Radical-Scavenging Activatable and Robust Polymeric Binder Based on Poly(acrylic acid) Cross-Linked with Tannic Acid for Silicon Anode of Lithium Storage System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
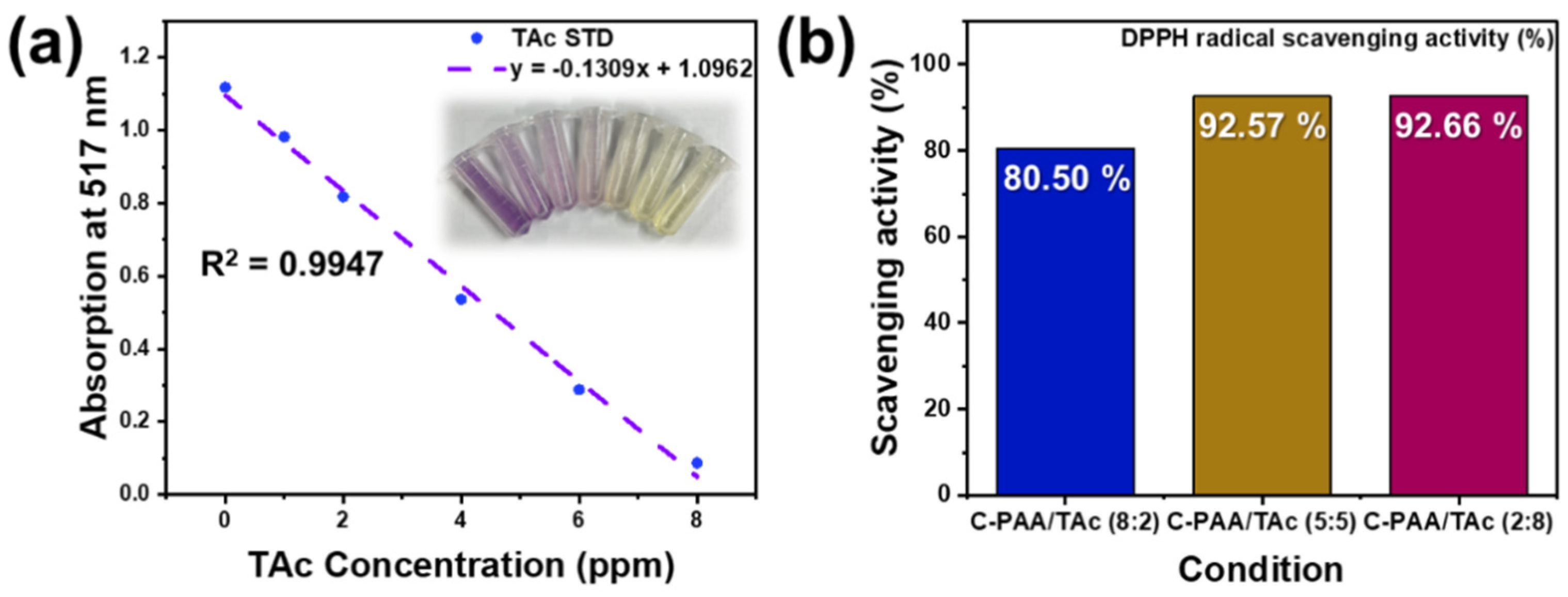
2.3. DPPH Radical-Scavenging Assay
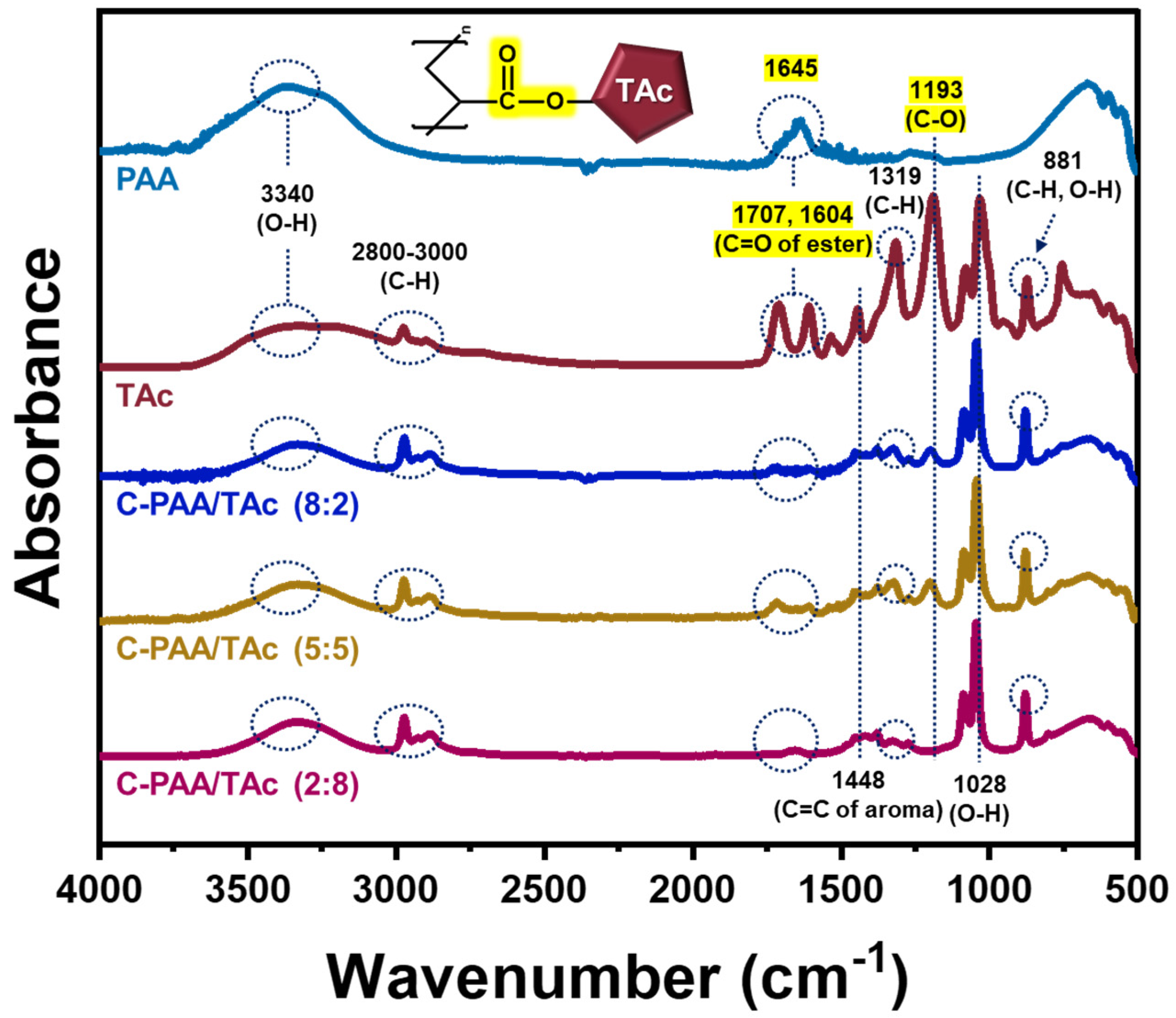
2.4. Characterization
2.5. Preparation of Silicon Anodes for Half Cells
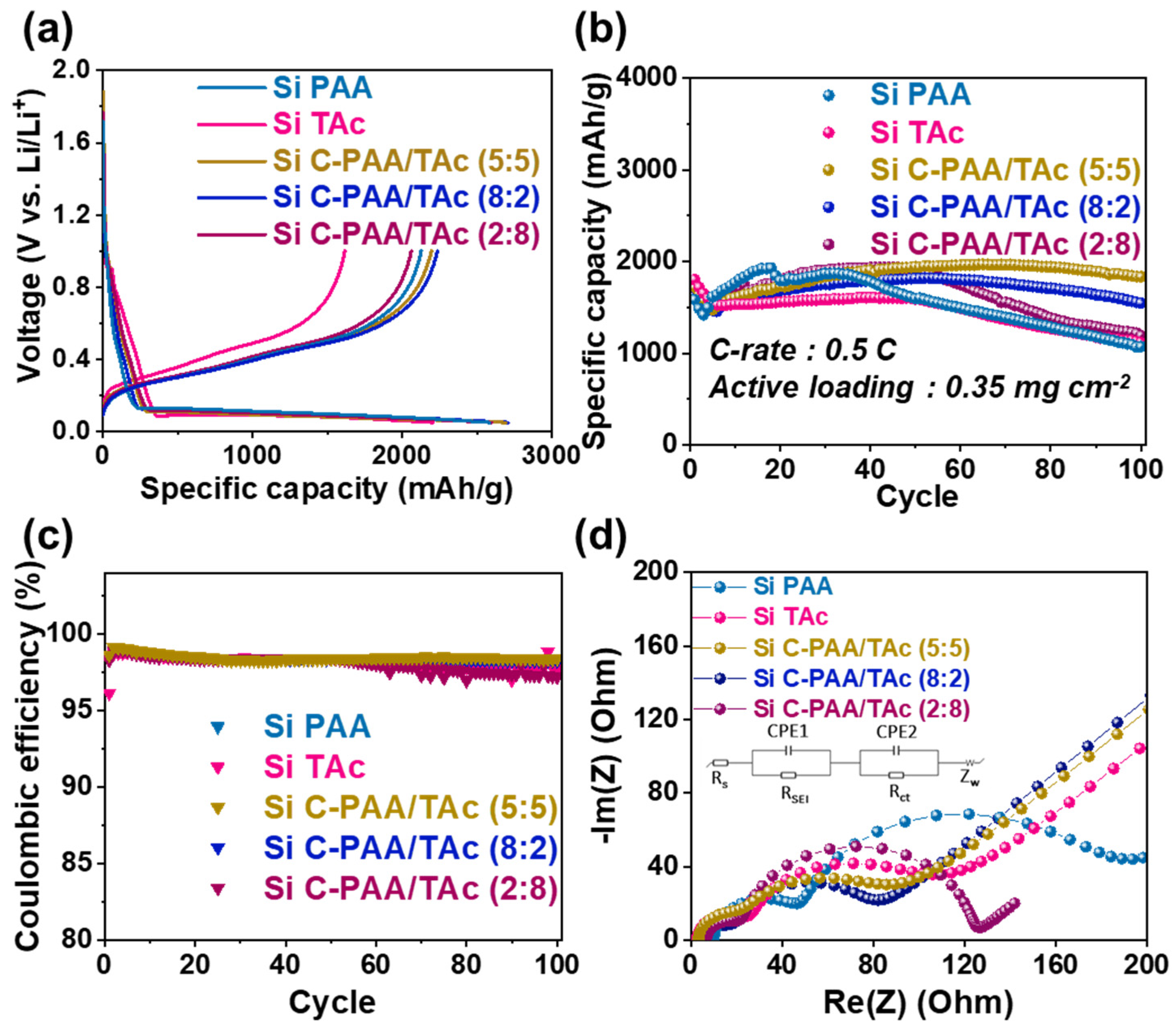
2.6. Electrochemical Measurements
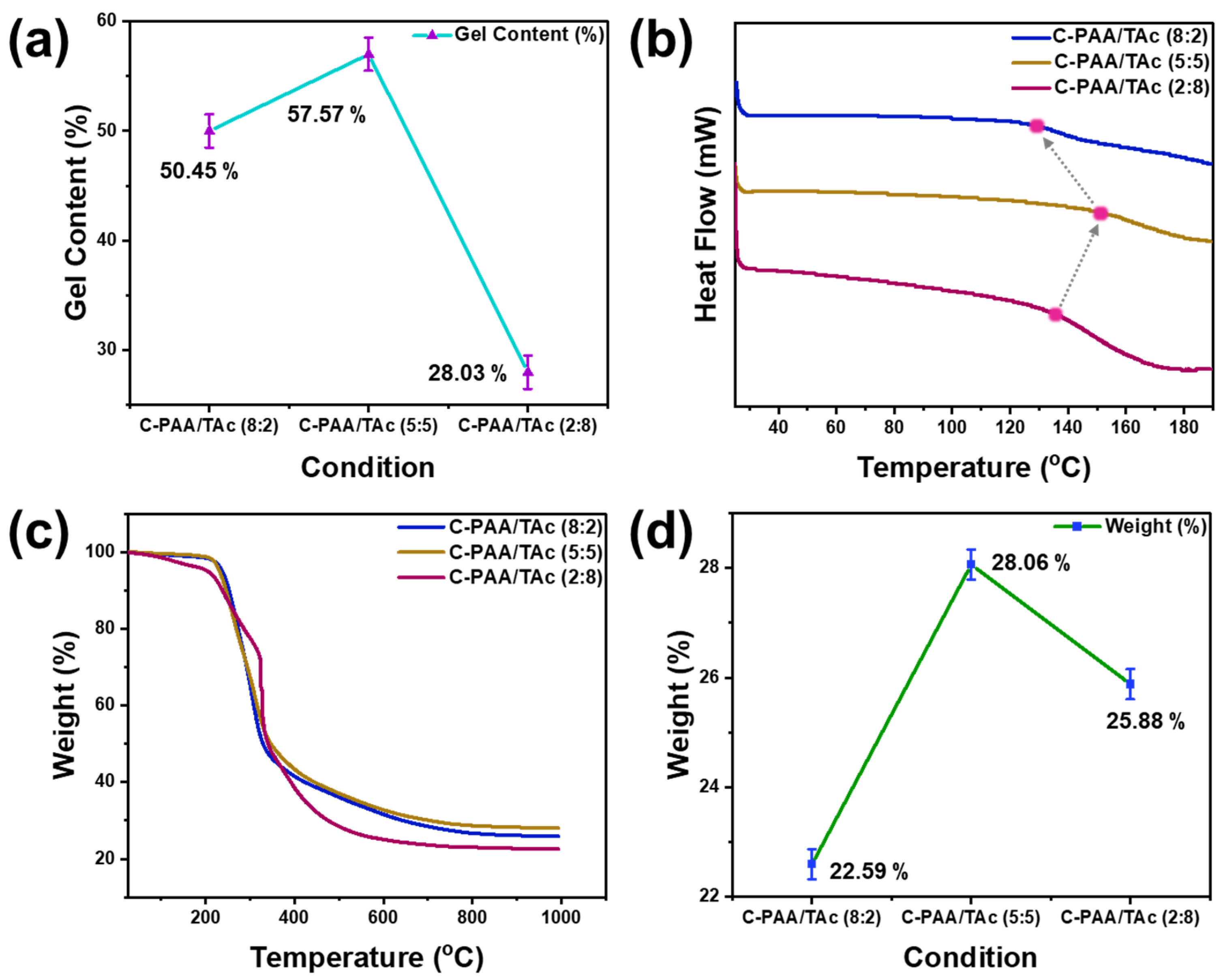
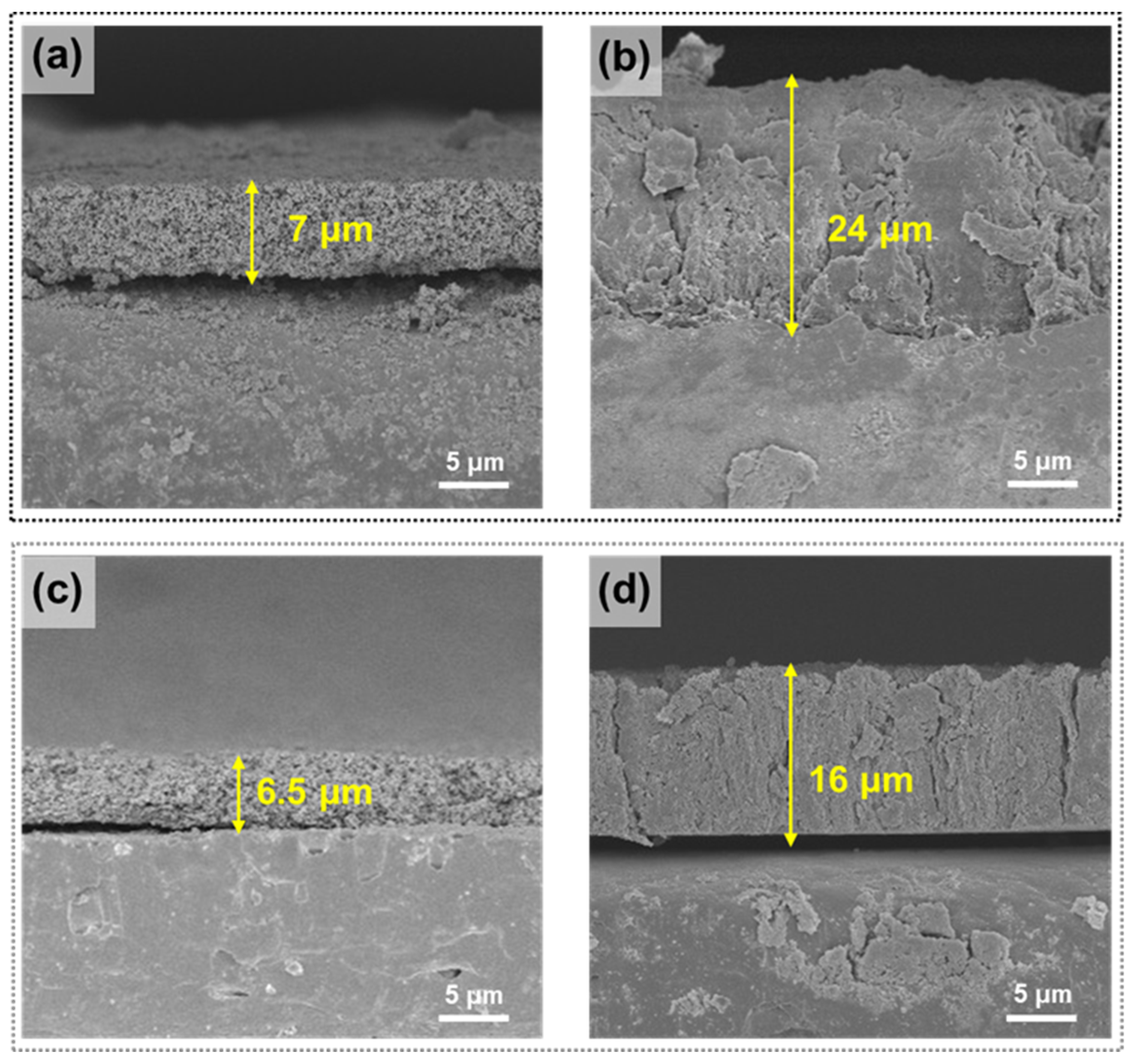
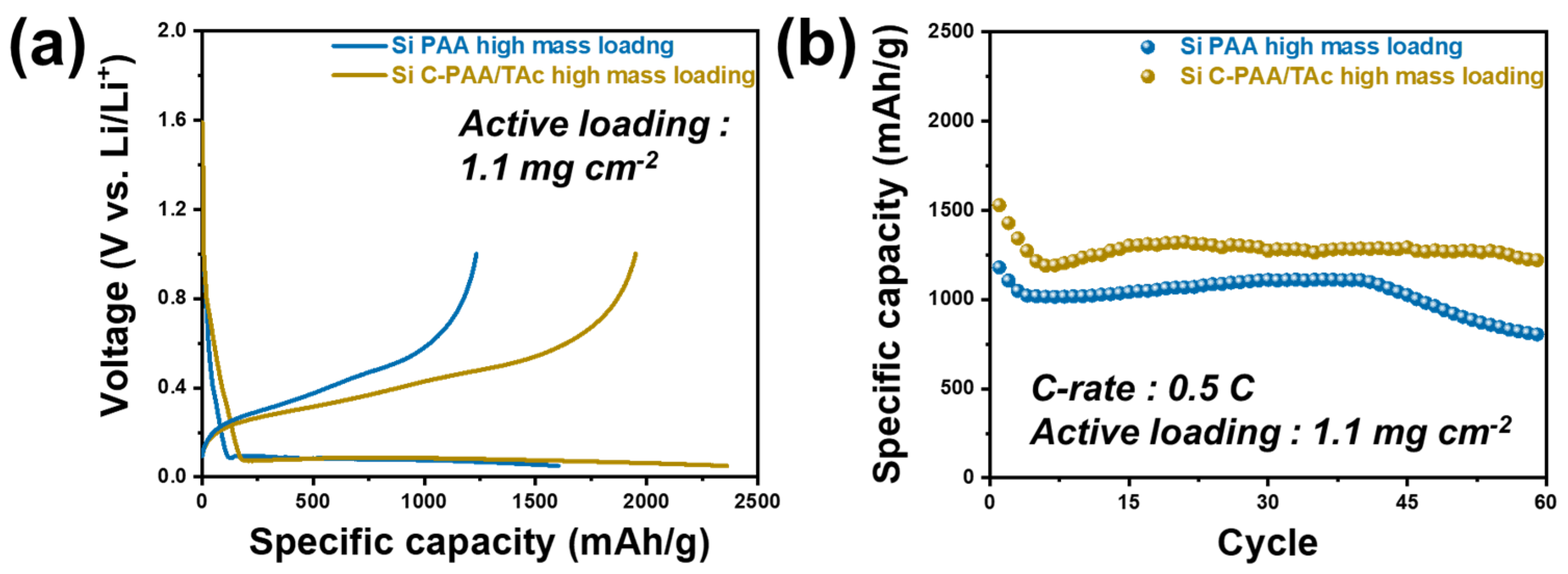
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cropper, M.; Griffiths, C. The Interaction of Population Growth and Environmental Quality. Am. Econ. Rev. 1994, 84, 250–254. [Google Scholar]
- IEA. Key World Energy Statistics, 2013; IEA Publication: Paris, France, 2013. [Google Scholar]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Narayan, T.; Chakraborty, S.; Konar, S.; Singh, S. Statistics as a technology to predict the seasonal variation of air pollution. IJITEE 2020, 9, 1426–1431. [Google Scholar]
- Hajilary, N.; Rezakazemi, M.; Shahi, A. CO2 emission reduction by zero flaring startup in gas refinery. Mater. Sci. Technol. 2020, 3, 218–224. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, T.; Kumari, M. Air pollution tolerance, metal accumulation and dust capturing capacity of common tropical trees in commercial and industrial sites. Sci. Total Environ. 2020, 722, 137622. [Google Scholar] [CrossRef]
- Rogelj, J.; Popp, A.; Calvin, K.V.; Luderer, G.; Emmerling, J.; Gernaat, D.; Fujimori, S.; Strefler, J.; Hasegawa, T.; Marangoni, G. Scenarios towards limiting global mean temperature increase below 1.5 C. Nat. Clim. Chang. 2018, 8, 325–332. [Google Scholar] [CrossRef]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovations 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Biomass power for energy and sustainable development. Environ. Eng. Manag. J. 2008, 7, 617–640. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Mozammil Hasnain, S.M.; Kumar Nayak, A.; Hasnain, M.S. Applications of biomass-derived materials for energy production, conversion, and storage. Mater. Sci. Technol. 2020, 3, 905–920. [Google Scholar] [CrossRef]
- Al Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of energy storage services, applications, limitations, and benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Gu, M.; He, Y.; Zheng, J.; Wang, C. Nanoscale silicon as anode for Li-ion batteries: The fundamentals, promises, and challenges. Nano Energy 2015, 17, 366–383. [Google Scholar] [CrossRef]
- Kwon, T.-w.; Jeong, Y.K.; Deniz, E.; AlQaradawi, S.Y.; Choi, J.W.; Coskun, A. Dynamic Cross-Linking of Polymeric Binders Based on Host–Guest Interactions for Silicon Anodes in Lithium Ion Batteries. ACS Nano 2015, 9, 11317–11324. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Beattie, S.D.; Loveridge, M.J.; Lain, M.J.; Ferrari, S.; Polzin, B.J.; Bhagat, R.; Dashwood, R. Understanding capacity fade in silicon based electrodes for lithium-ion batteries using three electrode cells and upper cut-off voltage studies. J. Power Sources 2016, 302, 426–430. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wu, J.; Shao, R.; Jiang, R.; Tie, Z.; Jin, Z. A Review on Recent Advances for Boosting Initial Coulombic Efficiency of Silicon Anodic Lithium Ion batteries. Small 2022, 18, 2102894. [Google Scholar] [CrossRef]
- Moon, J.; Lee, H.C.; Jung, H.; Wakita, S.; Cho, S.; Yoon, J.; Lee, J.; Ueda, A.; Choi, B.; Lee, S.; et al. Interplay between electrochemical reactions and mechanical responses in silicon–graphite anodes and its impact on degradation. Nat. Commun. 2021, 12, 2714. [Google Scholar] [CrossRef]
- Xu, Y.H.; Yin, G.; Ma, Y.; Zuo, P.; Cheng, X. Nanosized core/shell silicon@carbon anode material for lithium ion batteries with polyvinylidene fluoride as carbon source. J. Mater. Chem. 2010, 20, 3216–3220. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, Z.; Zhou, T.; Sencadas, V.; Zhang, J.; Zhang, L.; Konstantinov, K.; Guo, Z.; Liu, H.K. An All-Integrated Anode via Interlinked Chemical Bonding between Double-Shelled–Yolk-Structured Silicon and Binder for Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1703028. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Zhou, J.; Qian, T.; Wang, M.; Xu, N.; Zhang, Q.; Li, Q.; Yan, C. Core–Shell Coating Silicon Anode Interfaces with Coordination Complex for Stable Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5358–5365. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.A.; Safie, N.E.; Ahmad, A.S.; Yuza, N.A.; Zulkifli, N.S.A. Recent advances of silicon, carbon composites and tin oxide as new anode materials for lithium-ion battery: A comprehensive review. J Energy Storage 2021, 33, 102096. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Dong, T.; Mu, P.; Rong, X.; Li, Z. Uncovering the Chemistry of Cross-Linked Polymer Binders via Chemical Bonds for Silicon-Based Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 47164–47180. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Lee, H.A.; Shin, M.; Kim, J.; Choi, J.W.; Lee, H. Designing Adaptive Binders for Microenvironment Settings of Silicon Anode Particles. Adv. Mater. 2021, 33, 2007460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Y.; Chen, Y.; Huang, J.; Zhang, T.; Zeng, H.; Wang, C.; Liu, G.; Deng, Y. A Quadruple-Hydrogen-Bonded Supramolecular Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. Small 2018, 14, 1801189. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries. Sci. Rep. 2016, 6, 19583. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.-H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A Commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yang, S.; Zhu, G.; Yuan, Y.; Qu, Q.; Wang, Y.; Zheng, H. The effects of cross-linking cations on the electrochemical behavior of silicon anodes with alginate binder. Electrochim. Acta 2018, 269, 405–414. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Lestriez, B.; Bahri, S.; Sandu, I.; Roué, L.; Guyomard, D. On the binding mechanism of CMC in Si negative electrodes for Li-ion batteries. Electrochem. Commun. 2007, 9, 2801–2806. [Google Scholar] [CrossRef]
- Kwon, T.-W.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [PubMed]
- Baruah, P.; Duarah, R.; Karak, N. Tannic acid-based tough hyperbranched epoxy thermoset as an advanced environmentally sustainable high-performing material. Iran. Polym. J. 2016, 25, 849–861. [Google Scholar] [CrossRef]
- Tian, M.; Wu, P. Nature Plant Polyphenol Coating Silicon Submicroparticle Conjugated with Polyacrylic Acid for Achieving a High-Performance Anode of Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 5066–5073. [Google Scholar] [CrossRef]
- Jing, Y.; Diao, Y.; Yu, X. Free radical-mediated conjugation of chitosan with tannic acid: Characterization and antioxidant capacity. React. Funct. Polym. 2019, 135, 16–22. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-G.; Hwang, C.; Kim, S.H.; Park, C.; Kim, J.; Jung, G.Y.; Baek, K.; Chae, S.; Kang, S.J.; Cho, J.; et al. An Antiaging Electrolyte Additive for High-Energy-Density Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000563. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Shim, J.; Shin, H.; Lee, J.-C. Sustainable Lignin-Derived Cross-Linked Graft Polymers as Electrolyte and Binder Materials for Lithium Metal Batteries. ChemSusChem 2020, 13, 2642–2649. [Google Scholar] [CrossRef]
- Shim, J.; Bae, K.Y.; Kim, H.J.; Lee, J.H.; Kim, D.-G.; Yoon, W.Y.; Lee, J.-C. Solid Polymer Electrolytes Based on Functionalized Tannic Acids from Natural Resources for All-Solid-State Lithium-Ion Batteries. ChemSusChem 2015, 8, 4133–4138. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Yi, Z.; Zhao, Y.; Li, P.; Ho, K.; Blozowski, N.; Walker, G.; Jaffer, S.; Tjong, J.; Sain, M.; Lu, Z. The effect of tannic acids on the electrical conductivity of PEDOT:PSSFilms. Appl. Surf. Sci. 2018, 448, 583–588. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Wu, C.; Liao, C.; Li, L. Tannic-Acid-Coated Polypropylene Membrane as a Separator for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 16003–16010. [Google Scholar] [CrossRef]
- Lee, J.-S.; Jung, C.-H.; Jo, S.-Y.; Choi, J.-H.; Hwang, I.-T.; Nho, Y.-C.; Lee, Y.-M.; Lee, J.-S. Preparation of sulfonated crosslinked poly(2,6-dimethyl−1,4-phenylene oxide) membranes for direct methanol fuel cells by using electron beam irradiation. J. Polym. Sci. A Polym. Chem. 2010, 48, 2725–2731. [Google Scholar] [CrossRef]
- Koo, B.; Kim, H.; Cho, Y.; Lee, K.T.; Choi, N.S.; Cho, J. A highly cross-linked polymeric binder for high-performance silicon negative electrodes in lithium ion batteries. Angew. Chem. 2012, 124, 8892–8897. [Google Scholar] [CrossRef]
- Zhang, A.; Li, J.; Zhang, S.; Mu, Y.; Zhang, W.; Li, J. Characterization and acid-catalysed depolymerization of condensed tannins derived from larch bark. RSC Adv. 2017, 7, 35135–35146. [Google Scholar] [CrossRef] [Green Version]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Zhang, J.; Chen, W. Thermal behavior of collagen crosslinked with tannic acid under microwave heating. J. Therm. Anal. Calorim 2019, 135, 2329–2335. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; Groot, A.d.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Gang, Y.; Eom, T.-Y.; Marasinghe, S.D.; Lee, Y.; Jo, E.; Oh, C. Optimising the DPPH Assay for Cell-Free Marine Microorganism Supernatants. Mar. Drugs 2021, 19, 256. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Goyal, S.; Pandey, H.; Guleria, K.; Tewari, G. Variation in Antioxidant Activity and Antioxidant Constituents of Thymus serpyllum L. Grown in Different Climatic Conditions of Uttarakhand Himalayas. Def. Life Sci. J. 2021, 6, 109–116. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chem. 2016, 197, 864–871. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Luo, W.; Cui, L.; Wang, Y.-J.; Jian, T.; Li, X.; Yan, Q.; Liu, H.; Ouyang, C.; et al. Sequence-Defined Peptoids with OH and COOH Groups As Binders to Reduce Cracks of Si Nanoparticles of Lithium-Ion Batteries. Adv. Sci. 2020, 7, 2000749. [Google Scholar] [CrossRef]
- Wan, X.; Mu, T.; Shen, B.; Meng, Q.; Lu, G.; Lou, S.; Zuo, P.; Ma, Y.; Du, C.; Yin, G. Stable silicon anodes realized by multifunctional dynamic cross-linking structure with self-healing chemistry and enhanced ionic conductivity for lithium-ion batteries. Nano Energy 2022, 99, 107334. [Google Scholar] [CrossRef]
- Jiao, X.; Yuan, X.; Yin, J.; Boorboor Ajdari, F.; Feng, Y.; Gao, G.; Song, J. Multiple Network Binders via Dual Cross-Linking for Silicon Anodes of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 10306–10313. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Chen, X.; Jin, H.; Wang, J. Novel constructive self-healing binder for silicon anodes with high mass loading in lithium-ion batteries. Energy Storage Mater. 2021, 38, 121–129. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.G.; Jung, M.; Lee, S.; Song, W.-J.; Lee, J.-S. Radical-Scavenging Activatable and Robust Polymeric Binder Based on Poly(acrylic acid) Cross-Linked with Tannic Acid for Silicon Anode of Lithium Storage System. Nanomaterials 2022, 12, 3437. https://doi.org/10.3390/nano12193437
Park HG, Jung M, Lee S, Song W-J, Lee J-S. Radical-Scavenging Activatable and Robust Polymeric Binder Based on Poly(acrylic acid) Cross-Linked with Tannic Acid for Silicon Anode of Lithium Storage System. Nanomaterials. 2022; 12(19):3437. https://doi.org/10.3390/nano12193437
Chicago/Turabian StylePark, Hui Gyeong, Mincheol Jung, Shinyoung Lee, Woo-Jin Song, and Jung-Soo Lee. 2022. "Radical-Scavenging Activatable and Robust Polymeric Binder Based on Poly(acrylic acid) Cross-Linked with Tannic Acid for Silicon Anode of Lithium Storage System" Nanomaterials 12, no. 19: 3437. https://doi.org/10.3390/nano12193437
APA StylePark, H. G., Jung, M., Lee, S., Song, W.-J., & Lee, J.-S. (2022). Radical-Scavenging Activatable and Robust Polymeric Binder Based on Poly(acrylic acid) Cross-Linked with Tannic Acid for Silicon Anode of Lithium Storage System. Nanomaterials, 12(19), 3437. https://doi.org/10.3390/nano12193437






