In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
2.2. Experimental Design
2.3. Blood Sampling and Analysis
2.4. Ultrasound Analysis
2.5. Detection of Reactive Oxygen Species (ROS) and Nitrosyl Hemoglobin (HbNO) in Blood and ROS in Tissue Samples by Electron Paramagnetic Resonance (EPR)
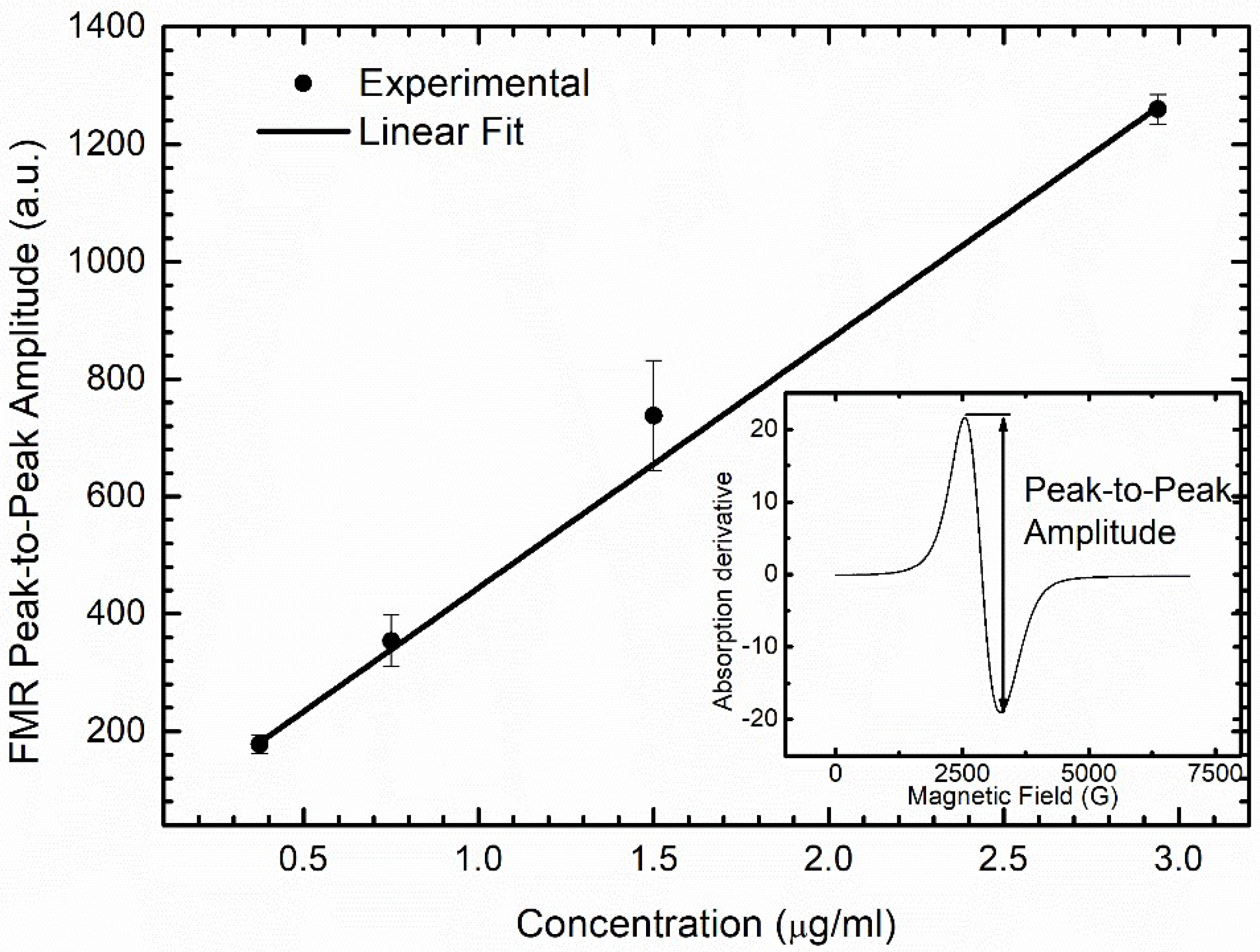
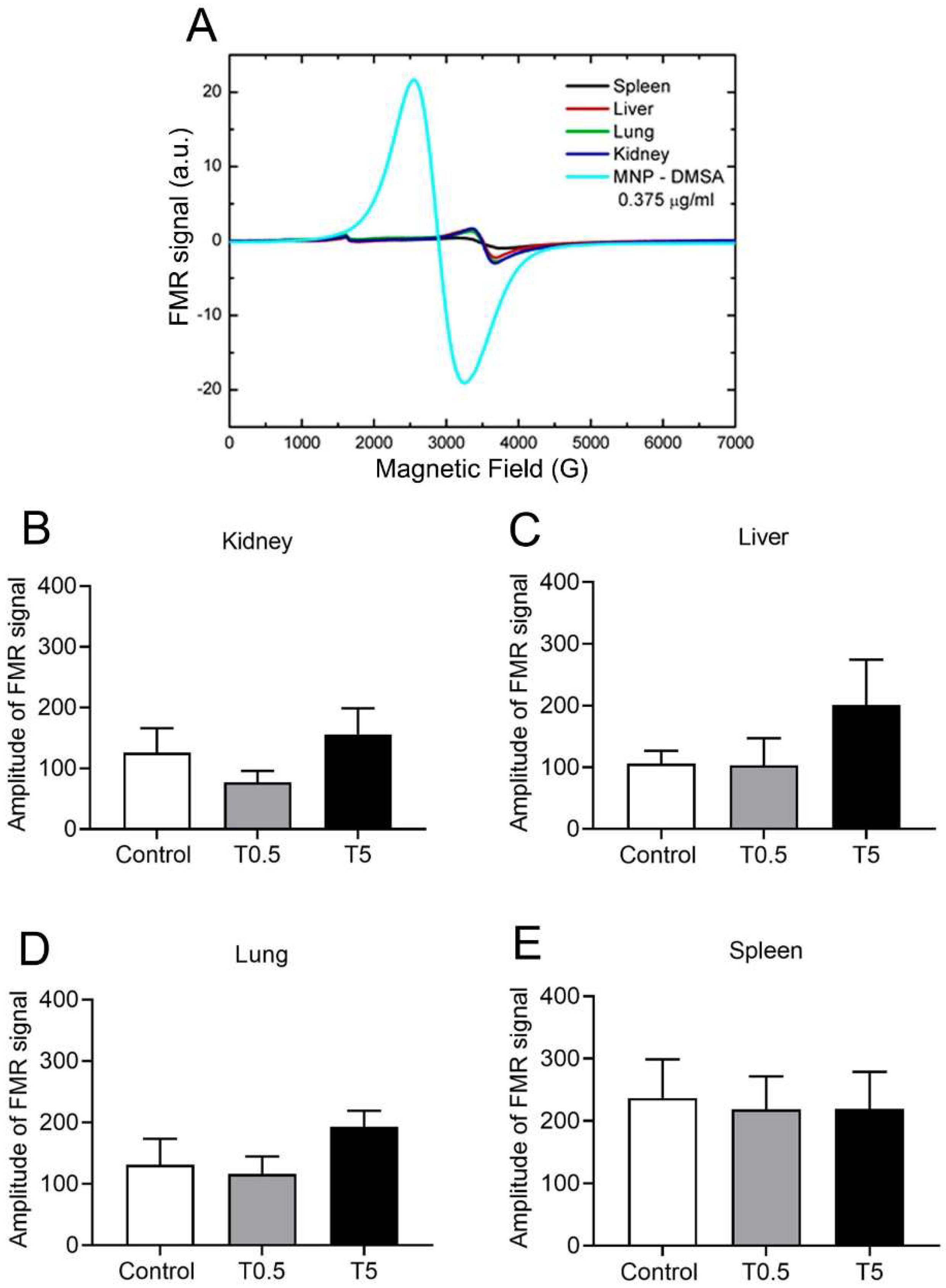
2.6. DMSA-MNP Detection and Quantification in Organs by Ferromagnetic Resonance (FMR)
2.7. Organ Micro-Computed Tomography (Micro-CT) and Histological Analysis
2.8. Statistical Analyses
3. Results
3.1. DMSA-MNP Characterization
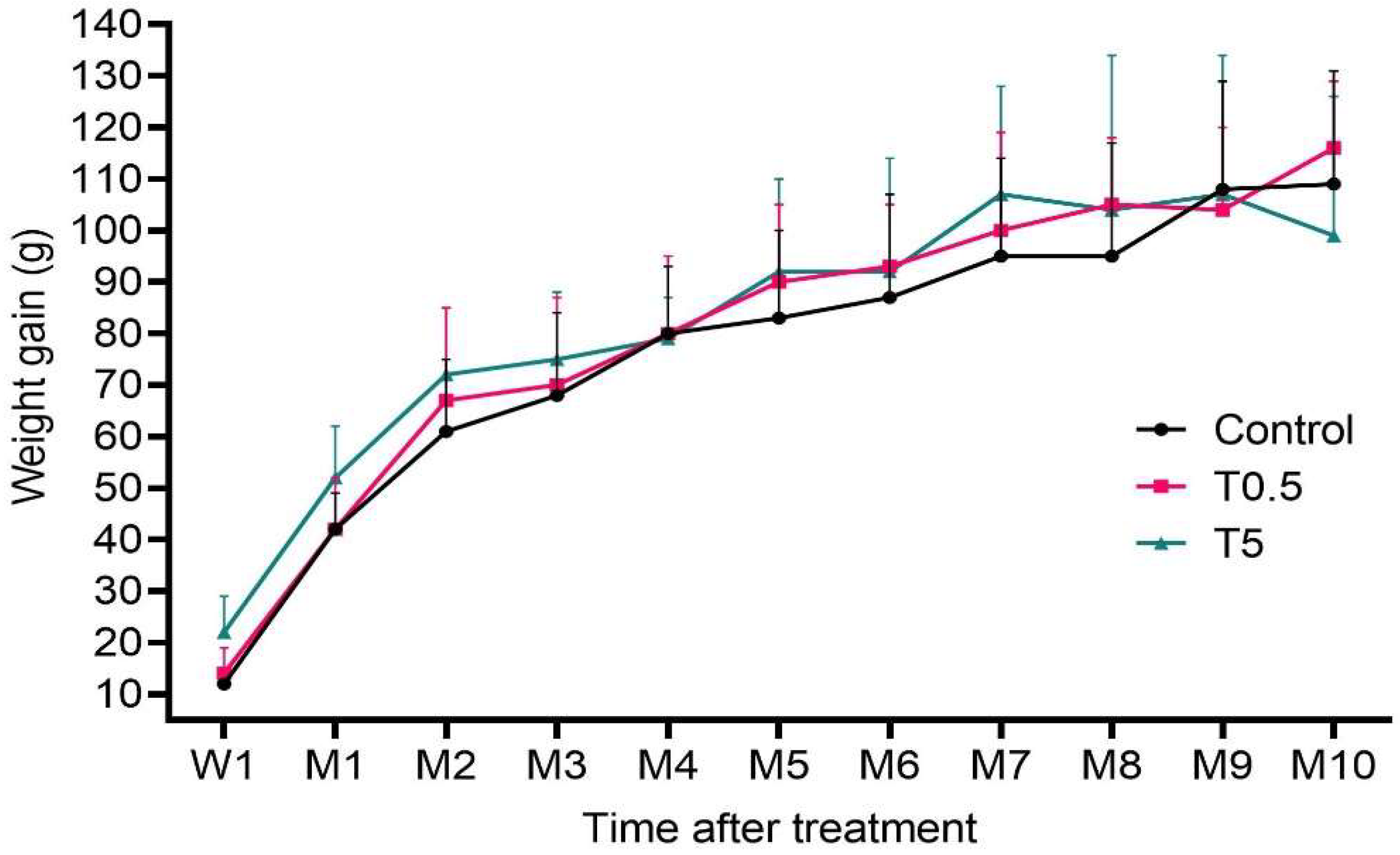
3.2. Clinical, Hematological, and Biochemical Analysis
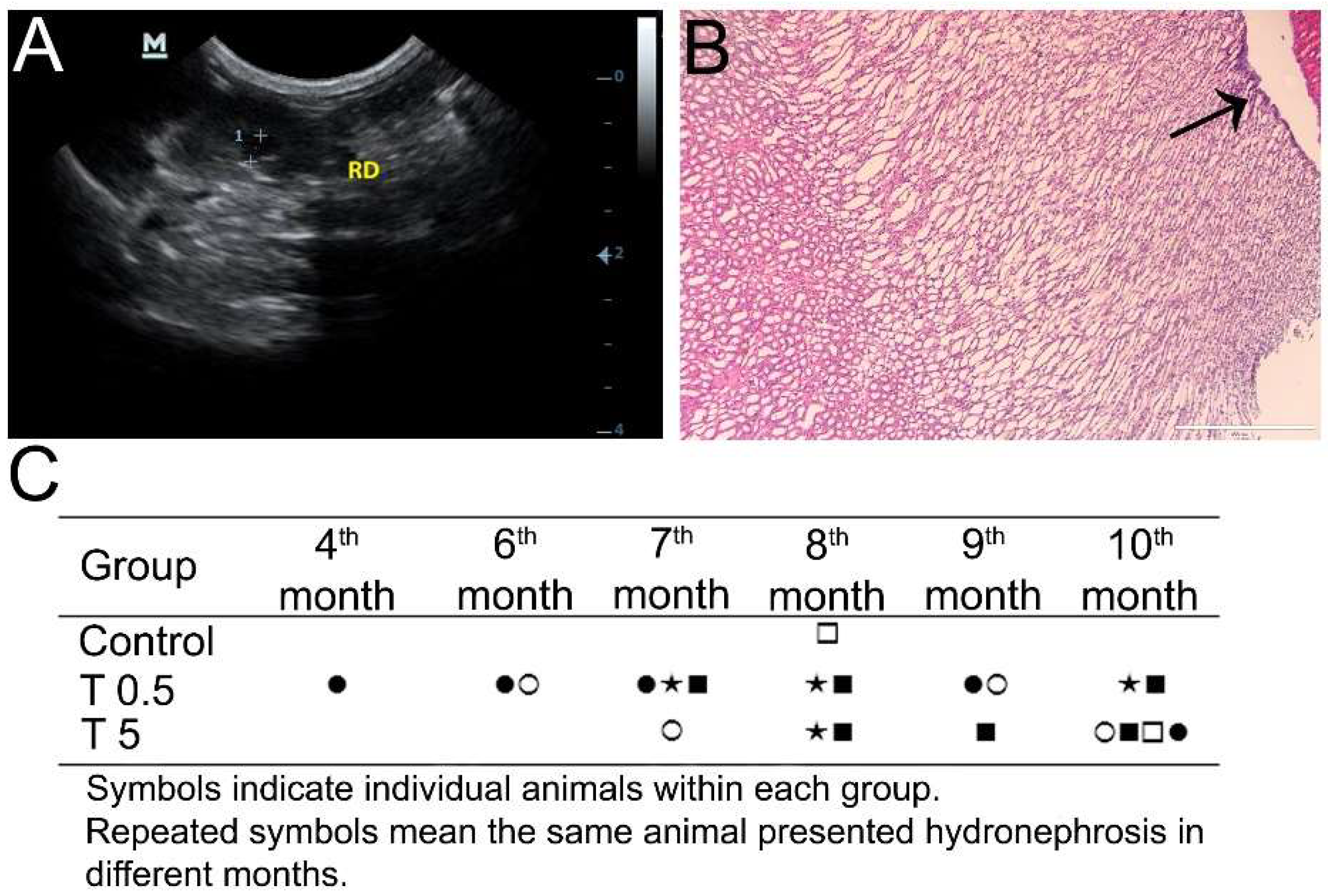
3.3. Ultrasonography Analysis
3.4. HbNO and ROS Detection in Blood and Different Tissues
3.5. DMSA-MNP Detection in the Organs
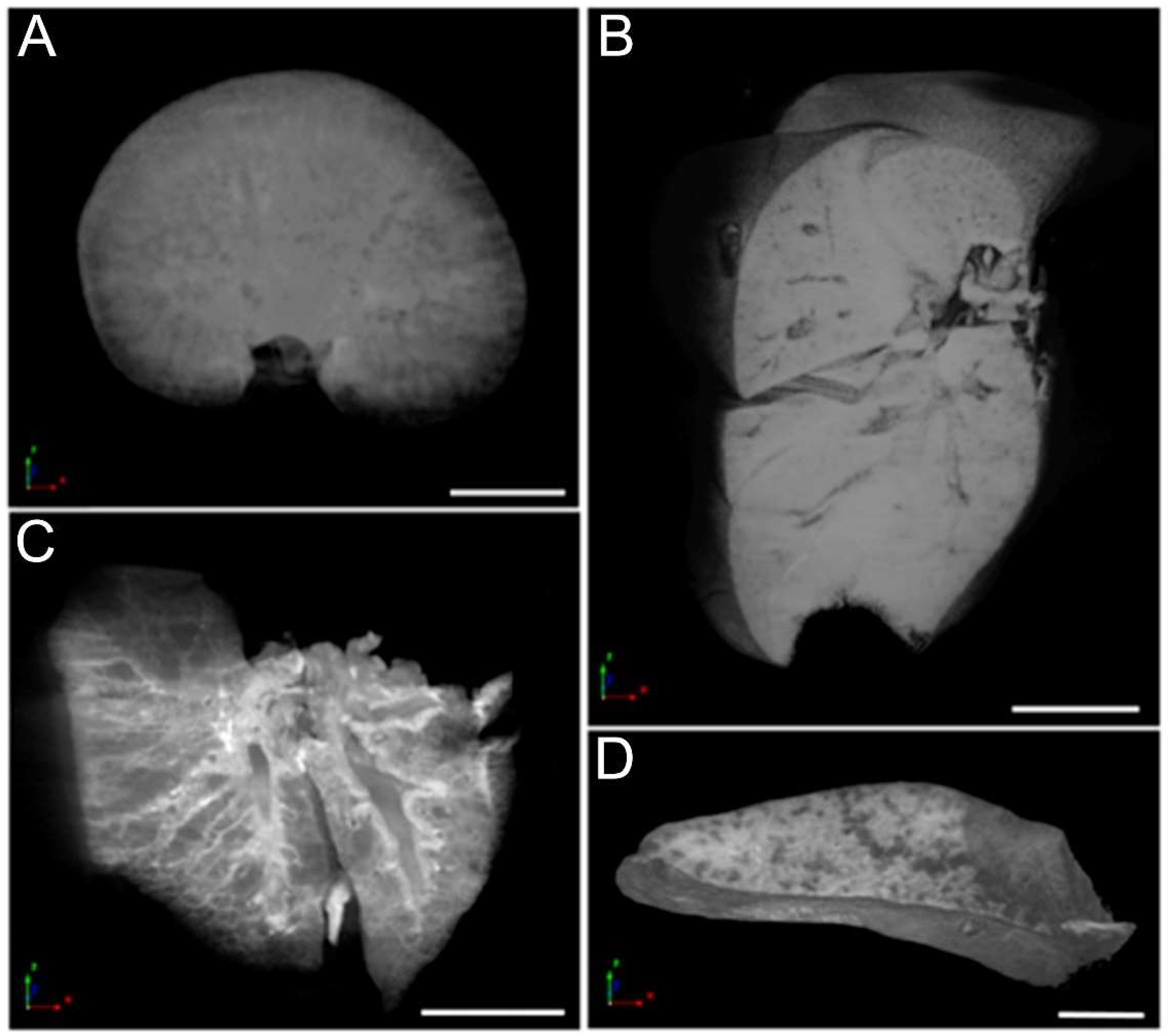
3.6. Micro-CT Analysis
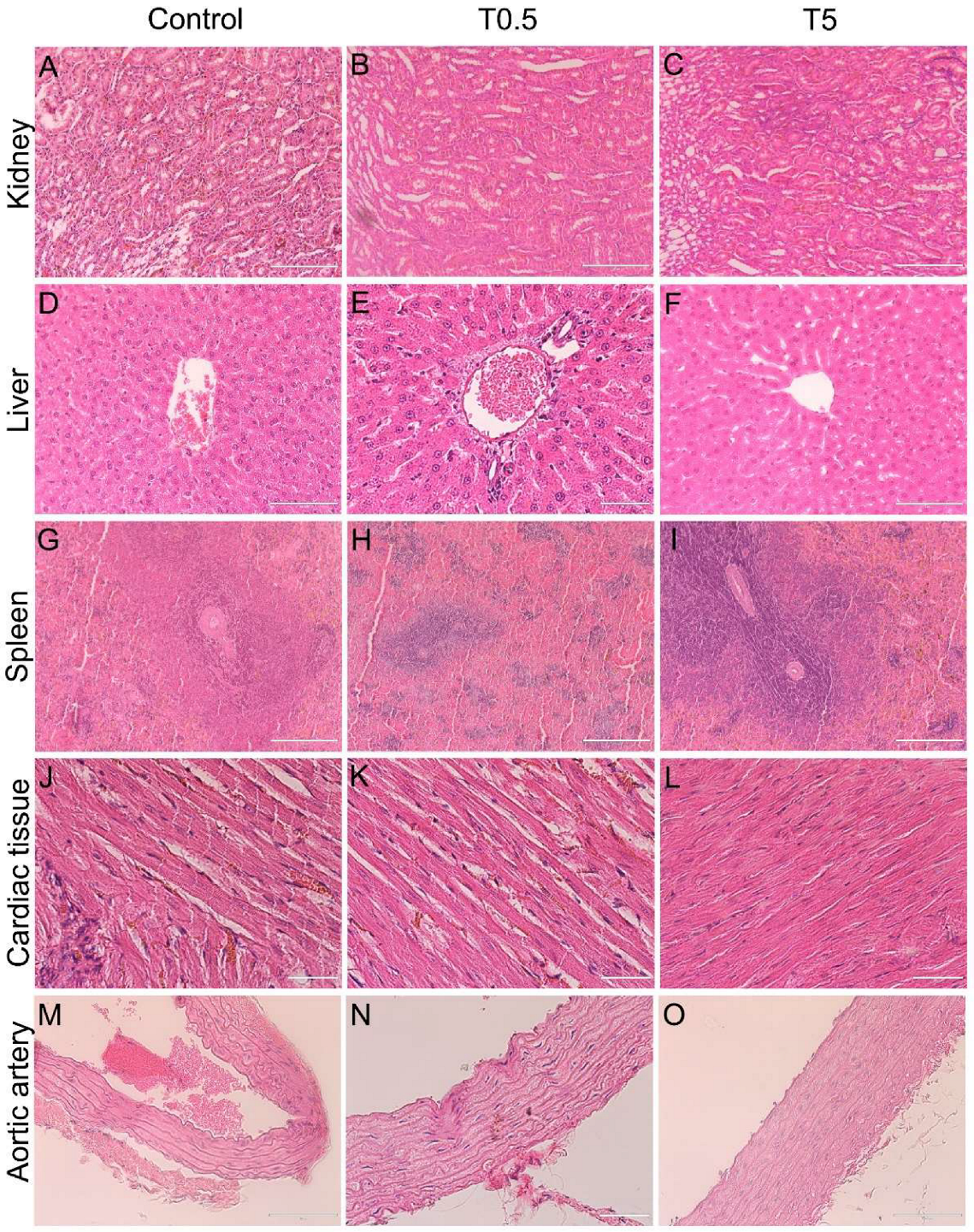
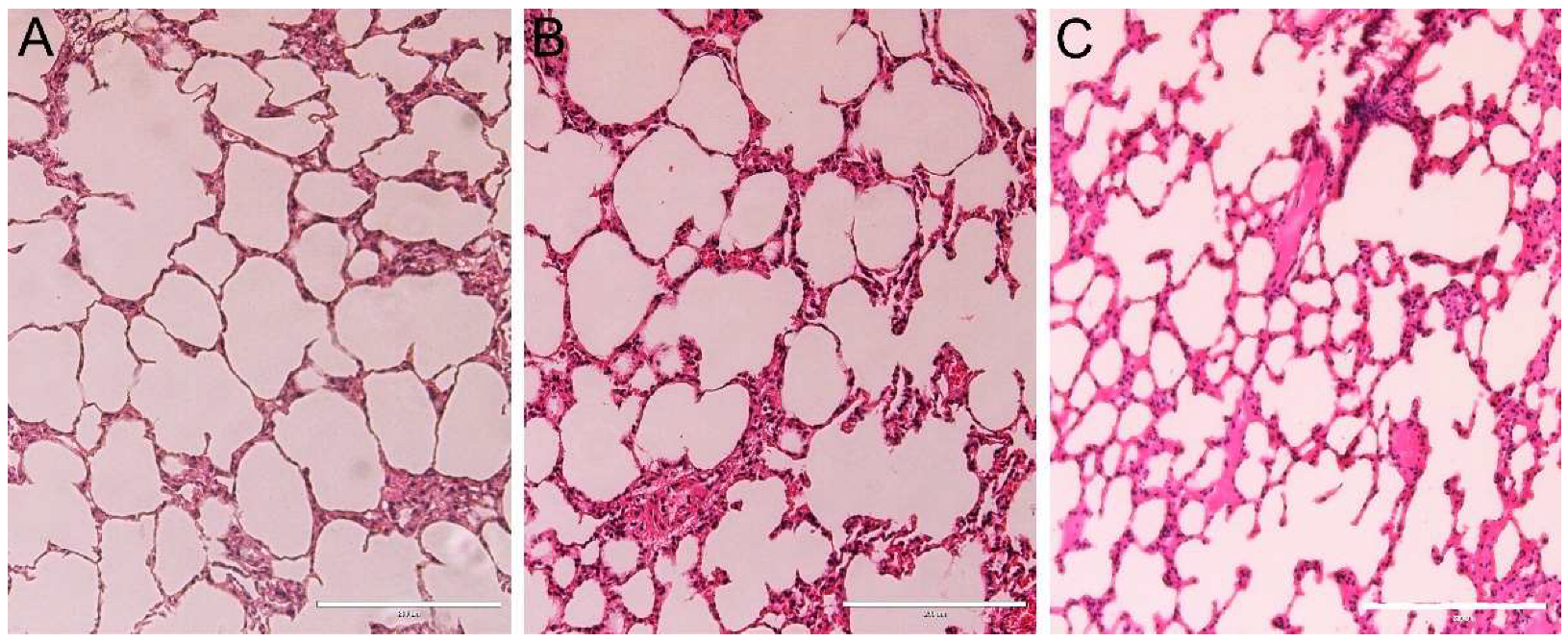
3.7. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonvico, F.; Mornet, S.; Vasseur, S.; Dubernet, C.; Jaillard, D.; Degrouard, J.; Hoebeke, J.; Duguet, E.; Colombo, P.; Couvreur, P. Folate-Conjugated Iron Oxide Nanoparticles for Solid Tumor Targeting as Potential Specific Magnetic Hyperthermia Mediators: Synthesis, Physicochemical Characterization, and in Vitro Experiments. Bioconjug. Chem. 2005, 16, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Thünemann, A.F.; Schütt, D.; Kaufner, L.; Pison, U.; Möhwald, H. Maghemite Nanoparticles Protectively Coated with Poly(ethylene imine) and Poly(ethylene oxide)-block-poly(glutamic acid). Langmuir 2006, 22, 2351–2357. [Google Scholar] [CrossRef]
- U.S Food & Drug Administration. Available online: https://www.fda.gov/ (accessed on 11 May 2020).
- Chaves, S.B.; Silva, L.P.; Lacava, Z.G.M.; Morais, P.C.; Azevedo, R.B. Interleukin-1 and interleukin-6 production in mice’s lungs induced by 2, 3 meso-dimercaptosuccinic-coated magnetic nanoparticles. Int. J. Appl. Phys. 2005, 97, 10Q915. [Google Scholar] [CrossRef]
- Garcia, M.P.; Parca, R.M.; Chaves, S.B.; Silva, L.P.; Santos, A.D.; Lacava, Z.G.M.; Morais, P.C.; Azevedo, R.B. Morphological analysis of mouse lungs after treatment with magnetite-based magnetic fluid stabilized with DMSA. J. Magn. Magn. Mater. 2005, 293, 277–282. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Garcia, M.P.; Tavares, M.C.H.; Valois, C.R.A.; Lima, E.C.D.; Teixeira, D.S.; Morais, P.C.; Tomaz, C.; Azevedo, R.B. Biodistribution and biocompatibility of DMSA stabilized maghemite magnetic nanoparticles in nonhuman primates (Cebus spp.). Nanomed. J. 2011, 6, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Ali, L.M.A.; Cáceres-Vélez, P.R.; Cornudella, R.; Gutiérrez, M.; Moreno, J.A.; Piñol, R.; Palacio, F.; Fascineli, M.L.; Azevedo, R.B.; et al. Hematotoxicity of magnetite nanoparticles coated with polyethylene glycol: In vitro and in vivo studies. Toxicol. Res. 2015, 4, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Huh, Y.M.; Jun, Y.W.; Song, H.T.; Kim, S.; Choi, J.S.; Lee, J.H.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. In Vivo Magnetic Resonance Detection of Cancer by Using Multifunctional Magnetic Nanocrystals. J. Am. Chem. Soc. 2005, 127, 12387–12391. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Berry, C.C. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224003. [Google Scholar] [CrossRef]
- Bee, A.; Bouchami, T.; Brossel, R.; Cabuil, V.; Carpentier, M.; Fruchart, J.M.; Massart, R.; Neveu, S.; Pons, J.N.; Robineau, M.; et al. Small particles. French Patent 9006484, 23 May 1990. [Google Scholar]
- Fauconnier, N.; Pons, J.N.; Roger, J.; Bee, A. Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J. Colloid Interface Sci. 1997, 194, 427–433l. [Google Scholar] [CrossRef] [PubMed]
- Massart, R.; Roger, J.; Cabuil, V. New trends in chemistry of magnetic colloids: Polar and non polar magnetic fluids, emulsions, capsules and vesicles. Braz. J. Phys. 1995, 25, 135–141. [Google Scholar]
- Pande, M.; Mehta, A.; Pant, B.P.; Flora, S.J.S. Combined administration of a chelating agent and an antioxidant in the prevention and treatment of acute lead intoxication in rats. Environ. Toxicol. Pharmacol. 2001, 9, 173–184. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pande, M.; Mehta, A. Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem. Biol. Interact. 2003, 145, 267–280. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Aposhian, M.M. Meso-2,3-Dimercaptosuccinic acid: Chemical, pharmacological and toxicological properties of an orally effective metal chelating agent. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Morais, P.C.; Azevedo, R.B.; Lacava, Z.G.M.; Villanueva, A.; Morales, M.P. Magnetic nanoparticles coated with dimercaptosuccinic acid: Development, characterization, and application in biomedicine. J. Nanopart. Res. 2014, 16, 2589. [Google Scholar] [CrossRef]
- Valois, C.R.A.; Braz, J.M.; Nunes, E.S.; Vinolo, M.A.R.; Lima, E.C.D.; Curi, R.; Kuebler, W.M.; Azevedo, R.B. The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via a b2 integrin-dependent pathway. Biomaterials 2010, 31, 366–374. [Google Scholar] [CrossRef]
- Edge, D.; Shortt, C.M.; Gobbo, O.L.; Teughels, S.; Prina-Mello, A.; Volkov, Y.; MacEneaney, P.; Radomski, M.W.; Markos, F. Pharmacokinetics and bio-distribution of novel super paramagnetic iron oxide nanoparticles (SPIONs) in the anaesthetized pig. Clin. Exp. Pharmacol. Physiol. 2016, 43, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Braz, S.V.; Monge-Fuentes, V.; Silva, J.R.; Tomaz, C.; Tavares, M.C.; Garcia, M.P.; Báo, S.N.; Lozzi, S.P.; Azevedo, R.B. Morphological Analysis of Reticuloendothelial System in Capuchin Monkeys (Sapajus spp.) after Meso-2,3-Dimercaptosuccinic Acid (DMSA) Coated Magnetic Nanoparticles Administration. PLoS ONE 2015, 10, e0140233. [Google Scholar] [CrossRef]
- Shrestha, S.; Jiang, P.; Sousa, M.H.; Morais, P.C.; Mao, Z.; Gao, C. Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison–Wesley: Boston, MA, USA, 1978; p. 555. [Google Scholar]
- Dikalov, S.; Fink, B. ESR techniques for the Detection of Nitric Oxide In Vivo and in Tissues. Methods Enzymol. 2005, 396, 597–610. [Google Scholar] [CrossRef]
- Gomes, B.R.B.; Firmino, M.; Jorge, J.S.; Ferreira, M.L.O.; Rodovalho, T.M.; Weis, S.N.; Souza, G.E.P.; Morais, P.C.; Sousa, M.V.; Souza, P.E.N.; et al. Increase of reactive oxygen species in different tissues during lipopolysaccharide-induced fever and antipyresis: An electron paramagnetic resonance study. Free Radic. Res. 2018, 52, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wierzbinski, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 3682. [Google Scholar] [CrossRef] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, W.; Eaton, J.W.; Qian, M. Molecular bases of cellular iron toxicity. Free Radic. Biol. Med. 2002, 32, 833–840. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, M.; Yang, F.; Mao, Y.; Xie, H.; Zhou, Q. Iron overload by Superparamagnetic Iron Oxide Nanoparticles is a High Risk Factor in Cirrhosis by a Systems Toxicology Assessment. Sci. Rep. 2016, 6, 29110. [Google Scholar] [CrossRef]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.H. In Vivo Clearance and Toxicity of Monodisperse Iron Oxide Nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef] [Green Version]
- Ng, R. Drugs: From Discovery to Approval, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 160–161. [Google Scholar]
- Kumar, V.; Farell, G.; Yu, F.; Harrington, S.; Fitzpatrick, L.; Rzewuska, E.; Miller, V.M.; Lieske, J.C. Cell Biology of Pathologic Renal Calcification: Contribution of Crystal Transcytosis, Cell-Mediated Calcification, and Nanoparticles. J. Investig. Med. 2006, 54, 412–424. [Google Scholar] [CrossRef]
- Quesenberry, K.E.; Carpenter, J.W. Ferrets, Rabbits, and Rodents Clinical Medicine and Surgery, 3rd ed.; Elsevier: St Louis, MI, USA, 2012; pp. 345–349. [Google Scholar]
- Cubas, Z.S.; Silva, J.C.R.; Catão-Dias, J.L. Tratado de Animais Selvagens, 2 ed.; Roca: São Paulo, Brazil, 2014; pp. 1169–1236. [Google Scholar]
- Briggs, C. Quality counts: New parameters in blood cell counting. Int. J. Lab. Hematol. 2009, 31, 277–297. [Google Scholar] [CrossRef]
- Aguirre, A.L.; Center, S.A.; Randolph, J.F.; Yeager, A.E.; Keegan, A.M.; Harvey, J.; Erb, H.N. Gallbladder disease in Shetland Sheepdogs: 38 cases (1995–2005). JAVMA 2007, 231, 79–88. [Google Scholar] [CrossRef] [PubMed]


| RBC (106/µL) | HB (g/dL) | HTC (%) | WBC (103/µL) | LP (%) | MP (%) | ||
|---|---|---|---|---|---|---|---|
| D0 * | 6.1–10.8 | 12.4–23.5 | 33.1–60.1 | 3.9–12.0 | 60.4–82.5 | 17.5–39.6 | |
| Day after injection | Group | ||||||
| D15 | Control | 10.8 ± 1.5 (10.2–11.8) | 22.2 ± 3.3 (20.8–24.7) | 60.2 ± 8.8 (43.6–66.5) | 8.9 ± 2.1 (6.2–12.2) | 70.1 ± 3.9 (64.4–74.9) | 29.9 ± 3.9 (25.1–33.1) |
| T0.5 | 8.2 ± 1.8 (5.9–10.3) | 17.5 ± 3.7 (12.4–22.3) | 46.8 ± 9.7 (33.9–59.5) | 8.9 ± 2.0 (6.1–11.7) | 72.5 ± 6.1 (65.1–79.5) | 27.5 ± 6.1 (23.8–34.9) | |
| T5 | 8.5 ± 1.2 (6.7–9.3) | 18.3 ± 2.5 (14.2–20.4) | 48.3 ± 6.2 (38.4–53.1) | 7.9 ± 0.8 (6.8–8.7) | 65.0 ± 8.2 (53.2–71.2) | 34.9 ± 8.2 (28.5–46.8) | |
| D30 | Control | 7.8 ± 0.3 (7.3–8.1) | 15.7 ± 0.7 (14.6–16.7) | 44.1 ± 1.7 (41.9–45.8) | 10.7 ± 1.6 (8.2–12.7) | 74.1 ± 3.8 (68.5–79.7) | 25.9 ± 3.8 (20.3–27.8) |
| T0.5 | 8.3 ± 0.4 (7.8–8.7) | 17.2 ± 1.1 (16.9–18.1) | 46.9 ± 2.4 (43.3–49.0) | 10.3 ± 2.2 (8.1–13.5) | 73.7 ± 3.7 (67.5–76.7) | 26.3 ± 3.7 (23.3–32.5) | |
| T5 | 8.9 ± 1.2 (7.5–10.6) | 18.5 ± 2.3 (15.9–21.5 | 50.6 ± 6.0 (44.4–58.8) | 10.5 ±4.4 (5.2–15.5) | 71.5 ± 11.8 (59.1–90.8) | 28.5 ± 11.8 (9.2–40.9) | |
| D60 | Control | 8.1 ± 0.2 (7.75–8.3) | 16.0 ± 0.5 (15.5–16.6) | 43.9 ± 0.8 (43.0–44.8) | 8.9 ± 1.3 (7.7–10.7) | 76.0 ± 2.1 (73.0–78.6) | 24.0 ± 2.1 (21.4–27.0) |
| T0.5 | 8.5 ± 0.7 (8.1–9.4) | 17.4 ± 1.5 (16.0–18.4) | 46.7 ± 3.7 (43.4–49.3) | 9.3 ± 1.8 (6.8–11.1) | 72.7 ± 2.4 (69.1–74.7) | 27.3 ± 2.4 (24.9–28.2) | |
| T5 | 8.2 ± 0.3 (7.9–8.5) | 16.6 ± 0.3 (16.2–16.9) | 45.8 ± 1.0 (44.5–46.1) | 6.3 ± 2.0 (4.7–9.0) | 68.1 ± 6.0 (59.8–76.5) | 31.9 ± 6.0 (23.5–40.2) | |
| D120 | Control | 7.8 ± 0.2 (7.4–8.0) | 15.4 ± 0.5 (14.7–16.0) | 40.8 ± 1.8 (38.4–43.0) | 5.7 ± 0.6 (4.8–6.6) | 66.5 ± 7.4 (53.8–73.2) | 33.5 ± 7.4 (26.8–46.2) |
| T0.5 | 7.7 ± 0.4 (7.2–8.4) | 15.7 ± 0.8 (14.4–16.7) | 42.0 ± 1.9 (39.2–44.4) | 5.2 ± 2.1 (3.4–8.3) | 65.8 ± 4.5 (61.0–71.0) | 34.2 ± 4.5 (29.0–37.7) | |
| T5 | 8.3 ± 0.7 (7.9–9.6) | 16.6 ± 1.1 (15.9–18.5) | 45.2 ± 3.3 (43.2–51.1) | 5.3 ± 1.3 (4.5–7.5) | 64.0 ± 6.8 (56.3–74.8) | 36.0 ± 6.8 (25.2–43.7) | |
| D180 | Control | 8.7 ± 1.2 (8.9–9.7) | 17.0 ± 2.3 (13.0–18.6) | 45.4 ± 6.1 (34.7–50.3) | 5.1 ± 0.8 (3.6–5.5) | 68.8 ± 1.9 (66.4–71.7) | 31.2 ± 1.9 (28.3–33.6) |
| T0.5 | 8.9 ± 1.3 (7.5–10.8) | 17.7 ± 2.7 (15.2–21.5) | 47.5 ± 6.8 (41.0–56.5) | 4.9 ± 0.2 (4.0–7.0) | 66.0 ± 4.6 (58.7–69.6) | 34.0 ± 4.5 (30.4–41.3) | |
| T5 | 8.3 ± 0.4 (7.8–8.8) | 16.6 ± 0.7 (16.2–17.3) | 44.5 ± 2.0 (41.6–46.6) | 3.7 ± 1.2 (2.9–5.8) | 64.3 ± 5.2 (56.9–69.7) | 35.7 ± 5.2 (30.3–43.1) | |
| D240 | Control | 8.5 ± 0.5 (7.7–9.2) | 17.0 ± 1.1 (15.6–18.4) | 44.9 ± 2.2 (42.7–48.5) | 5.5 ± 1.0 (4.4–6.9) | 65.2 ± 2.9 (61.3–67.9) | 34.8 ± 2.9 (32.1–38.7) |
| T0.5 | 7.9 ± 0.6 (7.3–8.4) | 16.1 ± 0.9 (15.0–16.9) | 42.0 ± 3.2 (38.0–47.4) | 6.1 ± 0.2 (5.9–6.3) | 68.0 ± 8.5 (56.2–76.4) | 32.1 ± 8.5 (23.6–43.8) | |
| T5 | 8.0 ± 0.6 (7.0–8.5) | 16.3 ± 1.6 (13.8–17.9) | 42.7 ± 4.0 (36.4–46.7) | 4.5 ± 1.9 (2.9–7.6) | 62.4 ± 4.9 (56.8–69.1) | 37.6 ± 4.9 (30.9–43.2) | |
| D300 | Control | 9.2 ± 1.3 (7.8–10.9) | 18.0 ± 2.7 (15.3–21.8) | 49.5 ± 7.6 (41.4–60.0) | 3.2 ± 0.5 (2.4–8.6) | 61.3 ± 5.5 (53.6–67.4) | 38.7 ± 5.5 (32.6–46.4) |
| T0.5 | 8.5 ± 0.7 (7.7–9.3) | 17.1 ± 1.2 (16.0–18.6) | 47.1 ± 3.9 (43.1–51.4) | 2.5 ± 1.0 (1.8–3.9) | 59.3 ± 6.3 (53.0–66.7) | 40.7 ± 6.3 (33.3–47.0) | |
| T5 | 7.8 ± 0.3 (7.7–10.9) | 15.6 ± 0.8 (15.3–21.8) | 42.9 ± 2.0 (41.1–60.0) | 2.5 ± 0.2 (1.8–4.1) | 53.6 ± 0.6 (51.3–68.7) | 36.6 ± 0.7 (31.1–48.7) |
| Creatinine (mg/dL) | Urea (mg/dL) | ALT (U/L) | AST (U/L) | Iron (μg/dL) | ||
|---|---|---|---|---|---|---|
| D0 * | 0.5–1.0 | 48–95 | 28–90 | 127–333 | 184–556 | |
| Day after injection | Group | |||||
| D15 | Control | 0.7 ± 0.1 (0.6–0.9) | 80.8 ± 9.8 (69–94) | 83.0 ± 24.0 (53–126) | 218.5 ± 51.0 (170–288) | 314.0 ± 26.9 (283–328) |
| T0.5 | 0.7 ± 0.1 (0.6–0.7) | 76.1 ± 7.2 (66–84) | 79.6 ± 16.5 (65–112) | 147.2 ± 16.8 (124–174) | 349.3 ± 39.7 (294–396) | |
| T5 | 0.7 ± 0.1 (0.6–0.8) | 76.4 ± 12.3 (63–92) | 76.6 ± 17.9 (62–107) | 142.8 ± 29.7 (109–178) | 479.3 ± 97.6 (376–570) | |
| D30 | Control | 0.9 ± 0.3 (0.8–1.4) | 94.7 ± 9.1 (78–103) | 90.8 ± 18.7 (68–117) | 263.3 ± 84.9 (173–379) | 394.6 ± 43.8 (317–420) |
| T0.5 | 0.7 ± 0.1 (0.5–0.8) | 66.0 ± 30.3 (15–87) | 78.8 ± 35.1 (51–125) | 238.0 ± 107.7 (136–336) | 319.5 ± 268.0 (130–509) | |
| T5 | 0.8 ± 0.1 (0.7–0.9) | 102.8 ± 7.9 (93–107) | 69.8 ± 30.7 (26–91) | 305.5 ± 48.8 (271–340) | 403.0 ± 38.2 (376–430) | |
| D60 | Control | 1.1 ± 0.6 (0.8–2.1) | 80.8 ± 8.0 (73–89) | 85.0 ± 50.6 (46–173) | 233.4 ± 85.0 (153–364) | 308.6 ± 73.7 (191–379) |
| T0.5 | 0.8 ± 0.1 (0.8–0.9) | 80.4 ± 4.3 (75–86) | 74.0 ± 7.6 (66–84) | 146.4 ± 71.3 (51–251) | 347.0 ± 30.1 (317–396) | |
| T5 | 0.8 ± 0.1 (0.8–0.9) | 79.8 ± 5.1 (74–85) | 65.3 ± 14.5 (53–86) | 206.8 ± 114.9 (137–378) | 345.5 ± 42.2 (294–386) | |
| D120 | Control | 0.5 ± 0.1 (0.4–0.7) | 56.4 ± 10.9 (39–69) | 46.4 ± 25.5 (19–80) | 186.0 ± 53.3 (130–243) | 263.0 ± 109.5 (140–434) |
| T0.5 | 0.4 ± 0.2 (0.1–0.6) | 52.2 ± 13.7 (36–70) | 70.8 ± 36.6 (51–136) | 201.2 ± 140.8 (106–445) | 236.4 ± 40.9 (167–273) | |
| T5 | 0.5 ± 0.1 (0.5–0.6) | 71.0 ± 4.8 (65–78) | 67.2 ± 11.5 (56–85) | 197.4 ± 64.4 (119–271) | 254.6 ± 14.8 (239–270) | |
| D180 | Control | 0.5 ± 0.1 (0.5–0.6) | 51.6 ± 6.5 (47–63) | 53.2 ± 23.9 (14–71) | 158.2 ± 63.5 (62–230) | 262.6 ± 44.3 (191–306) |
| T0.5 | 0.7 ± 0.1 (0.6–0.8) | 57.6 ± 4.3 (53–63) | 58.6 ± 10.0 (44–69) | 164.2 ± 39.0 (128–226) | 280.4 ± 39.9 (223–333) | |
| T5 | 0.6 ± 0.1 (0.5–0.7) | 53.8 ± 4.3 (49–60) | 55.6 ± 75.0 (49–66) | 176.0 ± 54.8 (116–266) | 271.4 ± 38.6 (212–310) | |
| D240 | Control | 0.7 ± 0.1 (0.6–0.8) | 44.5 ± 3.1 (42–48) | 51.0 ± 28.2 (18–89) | 154.8 ± 34.3 (127–210) | 286.8 ± 51.5 (250–375) |
| T0.5 | 0.8 ± 0.2 (0.5–1.0) | 52.8 ± 8.8 (39–61) | 60.2 ± 11.6 (43–71) | 213.4 ± 111.0 (122–374) | 240.2 ± 49.9 (163–295) | |
| T5 | 0.8 ± 0.1 (0.7–0.9) | 47.6 ± 6.4 (44–59) | 60.4 ± 14.5 (53–80) | 129.8 ± 40.7 (101–201) | 348.6 ± 85.5 (243–465) | |
| D300 | Control | 0.3 ± 0.3 (0.1–0.7) | 39.8 ± 10.1 (23–50) | 42.8 ± 22.8 (10–71) | 192.2 ± 50.3 (118–232) | 296,6 ± 131.5 (142–478) |
| T0.5 | 0.5 ± 0.1 (0.4–0.6) | 42.0 ± 12.0 (28–57) | 52.5 ± 14.6 (35–65) | 201.3 ± 52.2 (133–260) | 343.5 ± 103.0 (235–474) | |
| T5 | 0.5 ± 0.4 (0.1–0.9) | 49.2 ± 9.3 (43–64) | 62.2 ± 37.8 (39–129) | 185.4 ± 49.9 (126–226) | 303.8 ± 139.0 (205–542) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulini, F.; Marangon, A.R.M.; Azevedo, C.L.; Brito, J.L.M.; Lemos, M.S.; Sousa, M.H.; Veiga-Souza, F.H.; Souza, P.E.N.; Lucci, C.M.; Azevedo, R.B. In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up. Nanomaterials 2022, 12, 3513. https://doi.org/10.3390/nano12193513
Paulini F, Marangon ARM, Azevedo CL, Brito JLM, Lemos MS, Sousa MH, Veiga-Souza FH, Souza PEN, Lucci CM, Azevedo RB. In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up. Nanomaterials. 2022; 12(19):3513. https://doi.org/10.3390/nano12193513
Chicago/Turabian StylePaulini, Fernanda, Aline R. M. Marangon, Carolina L. Azevedo, Juliana L. M. Brito, Marcelle S. Lemos, Marcelo H. Sousa, Fabiane H. Veiga-Souza, Paulo E. N. Souza, Carolina M. Lucci, and Ricardo B. Azevedo. 2022. "In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up" Nanomaterials 12, no. 19: 3513. https://doi.org/10.3390/nano12193513






