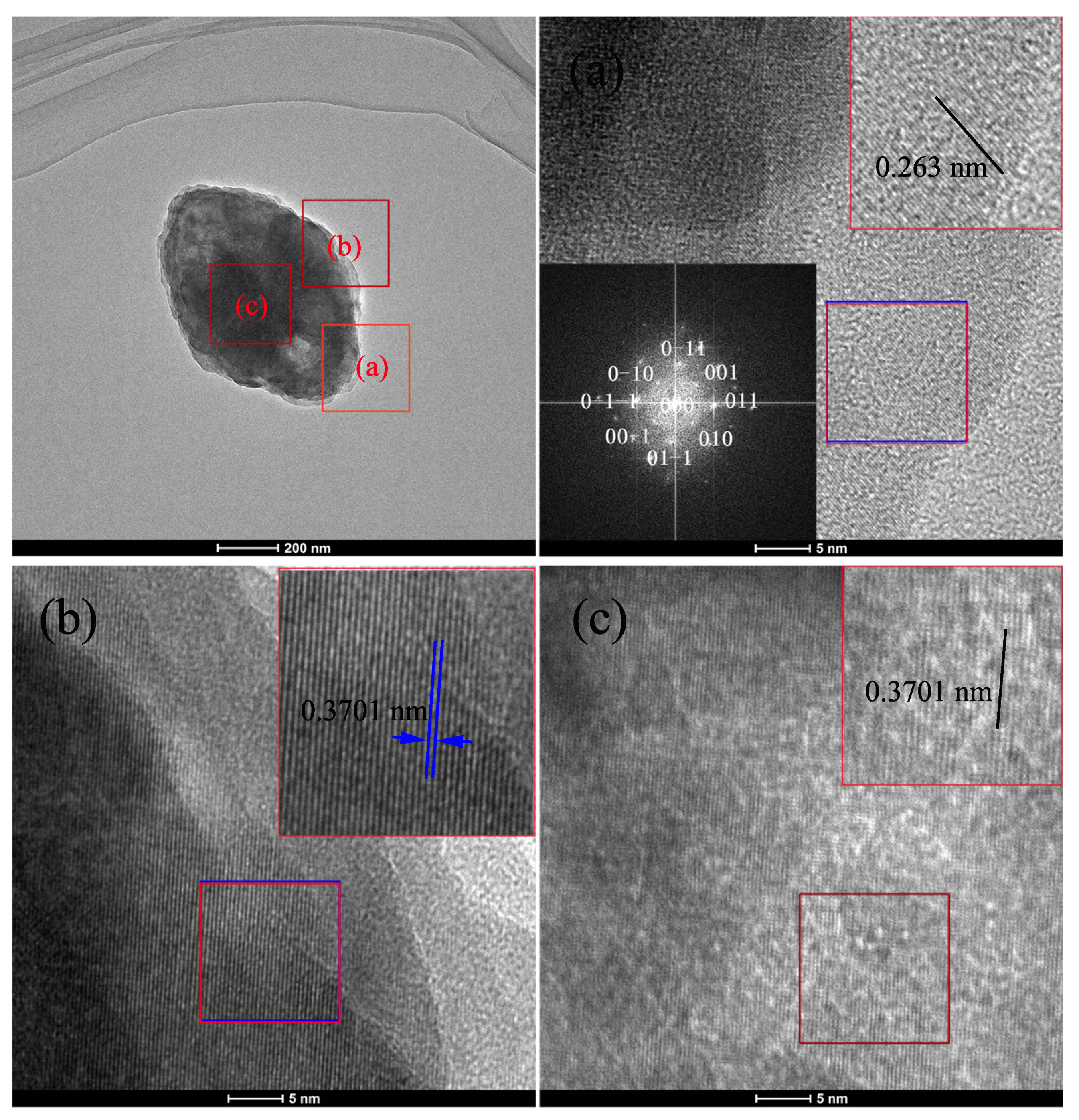
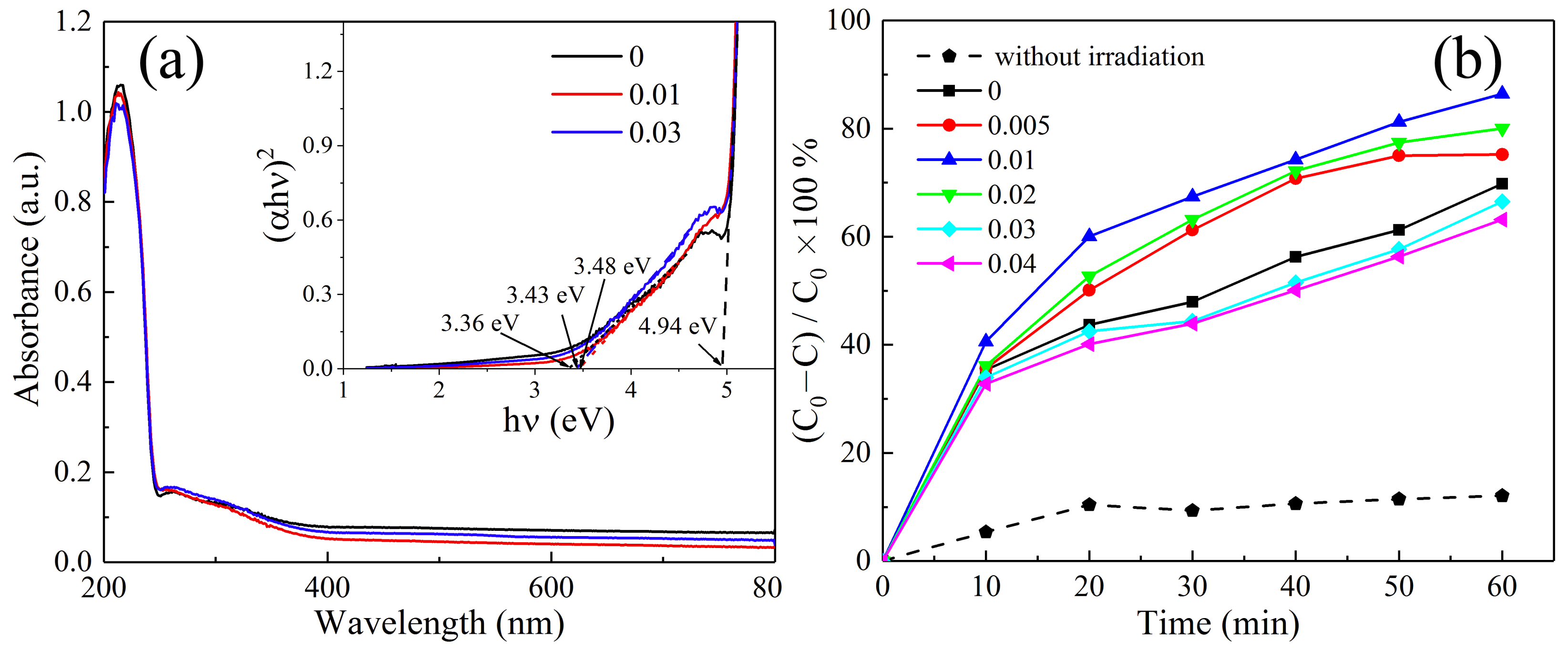
| Concentration | Primary Crystallite Size (nm) | Secondary Particle Size | |||
|---|---|---|---|---|---|
| L (nm) | W (nm) | T (nm) | L:W | ||
| 0 | 6.2 | 46.6 | 46.8 | 21.2 | 1:1 |
| 0.005 | 9.3 | 119.7 | 97.2 | 63.3 | 1.23:1 |
| 0.010 | 12.4 | 536.1 | 320.9 | 174.0 | 1.67:1 |
| 0.020 | 13.4 | 576.3 | 365.2 | 204.8 | 1.58:1 |
| 0.030 | 15.1 | 1995.0 | 1533.0 | 278.6 | 1.30:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Zhi, H.; Zhang, S.; Liu, S. Template-Free Hydrothermal Synthesis of Octahedron-, Diamond-, and Plate-like ZrO2 Mono-Dispersions. Nanomaterials 2022, 12, 3405. https://doi.org/10.3390/nano12193405
Gao L, Zhi H, Zhang S, Liu S. Template-Free Hydrothermal Synthesis of Octahedron-, Diamond-, and Plate-like ZrO2 Mono-Dispersions. Nanomaterials. 2022; 12(19):3405. https://doi.org/10.3390/nano12193405
Chicago/Turabian StyleGao, Ling, Hao Zhi, Shengnan Zhang, and Shifeng Liu. 2022. "Template-Free Hydrothermal Synthesis of Octahedron-, Diamond-, and Plate-like ZrO2 Mono-Dispersions" Nanomaterials 12, no. 19: 3405. https://doi.org/10.3390/nano12193405
APA StyleGao, L., Zhi, H., Zhang, S., & Liu, S. (2022). Template-Free Hydrothermal Synthesis of Octahedron-, Diamond-, and Plate-like ZrO2 Mono-Dispersions. Nanomaterials, 12(19), 3405. https://doi.org/10.3390/nano12193405






