The Ability and Mechanism of nHAC/CGF in Promoting Osteogenesis and Repairing Mandibular Defects
Abstract
:1. Introduction
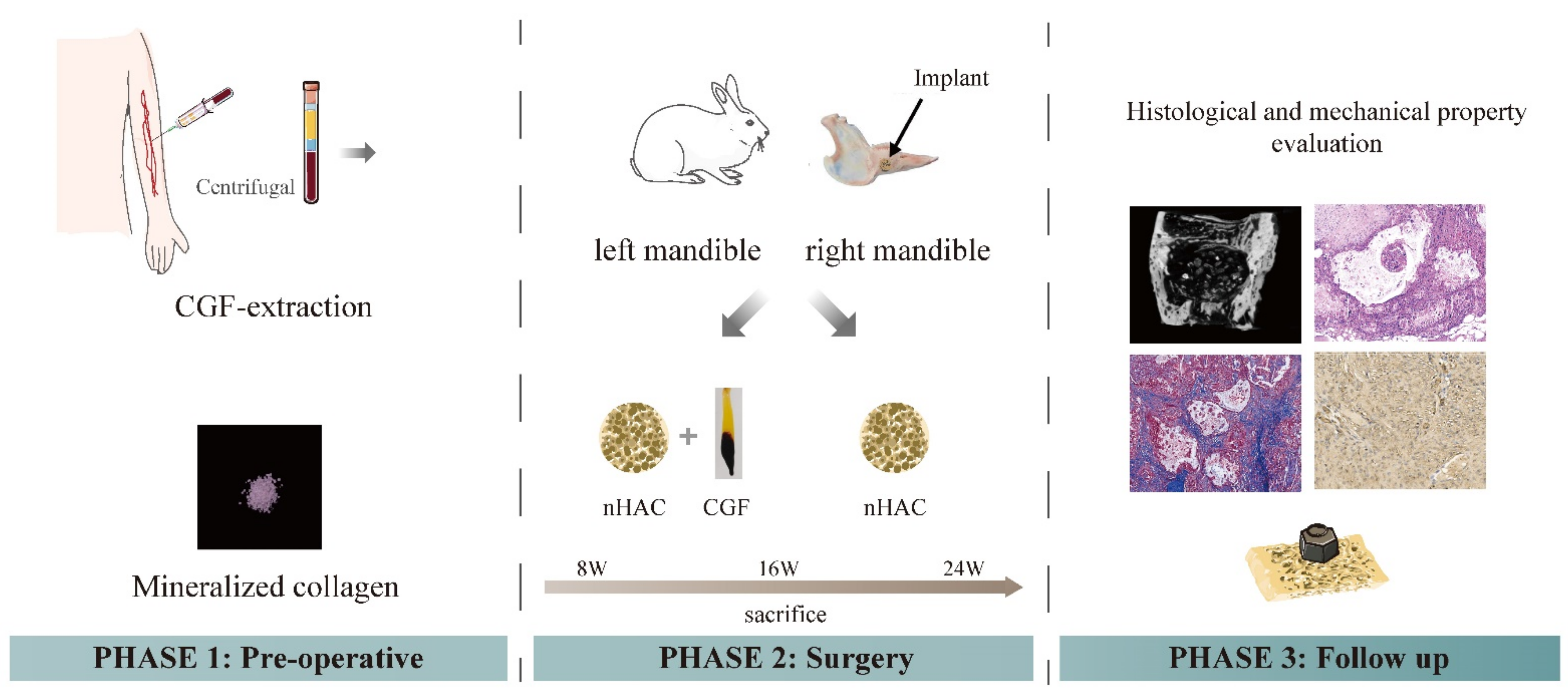
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Animals
2.3. Preparation of CGF
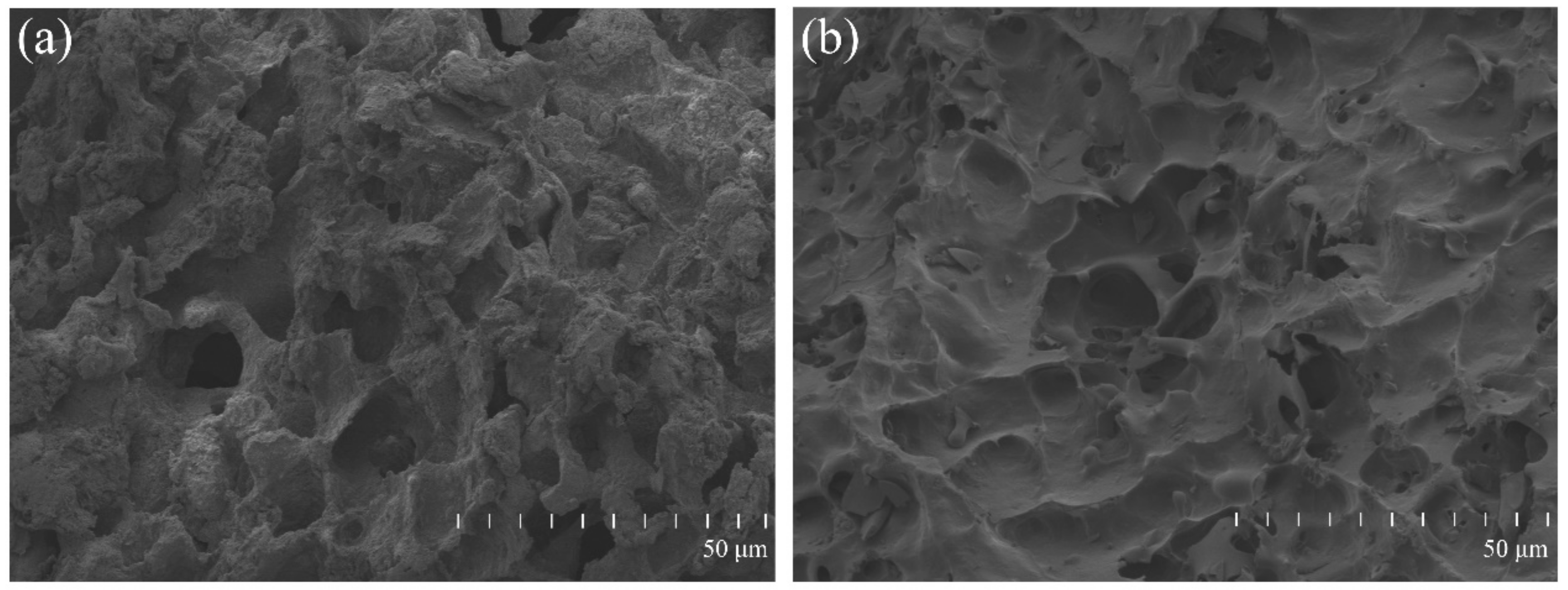
2.4. Surface Morphology of the Material
2.5. Establishment of Animal Models and Material Implantation
2.6. General Observations
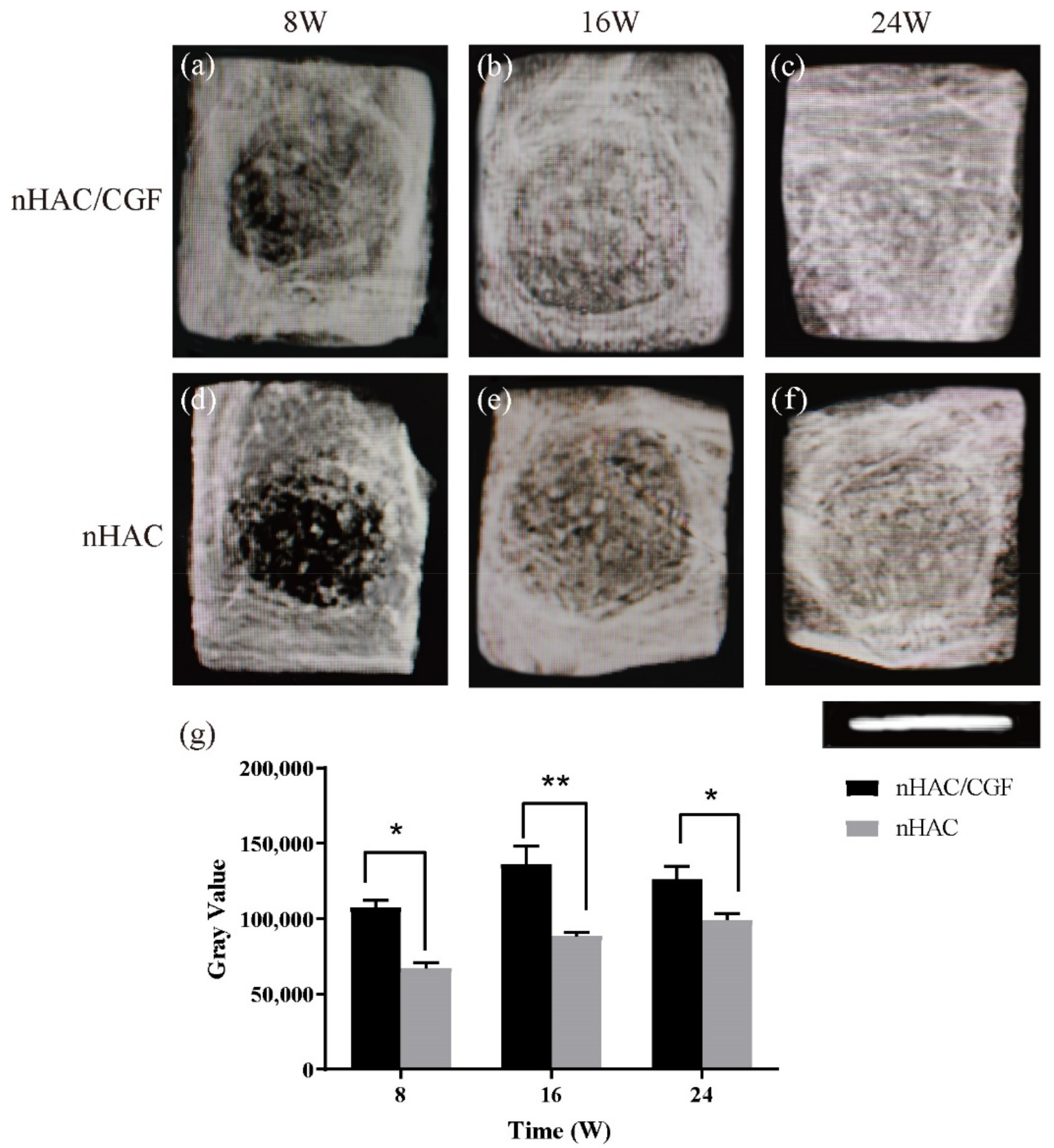
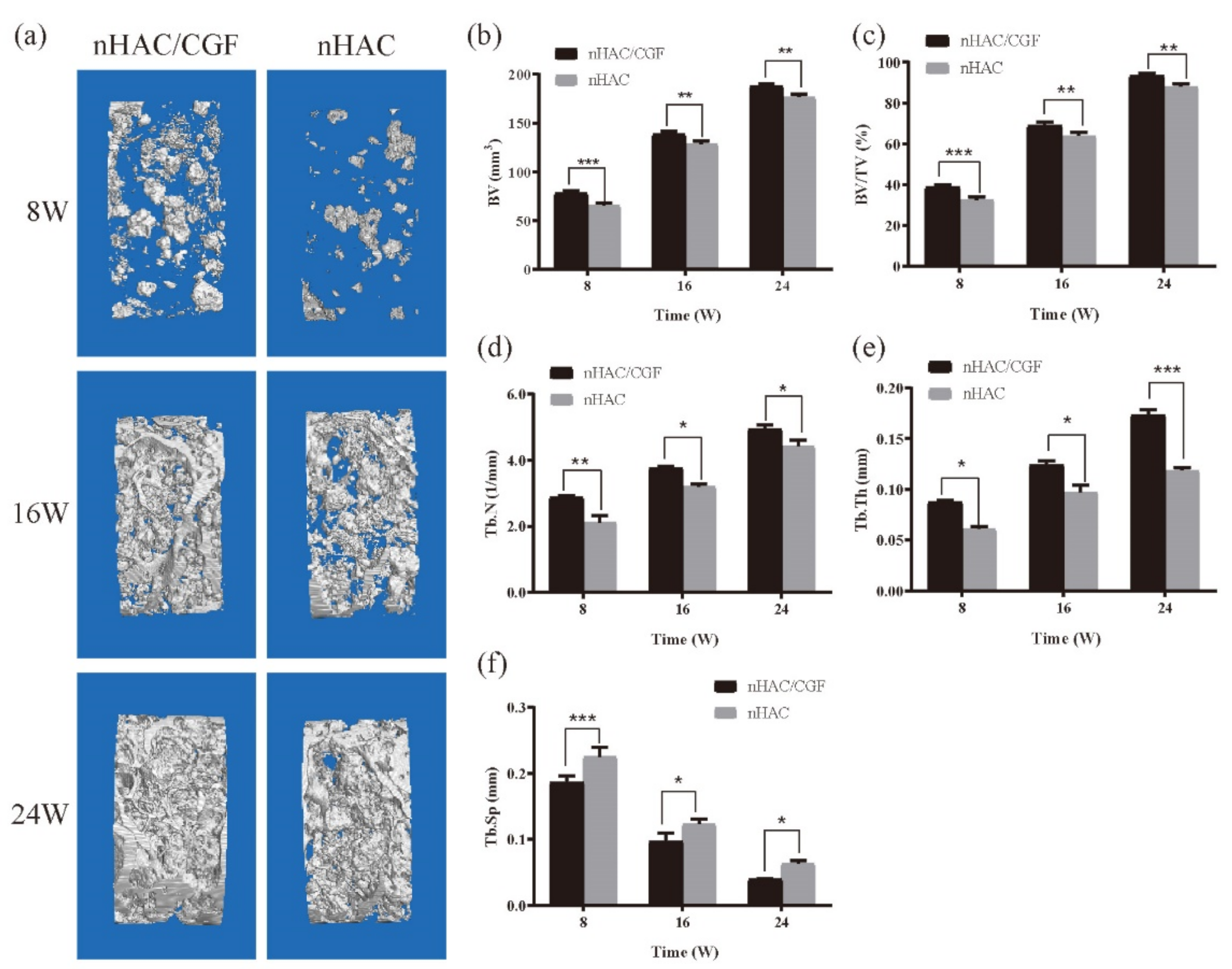
2.7. Imaging Examination
2.8. Histological Observations
2.9. Biomechanical Testing
2.10. Statistical Analysis
3. Results
3.1. The Surface Morphology of the Material
3.2. General Observation
3.3. Imaging Testing
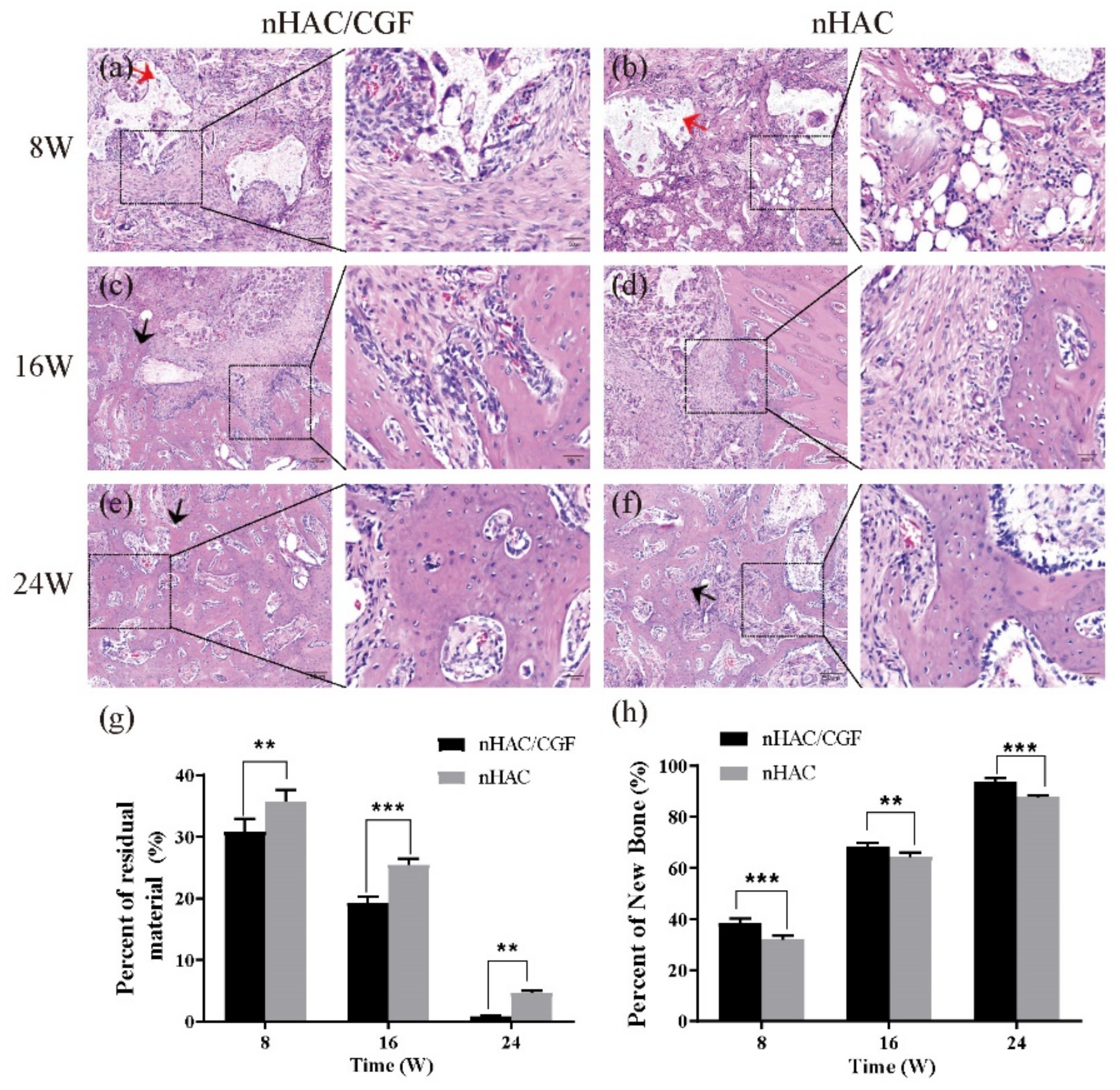
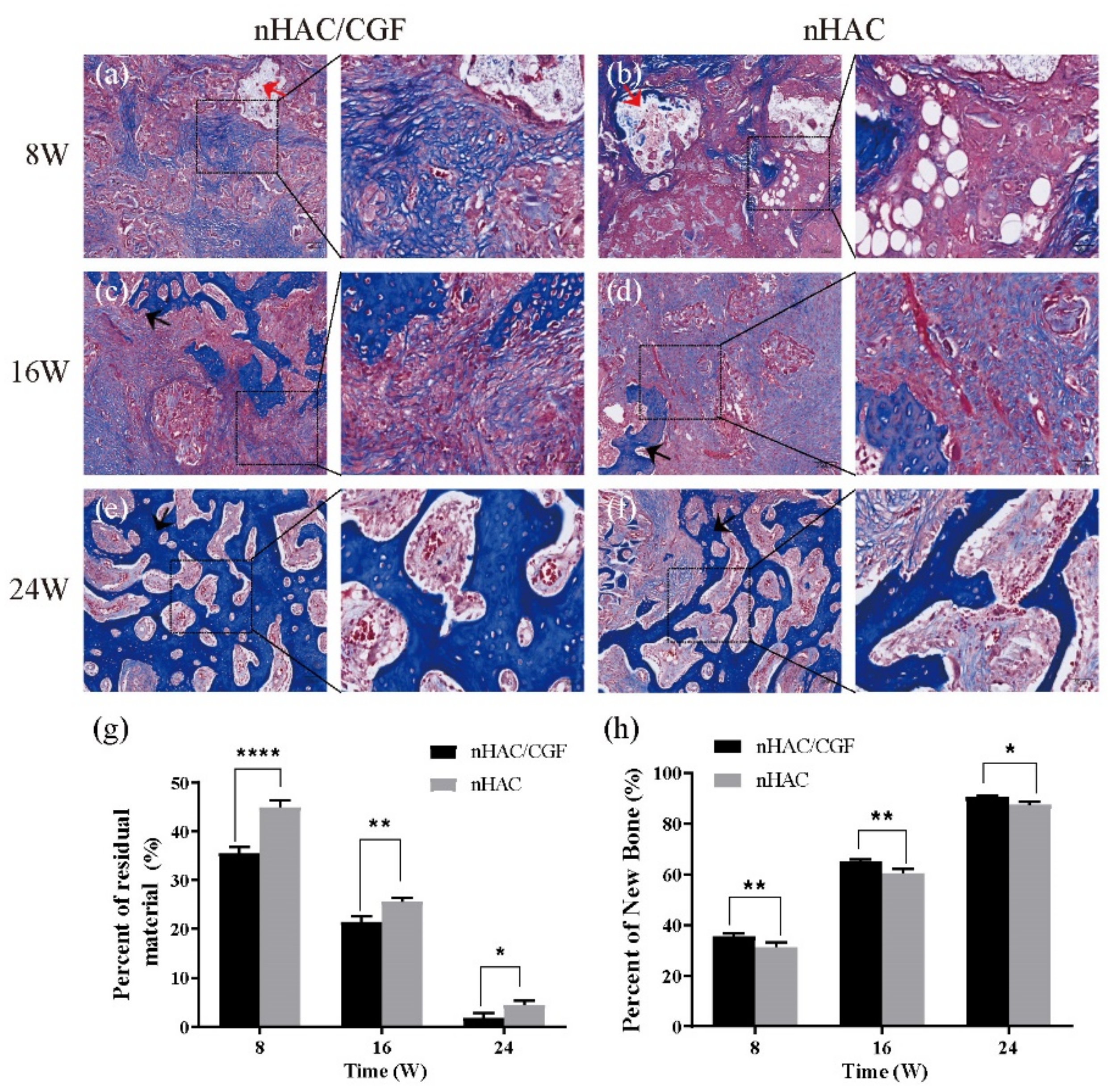
3.4. Histological Evaluation
3.5. Biomechanical Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. North Am. 2019, 31, 163–191. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Zara, S.; Tete, G.; Vinci, R.; Gherlone, E.; Cataldi, A. Biologic and clinical aspects of integration of different bone substitutes in oral surgery: A literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 392–402. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98-B, 6–9. [Google Scholar] [CrossRef]
- Wang, C.; Yu, B.; Fan, Y.; Ormsby, R.W.; McCarthy, H.O.; Dunne, N.; Li, X. Incorporation of multi-walled carbon nanotubes to PMMA bone cement improves cytocompatibility and osseointegration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109823. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Mao, Z.; Liu, B.; Yang, L.; He, W.; Fan, Y.; Li, X. The effects of silk layer-by-layer surface modification on the mechanical and structural retention of extracellular matrix scaffolds. Biomater. Sci. 2020, 8, 4026–4038. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.X.; Yang, G.G.; Li, Z.W.; Shi, Z.M.; Sun, Z.D. Clinical observation of biomimetic mineralized collagen artificial bone putty for bone reconstruction of calcaneus fracture. Regen. Biomater. 2018, 5, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.F.; Wang, C.Y.; Wan, P.; Wang, S.G.; Wang, X.M. Comparison of bone regeneration in alveolar bone of dogs on mineralized collagen grafts with two composition ratios of nano-hydroxyapatite and collagen. Regen. Biomater. 2016, 3, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhao, Z.; Yang, Y.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X.; Zhang, C. A high-strength mineralized collagen bone scaffold for large-sized cranial bone defect repair in sheep. Regen. Biomater. 2018, 5, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Zhou, W.J.; Liu, Z.H.; Hao, P.J. The extract of concentrated growth factor enhances osteogenic activity of osteoblast through PI3K/AKT pathway and promotes bone regeneration in vivo. Int. J. Implant. Dent. 2021, 7, 70. [Google Scholar] [CrossRef]
- Słota, D.; Głąb, M.; Tyliszczak, B.; Douglas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-Kupiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scari, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, H. A Comprehensive Review of Concentrated Growth Factors and Their Novel Applications in Facial Reconstructive and Regenerative Medicine. Aesthetic Plast. Surg. 2020, 44, 1047–1057. [Google Scholar] [CrossRef]
- Rochira, A.; Siculella, L.; Damiano, F.; Palermo, A.; Ferrante, F.; Carluccio, M.A.; Calabriso, N.; Giannotti, L.; Stanca, E. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, Z. A comparative study of the effect of Bio-Oss® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting. J. Oral Pathol. Med. 2017, 46, 528–536. [Google Scholar] [CrossRef]
- Wang, X.; Tong, S.; Huang, S.; Ma, L.; Liu, Z.; Zhang, D. Application of a New Type of Natural Calcined Bone Repair Material Combined with Concentrated Growth Factors in Bone Regeneration in Rabbit Critical-Sized Calvarial Defect. Biomed. Res. Int. 2020, 2020, 8810747. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, S.S.; Cui, F.Z. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003, 15, 3221–3226. [Google Scholar] [CrossRef]
- Doi, Y.; Horiguchi, T.; Moriwaki, Y.; Kitago, H.; Kajimoto, T.; Iwayama, Y. Formation of apatite-collagen complexes. J. Biomed. Mater. Res. 1996, 31, 43–49. [Google Scholar] [CrossRef]
- Weisgerber, D.W.; Caliari, S.R.; Harley, B.A. Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomater. Sci. 2015, 3, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wu, C.; Chen, S.; Zhang, Y.; Liu, A.; Ding, J.; Wei, D.; Guo, Z.; Sun, J.; Fan, H. Biomimetic mineralizable collagen hydrogels for dynamic bone matrix formation to promote osteogenesis. J. Mater. Chem. B 2020, 8, 3064–3075. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, J.; Sun, Q.; Yan, S.; Wang, W.; Yang, P.; Song, A. One-Year Results Evaluating the Effects of Concentrated Growth Factors on the Healing of Intrabony Defects Treated with or without Bone Substitute in Chronic Periodontitis. Med. Sci. Monit. 2019, 25, 4384–4389. [Google Scholar] [CrossRef]
- Fang, D.; Long, Z.; Hou, J. Clinical Application of Concentrated Growth Factor Fibrin Combined with Bone Repair Materials in Jaw Defects. J. Oral Maxillofac. Surg. 2020, 78, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Peyrin, F. Evaluation of bone scaffolds by micro-CT. Osteoporos. Int. 2011, 22, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Adali, E.; Yuce, M.O.; Gunbay, T.; Gunbay, S. Does Concentrated Growth Factor Used with Allografts in Maxillary Sinus Lifting Have Adjunctive Benefits? J. Oral Maxillofac. Surg. 2021, 79, 98–108. [Google Scholar] [CrossRef]
- Dai, Y.; Han, X.H.; Hu, L.H.; Wu, H.W.; Huang, S.Y.; Lu, Y.P. Efficacy of concentrated growth factors combined with mineralized collagen on quality of life and bone reconstruction of guided bone regeneration. Regen. Biomater. 2020, 7, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Topkara, A.; Ozkan, A.; Ozcan, R.H.; Oksuz, M.; Akbulut, M. Effect of Concentrated Growth Factor on Survival of Diced Cartilage Graft. Aesthet. Surg. J. 2016, 36, 1176–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durmuslar, M.C.; Balli, U.; Dede, F.O.; Misir, A.F.; Baris, E.; Kurkcu, M.; Kahraman, S.A. Histological Evaluation of the Effect of Concentrated Growth Factor on Bone Healing. J. Craniofac. Surg. 2016, 27, 1494–1497. [Google Scholar] [CrossRef]
- Du, Z.; Feng, X.; Cao, G.; She, Z.; Tan, R.; Aifantis, K.E.; Zhang, R.; Li, X. The effect of carbon nanotubes on osteogenic functions of adipose-derived mesenchymal stem cells in vitro and bone formation in vivo compared with that of nano-hydroxyapatite and the possible mechanism. Bioact. Mater. 2021, 6, 333–345. [Google Scholar] [CrossRef]
- McBane, J.E.; Sharifpoor, S.; Cai, K.; Labow, R.S.; Santerre, J.P. Biodegradation and in vivo biocompatibility of a degradable, polar/hydrophobic/ionic polyurethane for tissue engineering applications. Biomaterials 2011, 32, 6034–6044. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Kawakubo-Yasukochi, T.; Hirata, M. Osteocalcin and its endocrine functions. Biochem. Pharm. 2017, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Yang, C.; Li, Y.; Dai, Z. An overview of osteocalcin progress. J. Bone Min. Metab. 2016, 34, 367–379. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Kresnoadi, U.; Rahmania, P.N.; Caesar, H.U.; Djulaeha, E.; Agustono, B.; Ari, M.D.A. The role of the combination of Moringa oleifera leaf extract and demineralized freeze-dried bovine bone xenograft (xenograft) as tooth extraction socket preservation materials on osteocalcin and transforming growth factor-beta 1 expressions in alveolar bone of Cavia cobaya. J. Indian Prosthodont. Soc. 2019, 19, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109960. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Tao, C.S.; Cui, H.; Wang, C.M.; Cui, F.Z. High-strength mineralized collagen artificial bone. Front. Mater. Sci. 2014, 8, 53–62. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Cao, N.; Zhang, Y.; Cao, G.; Hao, C.; Liu, K.; Li, X.; Wang, W. The Ability and Mechanism of nHAC/CGF in Promoting Osteogenesis and Repairing Mandibular Defects. Nanomaterials 2022, 12, 212. https://doi.org/10.3390/nano12020212
Zhu Y, Cao N, Zhang Y, Cao G, Hao C, Liu K, Li X, Wang W. The Ability and Mechanism of nHAC/CGF in Promoting Osteogenesis and Repairing Mandibular Defects. Nanomaterials. 2022; 12(2):212. https://doi.org/10.3390/nano12020212
Chicago/Turabian StyleZhu, Yuhe, Nanjue Cao, Yue Zhang, Guangxiu Cao, Chunping Hao, Keda Liu, Xiaoming Li, and Wei Wang. 2022. "The Ability and Mechanism of nHAC/CGF in Promoting Osteogenesis and Repairing Mandibular Defects" Nanomaterials 12, no. 2: 212. https://doi.org/10.3390/nano12020212
APA StyleZhu, Y., Cao, N., Zhang, Y., Cao, G., Hao, C., Liu, K., Li, X., & Wang, W. (2022). The Ability and Mechanism of nHAC/CGF in Promoting Osteogenesis and Repairing Mandibular Defects. Nanomaterials, 12(2), 212. https://doi.org/10.3390/nano12020212





