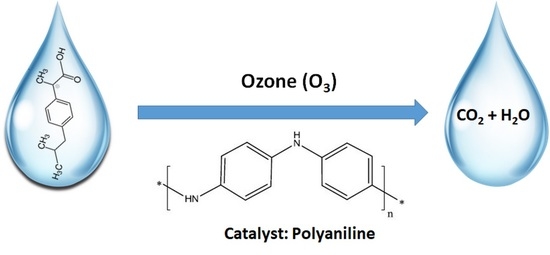
Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyaniline
2.3. Materials’ Characterization
2.4. Experimental Set-Up for Catalytic Ozonation and Aqueous Effluents’ Analysis
3. Results and Discussion
3.1. Materials’ Characterization
3.2. Catalytic Ozonation of Ibuprofen in Aqueous Solutions with Polyaniline-Based Catalysts
3.3. Kinetic Study
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominguez, I.; Arrebola, F.J.; Martinez Vidal, J.L.; Garrido Frenich, A. Assessment of wastewater pollution by gas chromatography and high resolution Orbitrap mass spectrometry. J. Chromatogr. A 2020, 1619, 460964. [Google Scholar] [CrossRef] [PubMed]
- Sires, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.J.; Maruya, K.A.; Snyder, S.A.; Zeng, E.Y. China’s water pollution by persistent organic pollutants. Environ. Pollut. 2012, 163, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, G.H.; Guo, H.; Zhang, W.; Hao, Z. Spatio-temporal patterns and source apportionment of coastal water pollution in eastern Hong Kong. Water Res. 2007, 41, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Reza, R.A.; Ahmaruzzaman, M. A facile approach for elimination of ibuprofen from wastewater: An experimental and theoretical study. Water Environ. J. 2020, 34, 435–443. [Google Scholar] [CrossRef]
- Liu, H.; Nkundabose, J.P.; Chen, H.; Yang, L.; Meng, C.; Ding, N. Decontamination of ibuprofen micropollutants from water based on visible-light-responsive hybrid photocatalyst. J. Environ. Chem. Eng. 2022, 10, 107154. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Fierascu, I.; Fierascu, R.C.; Brazdis, R.I.; Nica, A.V.; Butean, C.; Olaru, E.A.; Ulinici, S.; Verziu, M.N.; Dumitru, A. Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films. Nanomaterials 2022, 12, 613. [Google Scholar] [CrossRef]
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef]
- Holstege, C.P. Ibuprofen. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: New York, NY, USA, 2005; pp. 566–567. [Google Scholar]
- Liu, Y.; Zheng, X.; Zhang, S.; Sun, S. Enhanced removal of ibuprofen by heterogeneous photo-Fenton-like process over sludge-based Fe3O4-MnO2 catalysts. Water Sci. Technol. 2021, 85, 291–304. [Google Scholar] [CrossRef]
- Wen, S.; Chen, L.; Li, W.; Ren, H.; Li, K.; Wu, B.; Hu, H.; Xu, K. Insight into the characteristics, removal, and toxicity of effluent organic matter from a pharmaceutical wastewater treatment plant during catalytic ozonation. Sci. Rep. 2018, 8, 9581. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Jin, X.; Wu, C.; Fu, L.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. Development, dilemma and potential strategies for the application of nanocatalysts in wastewater catalytic ozonation: A review. J. Environ. Sci. 2023, 124, 330–349. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, F.; Xiang, L.; Yu, H.; Wang, Z.; Yan, B.; Chen, G. Synergistic effect for simultaneously catalytic ozonation of chlorobenzene and NO over MnCoO catalysts: Byproducts formation under practical conditions. Chem. Eng. J. 2022, 427, 130929. [Google Scholar] [CrossRef]
- Heidari, Z.; Pelalak, R.; Eshaghi Malekshah, R.; Pishnamazi, M.; Rezakazemi, M.; Aminabhavi, T.M.; Shirazian, S. A new insight into catalytic ozonation of sulfasalazine antibiotic by plasma-treated limonite nanostructures: Experimental, modeling and mechanism. Chem. Eng. J. 2022, 428, 131230. [Google Scholar] [CrossRef]
- Guan, Z.; Guo, Y.; Huang, Z.; Liao, X.; Chen, S.; Ou, X.; Sun, S.; Liang, J.; Cai, Y.; Xie, W.; et al. Simultaneous and efficient removal of organic Ni and Cu complexes from electroless plating effluent using integrated catalytic ozonation and chelating precipitation process in a continuous pilot-scale system. Chem. Eng. J. 2022, 428, 131250. [Google Scholar] [CrossRef]
- Zuo, X.; Ma, S.; Wu, Q.; Xiong, J.; He, J.; Ma, C.; Chen, Z. Nanometer CeO2 doped high silica ZSM-5 heterogeneous catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2021, 411, 125072. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, H.; Fu, M.-L.; Hu, Y.-B.; Liu, J.; Ji, Y.; Vasanthakumar, V.; Yuan, B. A new insight into catalytic ozonation of ammonia by MgO/Co3O4 composite: The effects, reaction kinetics and mechanism. Chem. Eng. J. 2021, 418, 129461. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, Z.; Zhan, T.; Hu, Z.T.; Chen, J. Catalytic Ozonation for the Degradation of 5-Sulfosalicylic Acid with Spinel-Type ZnAl2O4 Prepared by Hydrothermal, Sol-Gel, and Coprecipitation Methods: A Comparison Study. ACS Omega 2018, 3, 6506–6512. [Google Scholar] [CrossRef]
- He, C.; Chen, Y.; Guo, L.; Yin, R.; Qiu, T. Catalytic ozonation of NH4+–N in wastewater over composite metal oxide catalyst. J. Rare Earths 2020, 40, 73–84. [Google Scholar] [CrossRef]
- Gucheng, Z.; Jing, Z.; Yongli, Z.; Peng, Z.; Chenmo, W.; Wenshu, L.; Liwei, C. Effect of the composition and surface functional groups of Fe–Ni bimetal oxides catalysts in catalytic ozonation process. J. Water Supply Res. Technol. AQUA 2018, 67, 119–126. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.; Li, C. Fabrication of Surfactant-Enhanced Metal Oxides Catalyst for Catalytic Ozonation Ammonia in Water. Int. J. Environ. Res. Public Health 2018, 15, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.-Q.; Huang, C.; Li, J.-Y.; Yang, J.; Qu, B.; Yang, S.-Q.; Cui, Y.-H.; Yan, Y.; Sun, S.; Wu, X. Activated carbon catalytic ozonation of reverse osmosis concentrate after coagulation pretreatment from coal gasification wastewater reclamation for zero liquid discharge. J. Clean. Prod. 2021, 286, 124951. [Google Scholar] [CrossRef]
- Yuan, Y.; Xing, G.; Garg, S.; Ma, J.; Kong, X.; Dai, P.; Waite, T.D. Mechanistic insights into the catalytic ozonation process using iron oxide-impregnated activated carbon. Water Res. 2020, 177, 115785. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wang, Z.; Ouyang, C.; Cao, X.; Liang, P.; Huang, X.; Zhang, X. A hybrid fluidized-bed reactor (HFBR) based on arrayed ceramic membranes (ACMs) coupled with powdered activated carbon (PAC) for efficient catalytic ozonation: A comprehensive study on a pilot scale. Water Res. 2020, 173, 115536. [Google Scholar] [CrossRef] [PubMed]
- Nasseh, N.; Arghavan, F.S.; Rodriguez-Couto, S.; Hossein Panahi, A.; Esmati, M.; A-Musawi, T.J. Preparation of activated carbon@ZnO composite and its application as a novel catalyst in catalytic ozonation process for metronidazole degradation. Adv. Powder Technol. 2020, 31, 875–885. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, H.Y.; Liu, X.; Shi, H.X.; Zhou, T.H.; Wang, C.; Huo, Z.Y.; Wu, Q.Y. Combination of catalytic ozonation by regenerated granular activated carbon (rGAC) and biological activated carbon in the advanced treatment of textile wastewater for reclamation. Chemosphere 2019, 231, 369–377. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Duan, X.; Wang, S.; Wang, Y. Carbocatalytic ozonation toward advanced water purification. J. Mater. Chem. A 2021, 9, 18994–19024. [Google Scholar] [CrossRef]
- Du, M.-S.; Chen, K.-P.; Lin, Y.-P. Degradation of ibuprofen and acetylsulfamethoxazole by multi-walled carbon nanotube catalytic ozonation: Surface properties, kinetics and modeling. Environ. Sci. Water Res. Technol. 2019, 5, 1758–1768. [Google Scholar] [CrossRef]
- Zhuang, H.; Guo, J.; Hong, X. Advanced Treatment of Paper-Making Wastewater Using Catalytic Ozonation with Waste Rice Straw-Derived Activated Carbon-Supported Manganese Oxides as a Novel and Efficient Catalyst. Pol. J. Environ. Stud. 2018, 27, 451–457. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Ding, K.; Liu, Z.; Wang, L.; Xu, Y. Heterogeneous catalytic ozonation of hydroquinone using sewage sludge-derived carbonaceous catalysts. Water Sci. Technol. 2018, 77, 1410–1417. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Quan, X.; Yu, H. Fluorine-doped carbon nanotubes as an efficient metal-free catalyst for destruction of organic pollutants in catalytic ozonation. Chemosphere 2018, 190, 135–143. [Google Scholar] [CrossRef]
- Lu, S.; Liu, Y.; Feng, L.; Sun, Z.; Zhang, L. Characterization of ferromagnetic sludge-based activated carbon and its application in catalytic ozonation of p-chlorobenzoic acid. Environ. Sci. Pollut. Res. Int. 2018, 25, 5086–5094. [Google Scholar] [CrossRef]
- Ferrari-Lima, A.M.; Marques, R.G.; Gimenes, M.L.; Fernandes-Machado, N.R.C. Synthesis, characterisation and photocatalytic activity of N-doped TiO2–Nb2O5 mixed oxides. Catal. Today 2015, 254, 119–128. [Google Scholar] [CrossRef]
- Sayılkan, F.; Asiltürk, M.; Tatar, P.; Kiraz, N.; Şener, Ş.; Arpaç, E.; Sayılkan, H. Photocatalytic performance of Sn-doped TiO2 nanostructured thin films for photocatalytic degradation of malachite green dye under UV and VIS-lights. Mater. Res. Bull. 2008, 43, 127–134. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, Y.; Zou, Y.; Dai, L. Carbon-Based Metal-Free Electrocatalysis for Energy Conversion, Energy Storage, and Environmental Protection. Electrochem. Energy Rev. 2018, 1, 84–112. [Google Scholar] [CrossRef] [Green Version]
- Rangraz, Y.; Heravi, M.M.; Elhampour, A. Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations. Chem. Rec. 2021, 21, 1985–2073. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Shen, H.; Yang, Y.; Huang, Z.; Xiang, X.; Tian, Y. Charge storage performance of doped carbons prepared from polyaniline for supercapacitors. J. Solid State Electrochem. 2011, 15, 175–182. [Google Scholar] [CrossRef]
- Iftimie, S.; Dumitru, A.; Bradu, C. Carbon nanotubes and carbonized polyaniline nanostructures as 3D modified anode for microbial fuel cells. Proc. Rom. Acad. Ser. A 2019, 20, 45–50. [Google Scholar]
- Stejskal, J.; Kohl, M.; Trchová, M.; Kolská, Z.; Pekárek, M.; Křivka, I.; Prokeš, J. Conversion of conducting polypyrrole nanostructures to nitrogen-containing carbons and its impact on the adsorption of organic dye. Mater. Adv. 2021, 2, 706–717. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised polyaniline and polypyrrole: Towards advanced nitrogen-containing carbon materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Pašti, I.A.; Janošević Ležaić, A.; Gavrilov, N.M.; Ćirić-Marjanović, G.; Mentus, S.V. Nanocarbons derived from polymers for electrochemical energy conversion and storage—A review. Synth. Met. 2018, 246, 267–281. [Google Scholar] [CrossRef]
- Ding, H.; Shen, J.; Wan, M.; Chen, Z. Formation Mechanism of Polyaniline Nanotubes by a Simplified Template-Free Method. Macromol. Chem. Phys. 2008, 209, 864–871. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. Int. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Umh, H.N.; Kim, Y. Sensitivity of nanoparticles’ stability at the point of zero charge (PZC). J. Ind. Eng. Chem. 2014, 20, 3175–3178. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Hernandez-Esparza, M.; Doria-Serrano, C.; Fregoso-Infante, A.; Singh, M.M. The Point of Zero Charge of Oxides. In Environmental Chemistry: Microscale Laboratory Experiments; Ibanez, J.G., Hernandez-Esparza, M., Doria-Serrano, C., Fregoso-Infante, A., Singh, M.M., Eds.; Springer New York: New York, NY, USA, 2008; pp. 70–78. [Google Scholar]
- Gulicovski, J.J.; Čerović, L.S.; Milonjić, S.K. Point of Zero Charge and Isoelectric Point of Alumina. Mater. Manuf. Process. 2008, 23, 615–619. [Google Scholar] [CrossRef]
- Čerović, L.S.; Milonjić, S.K.; Todorović, M.B.; Trtanj, M.I.; Pogozhev, Y.S.; Blagoveschenskii, Y.; Levashov, E.A. Point of zero charge of different carbides. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 1–6. [Google Scholar] [CrossRef]
- Mustafa, S.; Dilara, B.; Nargis, K.; Naeem, A.; Shahida, P. Surface properties of the mixed oxides of iron and silica. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 273–282. [Google Scholar] [CrossRef]
- Trchová, M.; Morávková, Z.; Bláha, M.; Stejskal, J. Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim. Acta 2014, 122, 28–38. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. The chemical oxidative polymerization of aniline in water: Raman spectroscopy. J. Raman Spectrosc. 2008, 39, 1375–1387. [Google Scholar] [CrossRef]
- Milakin, K.A.; Acharya, U.; Hromádková, J.; Trchová, M.; Stejskal, J.; Bober, P. nitrogen-containing carbon enriched with tungsten atoms prepared by carbonization of polyaniline. Chem. Pap. 2021, 75, 5153–5161. [Google Scholar] [CrossRef]
- Rozlívková, Z.; Trchová, M.; Exnerová, M.; Stejskal, J. The carbonization of granular polyaniline to produce nitrogen-containing carbon. Synth. Met. 2011, 161, 1122–1129. [Google Scholar] [CrossRef]
- Mentus, S.; Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. Conducting carbonized polyaniline nanotubes. Nanotechnology 2009, 20, 245601. [Google Scholar] [CrossRef]
- Han, M.G.; Im, S.S. X-ray photoelectron spectroscopy study of electrically conducting polyaniline/polyimide blends. Polymer 2000, 41, 3253–3262. [Google Scholar] [CrossRef]
- Cho, S.; Kwon, O.S.; You, S.A.; Jang, J. Shape-controlled polyaniline chemiresistors for high-performance DMMP sensors: Effect of morphologies and charge-transport properties. J. Mater. Chem. A 2013, 1, 5679–5688. [Google Scholar] [CrossRef]
- Kebiche, H.; Poncin-Epaillard, F.; Haddaoui, N.; Debarnot, D. A route for the synthesis of polyaniline-based hybrid nanocomposites. J. Mater. Sci. 2020, 55, 5782–5794. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synthetic Metals 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Gao, Y.; Ying, J.; Xu, X.; Cai, L. Nitrogen-Enriched Carbon Nanofibers Derived from Polyaniline and Their Capacitive Properties. Appl. Sci. 2018, 8, 1079. [Google Scholar] [CrossRef]
- Carroll-Webb, S.A.; Walther, J.V. A surface complex reaction model for the pH-dependence of corundum and kaolinite dissolution rates. Geochim. Cosmochim. Acta 1988, 52, 2609–2623. [Google Scholar] [CrossRef]
- Kollannur, N.; Arnepalli, D.N. Methodology for Determining Point of Zero Salt Effect of Clays in Terms of Surface Charge Properties. J. Mater. Civ. Eng. 2019, 31, 04019286. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Raczyk-Stanisławiak, U.; Świetlik, J.; Nawrocki, J. Catalytic ozonation of natural organic matter on alumina. Appl. Catal. B Environ. 2006, 62, 345–358. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004, 110, 19–48. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
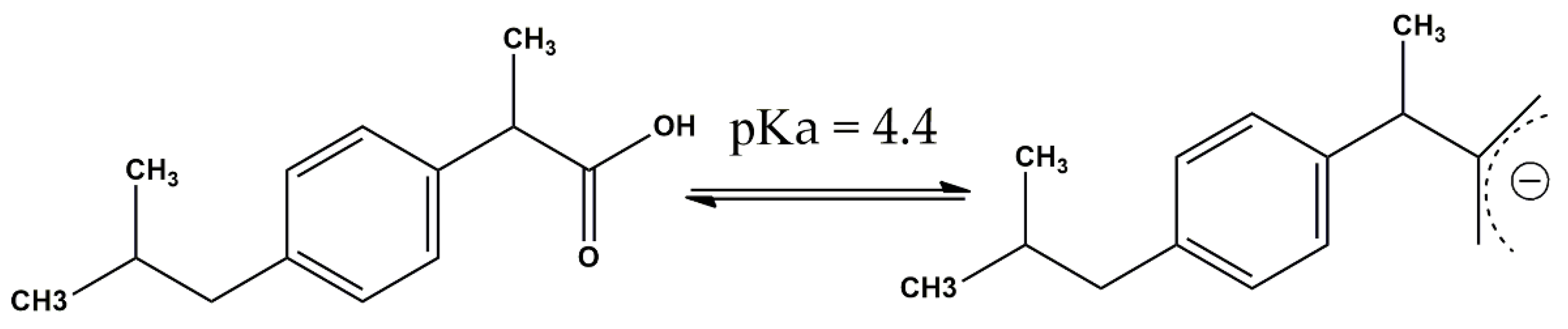
- Vicenteno-Vera, A.G.; Campos-Hernandez, T.; Ramirez-Silva, M.T.; Galano, A.; Rojas-Hernandez, A. Determination of pKa Values of Diclofenac and Ibuprofen in Aqueous Solutions by Capillary Zone Electrophoresis. ECS Trans. 2010, 29, 443–448. [Google Scholar] [CrossRef]



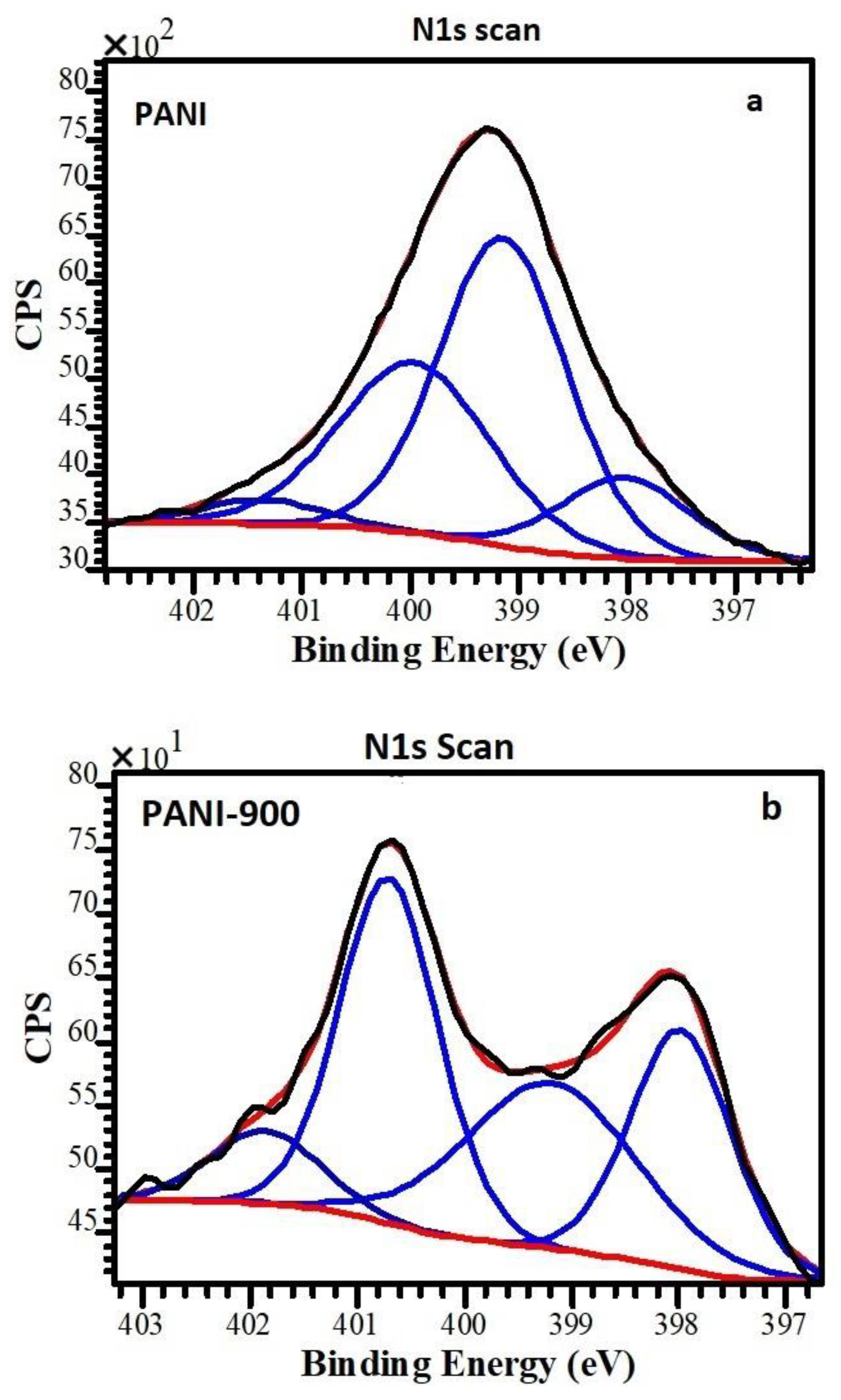
















| Sample | C1s | O1s | N1s | S2p | ||||
|---|---|---|---|---|---|---|---|---|
| BE | Wt% | BE | Wt% | BE | Wt% | BE | Wt% | |
| PANI | 284.5 | 77.8 | 530.5 | 11.2 | 399.5 | 8.9 | 168.5 | 2.1 |
| PANI 900 | 284.5 | 90.6 | 532.5 | 3.9 | 401.5 | 5.5 | – | – |
| Sample/N Functionality | =N– | –NH– | –NH+ | =NH+ | N+/N Ratio |
|---|---|---|---|---|---|
| PANI | 17.7 | 45.0 | 30.1 | 7.2 | 0.37 |
| Sample/N functionality | Pyridinic N | Pyrrolic N | quaternary–N | pyridine–N–oxide | Pyridinic N and Pyrrolic N |
| PANI 900 | 25.5 | 29.9 | 36.2 | 8.4 | 55.4 |
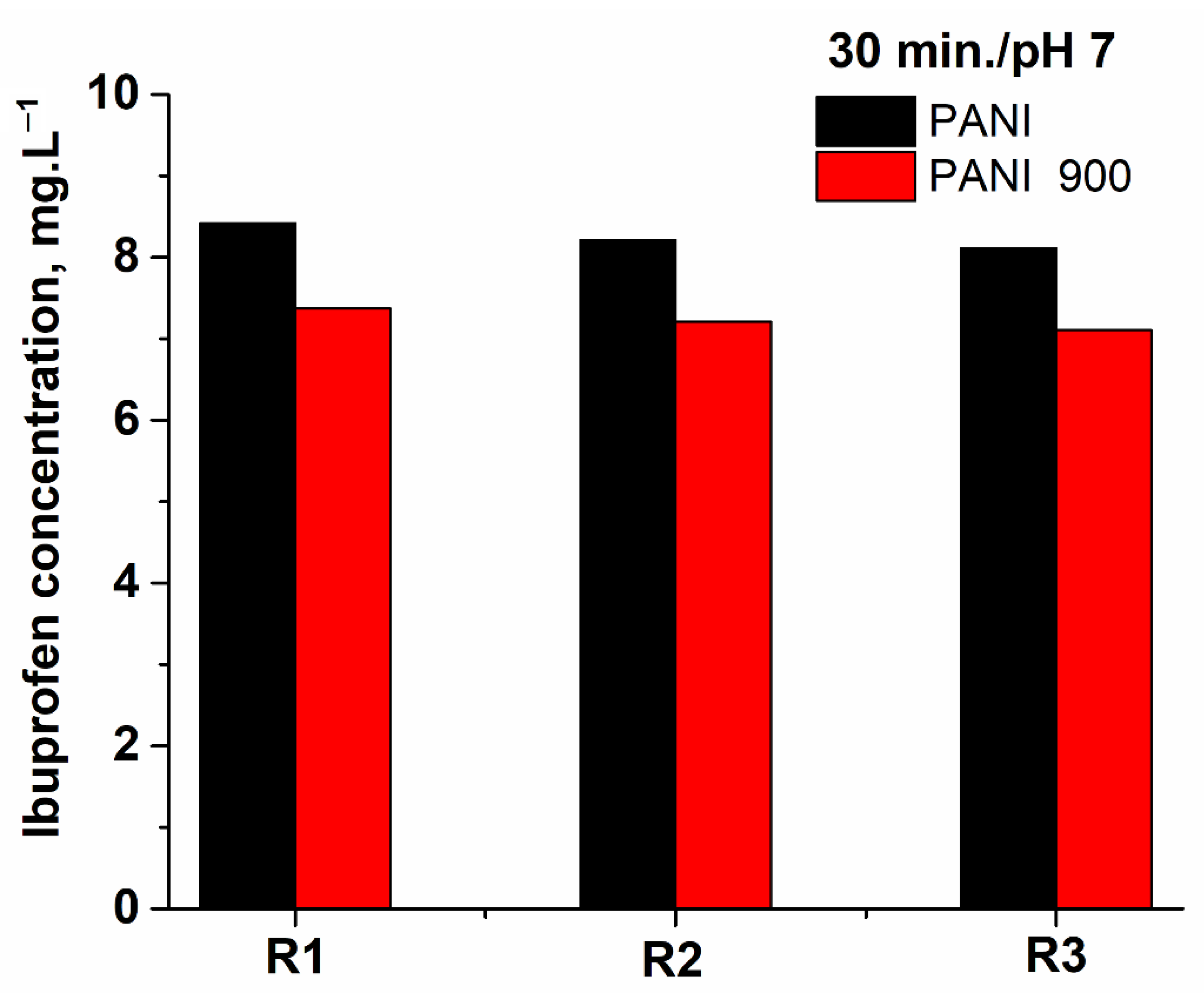
| Catalytic System/pH | Total Ozone Consumed in 180 min (mg) | mgO3/mg TOC Removed | Time for Total Ibuprofen Removal (Minutes) | Ozone Consumed for Total Ibuprofen Removal (mg) |
|---|---|---|---|---|
| Non-Catalytic pH 4 | 52.43 | 3.40 | 180 | 52.43 |
| Non-Catalytic pH 7 | 54.57 | 2.38 | 89 | 26.98 |
| Non-Catalytic pH 10 | 95.26 | 3.52 | 56 | 29.64 |
| PANI pH 4 | 93.46 | 3.36 | 60 | 31.15 |
| PANI pH 7 | 101.77 | 2.58 | 45 | 25.44 |
| PANI pH 10 | 105.32 | 2.39 | 30 | 17.55 |
| PANI 900 pH 4 | 81.35 | 2.09 | 60 | 27.12 |
| PANI 900 pH 7 | 98.39 | 1.92 | 45 | 24.60 |
| PANI 900 pH 10 | 100.58 | 1.87 | 20 | 11.18 |
| Catalytic System/pH | kobs (min−1) 102 | R2 |
|---|---|---|
| Non-Catalytic pH 4 | 2.938 | 0.95869 |
| Non-Catalytic pH 7 | 5.312 | 0.98125 |
| Non-Catalytic pH 10 | 11.204 | 0.99521 |
| PANI pH 4 | 5.067 | 0.96698 |
| PANI pH 7 | 9.383 | 0.9824 |
| PANI pH 10 | 17.064 | 0.98936 |
| PANI 900 pH 4 | 7.125 | 0.94914 |
| PANI 900 pH 7 | 9.233 | 0.9955 |
| PANI 900 pH 10 | 17.525 | 0.99003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, A.-V.; Olaru, E.A.; Bradu, C.; Dumitru, A.; Avramescu, S.M. Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures. Nanomaterials 2022, 12, 3468. https://doi.org/10.3390/nano12193468
Nica A-V, Olaru EA, Bradu C, Dumitru A, Avramescu SM. Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures. Nanomaterials. 2022; 12(19):3468. https://doi.org/10.3390/nano12193468
Chicago/Turabian StyleNica, Angel-Vasile, Elena Alina Olaru, Corina Bradu, Anca Dumitru, and Sorin Marius Avramescu. 2022. "Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures" Nanomaterials 12, no. 19: 3468. https://doi.org/10.3390/nano12193468
APA StyleNica, A.-V., Olaru, E. A., Bradu, C., Dumitru, A., & Avramescu, S. M. (2022). Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures. Nanomaterials, 12(19), 3468. https://doi.org/10.3390/nano12193468