Understanding of Contradiction on Concentration Effect on Stability, Physical Properties, Evaporation and Microexplosion Characteristics of Al/JP-10/Oleic Acid Nanofluid Fuel
Abstract
:1. Introduction
2. Materials and Experimental Methods
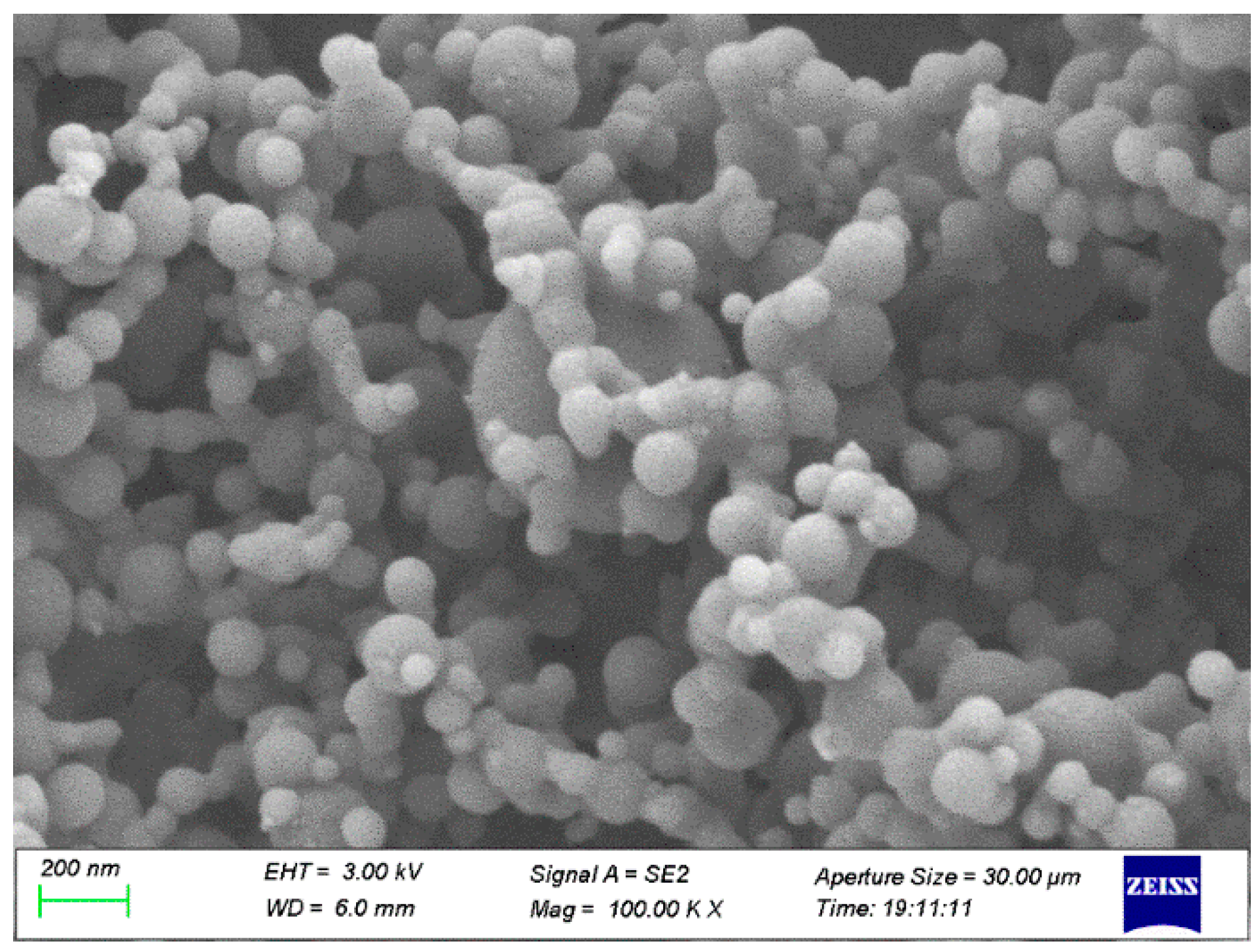
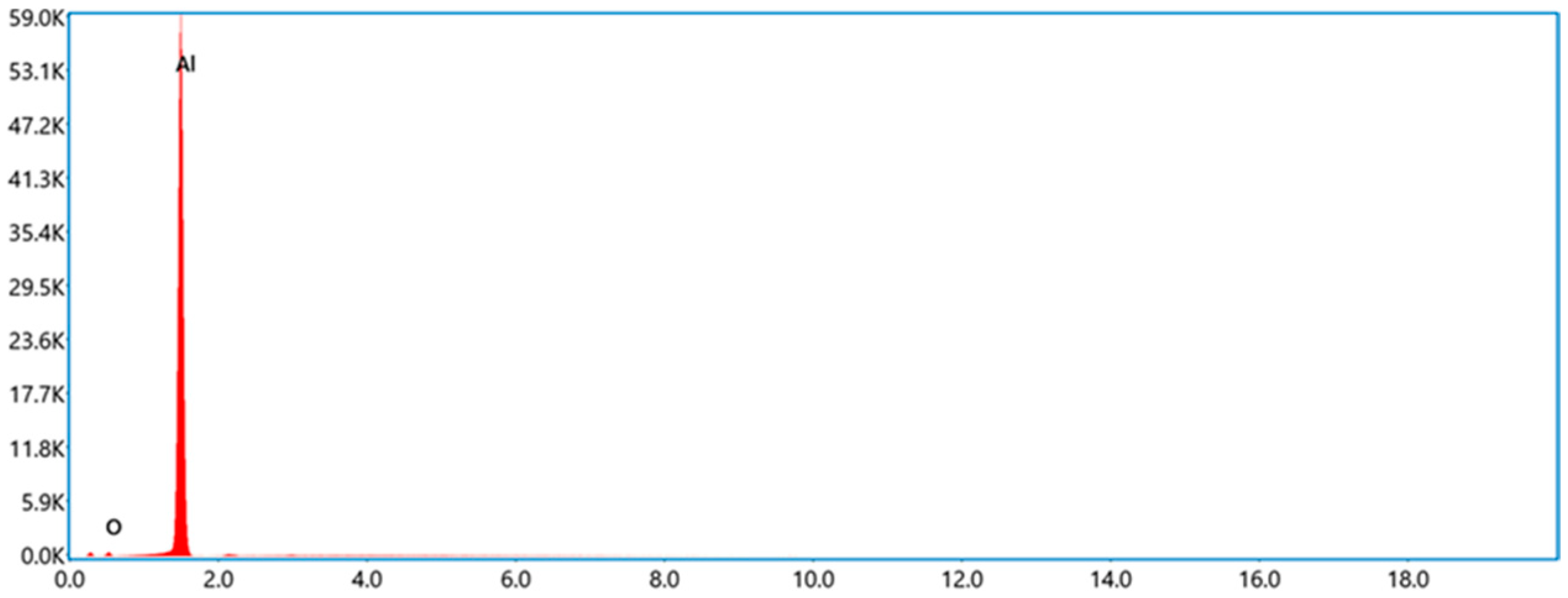
2.1. Materials
2.2. Preparation of Al/JP-10/Oleic Acid Nanofluid Fuels
2.3. Stability and Physical Property Test Method
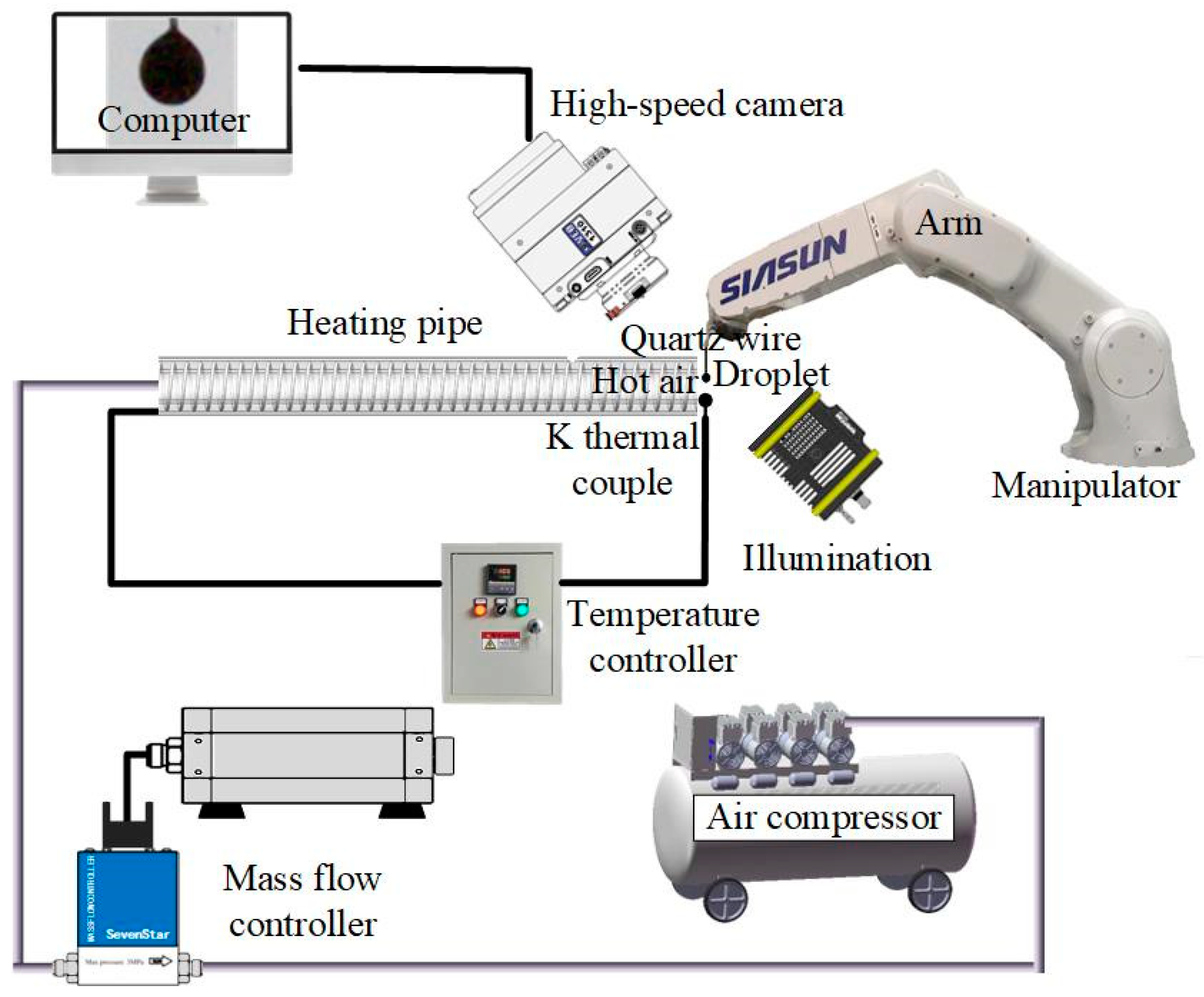
2.4. Experimental Setup for Evaporation
3. Results and Discussion
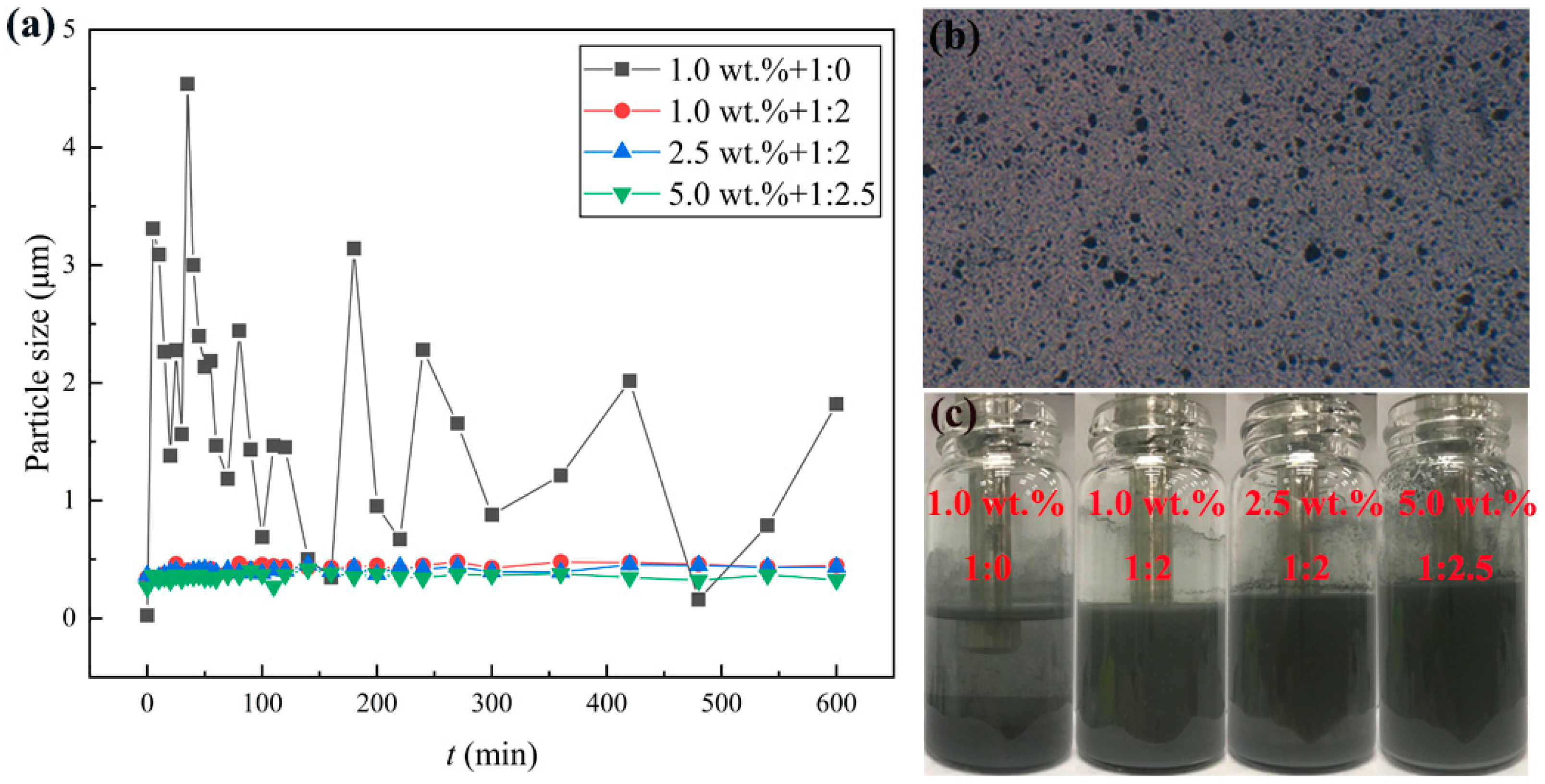
3.1. Effect of the Mass Ratio of Al and Oleic Acid on the Stability of Al/JP-10/Oleic Acid Nanofluid Fuels
3.2. Effect of Al Concentration on Physical Properties of Al/JP-10/Oleic Acid Nanofluid Fuels
3.3. Effect of Al Concentration on Evaporation Characteristics of Al/JP-10/Oleic Acid Nanofluid Fuels
3.3.1. Evaporation Characteristics of JP-10
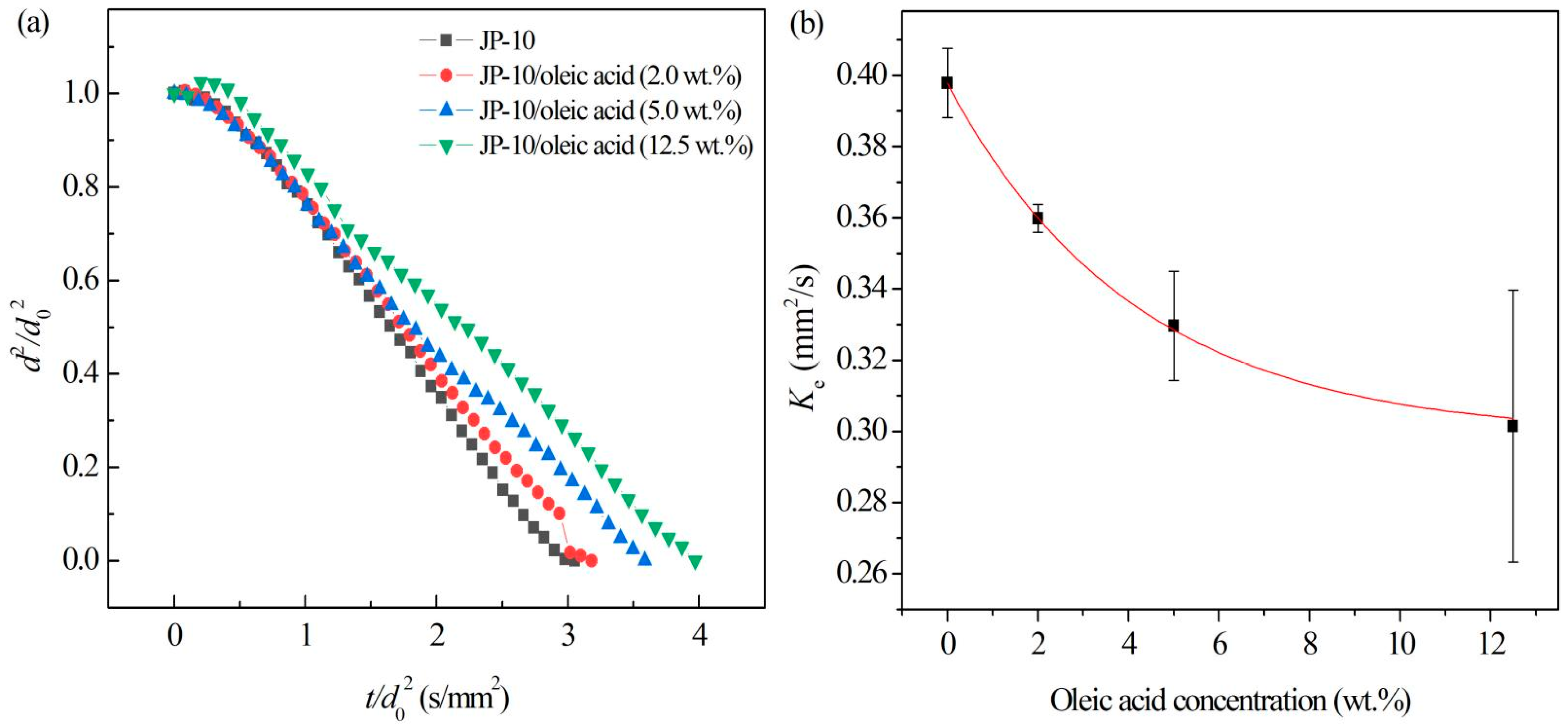
3.3.2. Effect of Oleic Acid Concentration on Evaporation of JP-10
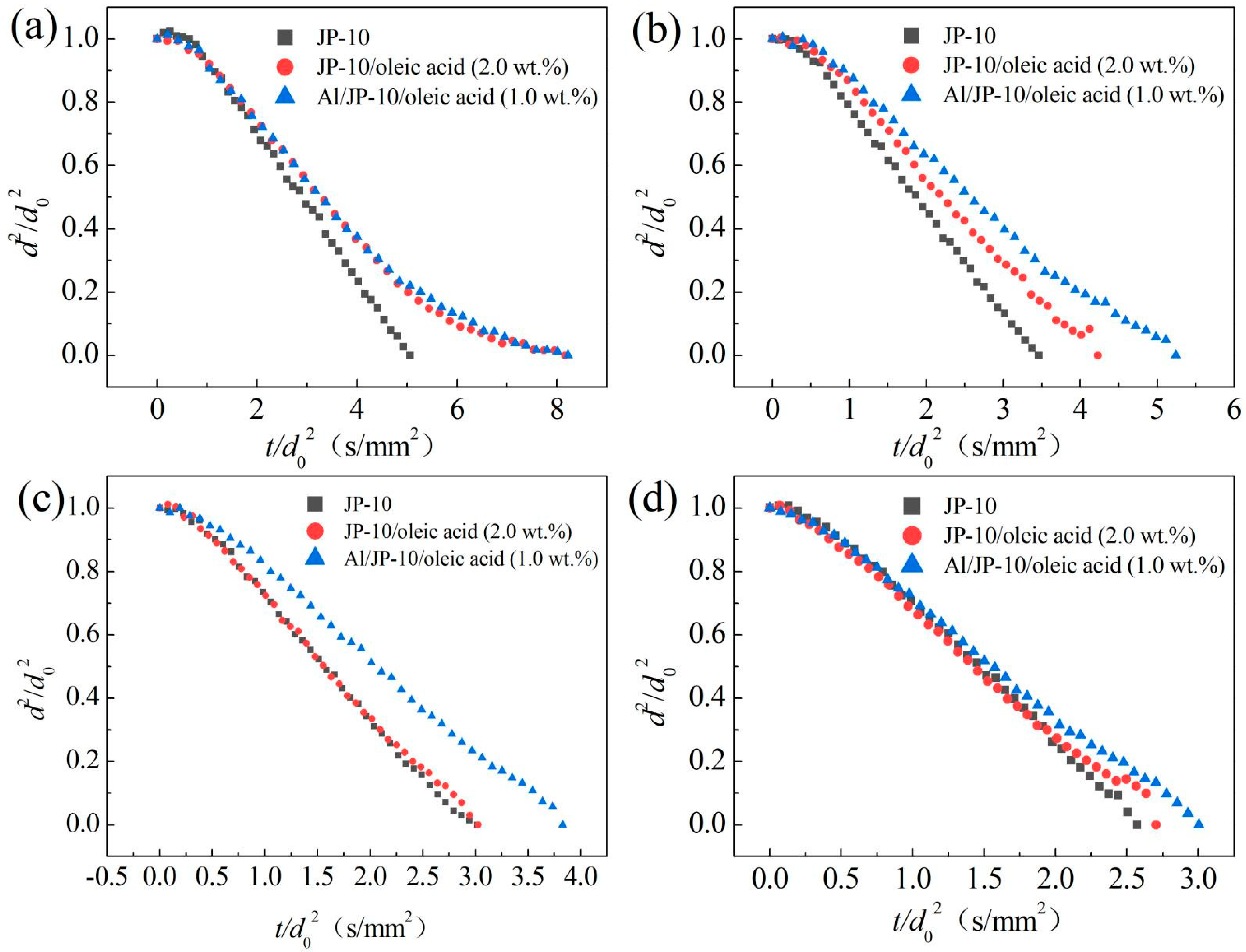
3.3.3. Effect of Al Concentration on Evaporation of Al/JP-10/Oleic Acid Nanofluid Fuel
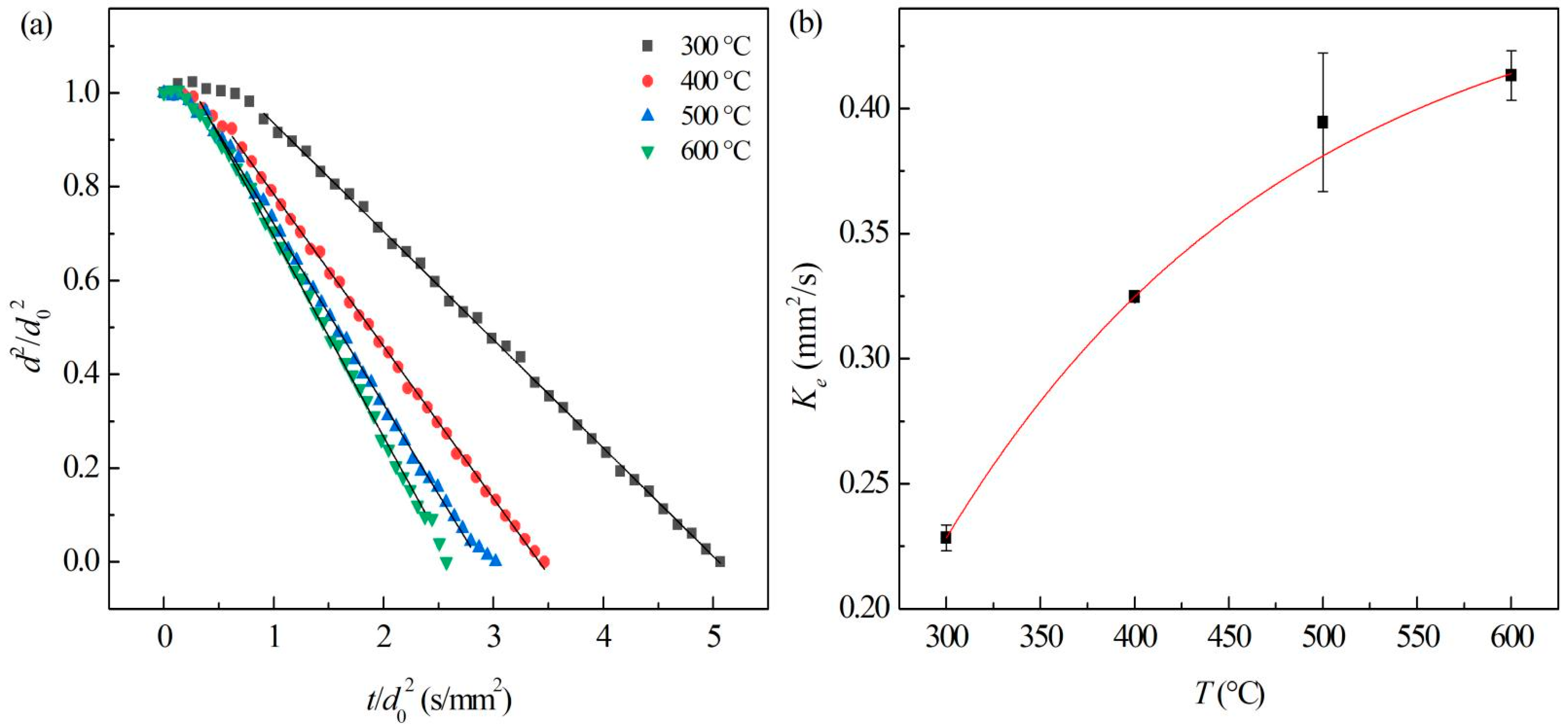
3.3.4. Effect of Temperature on Evaporation of Al/JP-10/Oleic Acid Nanofluid Fuel
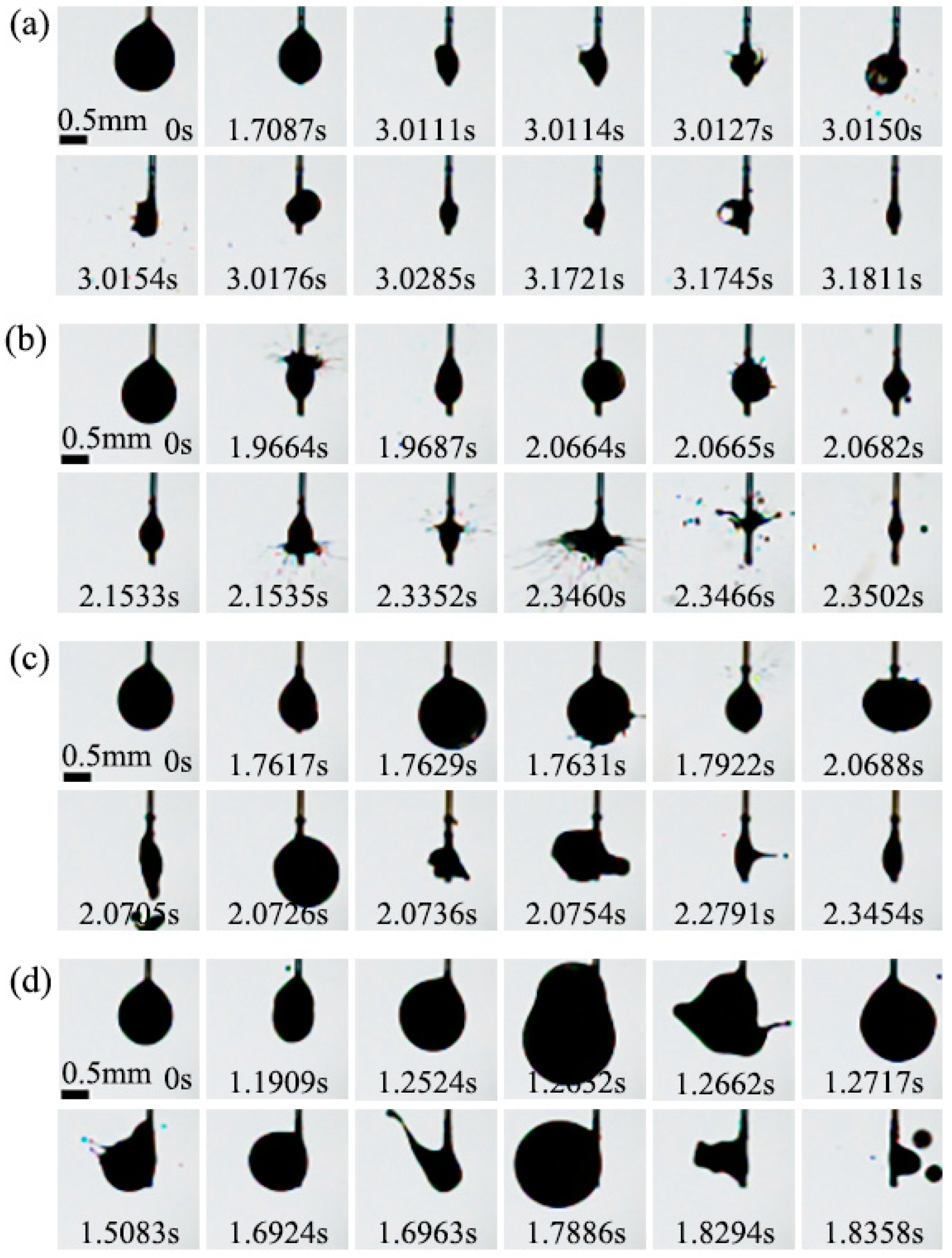
3.3.5. Effect of Al Concentration on Microexplosion of Al/JP-10/Oleic Acid Nanofluid Fuel
4. Conclusions
- (1)
- When the mass ratio of Al to oleic acid is 1:2, the dispersion stability of the nanofluid fuels is the best at Al concentrations of 1.0 wt.% and 2.5 wt.%, while when the mass ratio of Al to oleic acid becomes 1:2.5, the nanofluid fuel of 5.0 wt.% Al concentration can maintain the best stability.
- (2)
- The density of the Al/JP-10/oleic acid nanofluid fuel increases linearly with the mass concentration of Al nanoparticles, and the viscosity coefficient is a quadratic function of the Al concentration. The surface tension of the nanofluid fuel oscillates as a quartic function of the Al concentration, lower than that of JP-10 at all concentrations.
- (3)
- The evaporation of JP-10 includes non-isothermal and isothermal evaporation, while the JP-10/oleic acid and Al/JP-10/oleic acid fuels experience non-isothermal, isothermal evaporation, and microexplosion. The evaporation follows d2-law in the isothermal stage, and the evaporation rate decreases with the increasing concentrations of oleic acid and Al nanoparticles. The enhancement of the ambient temperature (300–600 °C) can significantly increase the evaporation rates of the fuels, compensating for the adverse effect of adding oleic acid and Al nanoparticles. At 600 °C, the evaporation rates of the JP-10, JP-10/oleic acid, Al/JP-10/oleic acid fuels are equivalent.
- (4)
- At low ambient temperatures of 300 °C or below, the nanofluid fuels scarcely produce the microexplosion phenomenon. At high temperatures of 400 °C or above, the droplets undergo obvious microexplosion after adding oleic acid and Al nanoparticles. Moreover, with the increase in the Al concentration (1.0 wt.%, 2.5 wt.%, 5.0 wt.%, 10.0 wt.%), the time of microexplosion is advanced, the bubble size before microexplosion becomes larger, and the degree of microexplosion is more intense.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagaut, P.; Cathonnet, M. The ignition, oxidation, and combustion of kerosene: A review of experimental and kinetic modeling. Prog. Energy Combust. Sci. 2006, 32, 48–92. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.T.; Dasgupta, S.; Iii, W.A.G. Initiation mechanisms and kinetics of pyrolysis and combustion of JP-10 hydrocarbon jet fuel. J. Phys. Chem. A 2009, 113, 1740–1746. [Google Scholar] [CrossRef] [Green Version]
- Li, S.J.; Zhuo, Z.; He, L.J.; Huang, X.F. Atomization characteristics of nano-Al/ethanol nanofluid fuel in electrostatic field. Fuel 2019, 236, 811–819. [Google Scholar] [CrossRef]
- Huang, X.F.; Li, S.J. Ignition and combustion characteristics of jet fuel liquid film containing graphene powders at meso-scale. Fuel 2016, 177, 113–122. [Google Scholar] [CrossRef]
- Yadav, A.K.; Nandakumar, K.; Srivastava, A.; Chowdhury, A. Combustion of rocket-grade kerosene droplets loaded with graphene nanoplatelets—A search for reasons behind optimum mass loadings. Combust Flame 2019, 203, 1–13. [Google Scholar] [CrossRef]
- Sabourin, J.L.; Dabbs, D.M.; Yetter, R.A.; Dryer, F.L.; Aksay, I.A. Functionalized Graphene Sheet Colloids for Enhanced Fuel/Propellant Combustion. ACS Nano 2009, 3, 3945–3954. [Google Scholar] [CrossRef]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Nanofuels: Combustion, engine performance and emissions. Fuel 2014, 120, 91–97. [Google Scholar] [CrossRef]
- Nakhchi, M.E.; Esfahani, J.A. Numerical investigation of turbulent CuO–water nanofluid inside heat exchanger enhanced with double V-cut twisted tapes. J. Therm. Anal. Calorim. 2021, 145, 2535–2545. [Google Scholar] [CrossRef]
- Wen, D. Nanofuel as a potential secondary energy carrier. Energy Environ. Sci. 2010, 3, 591–600. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammad, H.A. A review on applications and challenges of nanofluids. Renew. Sust. Energ. Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Li, G.; Hou, B.; Wang, A.; Xin, X.; Cong, Y.; Wang, X.; Li, N.; Zhang, T. Making jp-10 superfuel affordable with a lignocellulosic platform compound. Angew. Chem. Int. Ed. 2019, 131, 12282–12286. [Google Scholar] [CrossRef]
- Chung, H.S.; Chen, C.; Kremer, R.A.; Boulton, J.R.; Burdette, G.W. Recent Developments in High-Energy Density Liquid Hydrocarbon Fuels. Energy Fuels 1999, 13, 641–649. [Google Scholar] [CrossRef]
- Basu, S.; Miglani, A. Combustion and heat transfer characteristics of nanofluid fuel droplets: A short review. Int. J. Heat Mass Transf. 2016, 96, 482–503. [Google Scholar] [CrossRef]
- Senthilraja, S.; Karthikeyan, M.; Gangadevi, R. Nanofluid applications in future automobiles: Comprehensive review of existing data. Nanomicro Lett. 2010, 2, 306–310. [Google Scholar] [CrossRef]
- Li, S.; Yang, Q.; Ye, L.; Du, H.; Zhang, Z.; Huang, X.; Xu, J. Effect of Nanoparticle Concentration on Physical and Heat-Transfer Properties and Evaporation Characteristics of Graphite/n-Decane Nanofluid Fuels. ACS Omega 2022, 7, 3284–3292. [Google Scholar] [CrossRef]
- Sundaram, D.S.; Yang, V.; Zarko, V.E. Combustion of nano aluminum particles (Review). Combust. Explos. Shock Waves 2015, 51, 173–196. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Yang, Y.; Huang, X. Comparison on Laser Ignition and Combustion Characteristics of Nano- and Micron-Sized Aluminum. Combust. Sci. Technol. 2021, 193, 341–353. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Autoignition and combustion characteristics of heptane droplets with the addition of aluminium nanoparticles at elevated temperatures. Combust. Flame 2015, 162, 191–206. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Autoignition and combustion characteristics of kerosene droplets with dilute concentrations of aluminum nanoparticles at elevated temperatures. Combust. Flame 2015, 162, 774–787. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Effects of dense concentrations of aluminum nanoparticles on the evaporation behavior of kerosene droplet at elevated temperatures: The phenomenon of microexplosion. Exp. Therm. Fluid Sci. 2014, 56, 33–44. [Google Scholar] [CrossRef]
- Yu, L.; Xu, X.; Zou, J.J.; Zhang, X. Combustion of JP-10-Based Slurry with Nanosized Aluminum Additives. J. Propuls. Power 2016, 32, 1–11. [Google Scholar]
- Babar, H.; Ali, H.M. Towards hybrid nanofluids: Preparation, thermophysical properties, applications, and challenges. J. Mol. Liq. 2019, 281, 598–633. [Google Scholar] [CrossRef]
- Zhuo, Z.; Li, S.J.; Lu, Y.B.; Huang, X.F. Synergetic effects of nanoparticle concentration and electrification on the breakup performance of nanofluid fuel. Int. J. Heat Mass Transf. 2019, 137, 940–950. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; He, Y.; Tan, C. Rheological behaviour of ethylene glycol based titania nanofluids. Chem. Phys. Lett. 2007, 444, 333–337. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.U.; Saidur, R.; Amalina, M.A.; Islam, A.K.M.S. Effect of nanoparticles concentration and their sizes on surface tension of nanofluids. Proc. Eng. 2015, 105, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Chinnam, J.; Das, D.K.; Vajjha, R.S.; Satti, J.R. Measurements of the surface tension of nanofluids and development of a new correlation. Int. J. Therm. Sci. 2015, 98, 68–80. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K.; Ali, G.; Cho, S.O. Evaporation characteristics of kerosene droplets with dilute concentrations of ligand-protected aluminum nanoparticles at elevated temperatures. Combust. Flame 2013, 160, 2955–2963. [Google Scholar] [CrossRef]
- Wang, X.; Dai, M.; Wang, J.; Xie, Y.; Ren, G.; Jiang, G. Effect of ceria concentration on the evaporation characteristics of diesel fuel droplets. Fuel 2019, 236, 1577–1585. [Google Scholar] [CrossRef]
- Dai, M.; Wang, J.; Wei, N.; Wang, X.; Xu, C. Experimental study on evaporation characteristics of diesel/cerium oxide nanofluid fuel droplets. Fuel 2019, 254, 115633. [Google Scholar] [CrossRef]
- Law, C.K. Recent advances in droplet vaporization and combustion. Prog. Energy Combust. Sci. 1982, 8, 171–201. [Google Scholar] [CrossRef]
- Wong, S.C.; Lin, A.C.; Chi, H.Y. Effects of surfactant on the evaporation, shell formation and disruptive behavior of slurry droplets. Symp. (Int.) Combust. 1991, 23, 1391–1397. [Google Scholar] [CrossRef]
- Ao, W.; Gao, Y.; Zhou, S.; Li, L.; Yan, Q.L. Enhancing the stability and combustion of a nanofluid fuel with polydopamine-coated aluminum nanoparticles. Chem. Eng. J. 2021, 418, 129527. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Li, S.; Ye, L.; Huang, X. Understanding of Contradiction on Concentration Effect on Stability, Physical Properties, Evaporation and Microexplosion Characteristics of Al/JP-10/Oleic Acid Nanofluid Fuel. Nanomaterials 2022, 12, 3446. https://doi.org/10.3390/nano12193446
Yang Q, Li S, Ye L, Huang X. Understanding of Contradiction on Concentration Effect on Stability, Physical Properties, Evaporation and Microexplosion Characteristics of Al/JP-10/Oleic Acid Nanofluid Fuel. Nanomaterials. 2022; 12(19):3446. https://doi.org/10.3390/nano12193446
Chicago/Turabian StyleYang, Qianmei, Shengji Li, Linhui Ye, and Xuefeng Huang. 2022. "Understanding of Contradiction on Concentration Effect on Stability, Physical Properties, Evaporation and Microexplosion Characteristics of Al/JP-10/Oleic Acid Nanofluid Fuel" Nanomaterials 12, no. 19: 3446. https://doi.org/10.3390/nano12193446





