Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved Formaldehyde Gas Sensing
Abstract
:1. Introduction
2. Materials and Methods
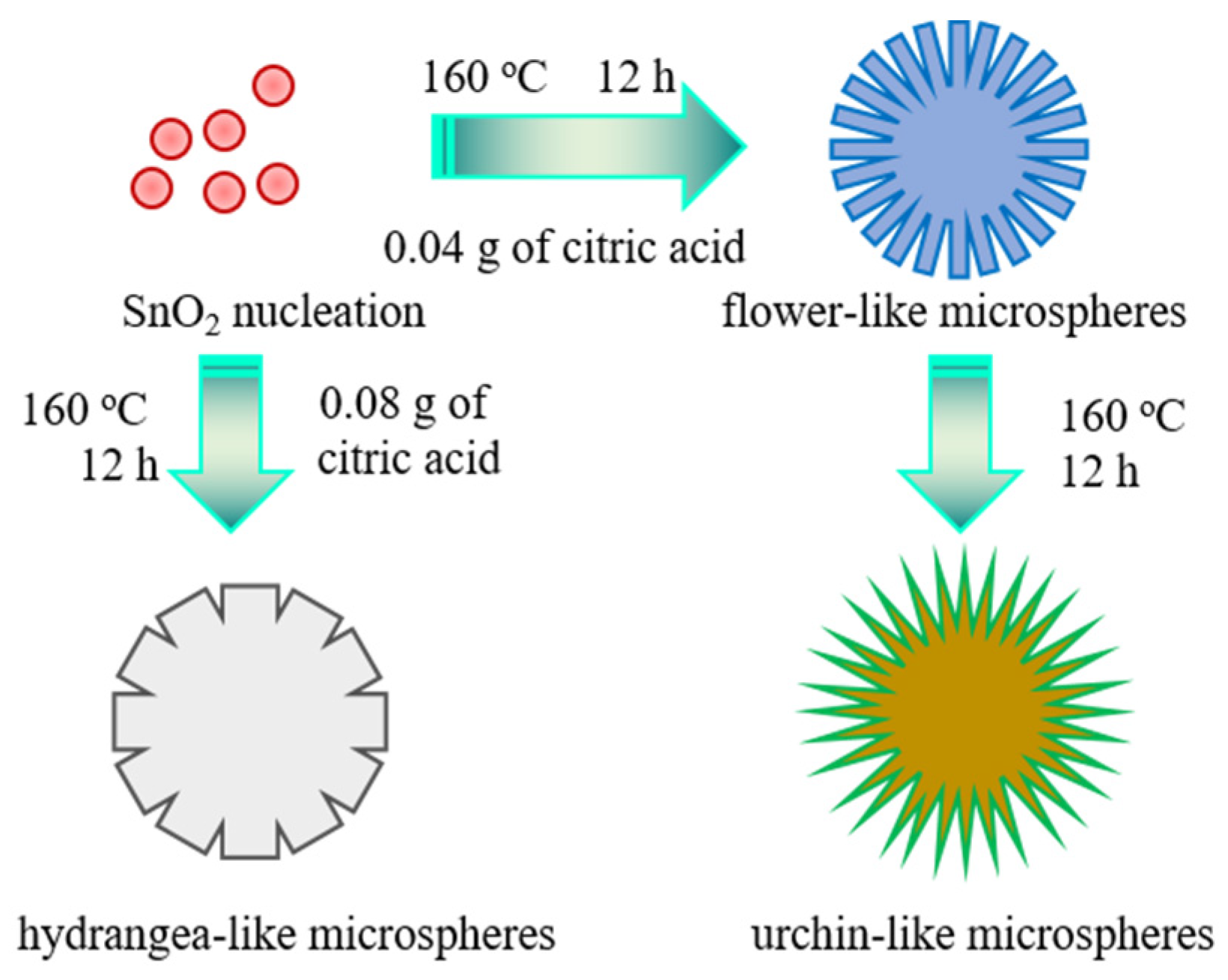
2.1. Chemical Preparation
2.2. Characterization
2.3. Gas Sensor Fabrication and Measurement
3. Results and Discussion
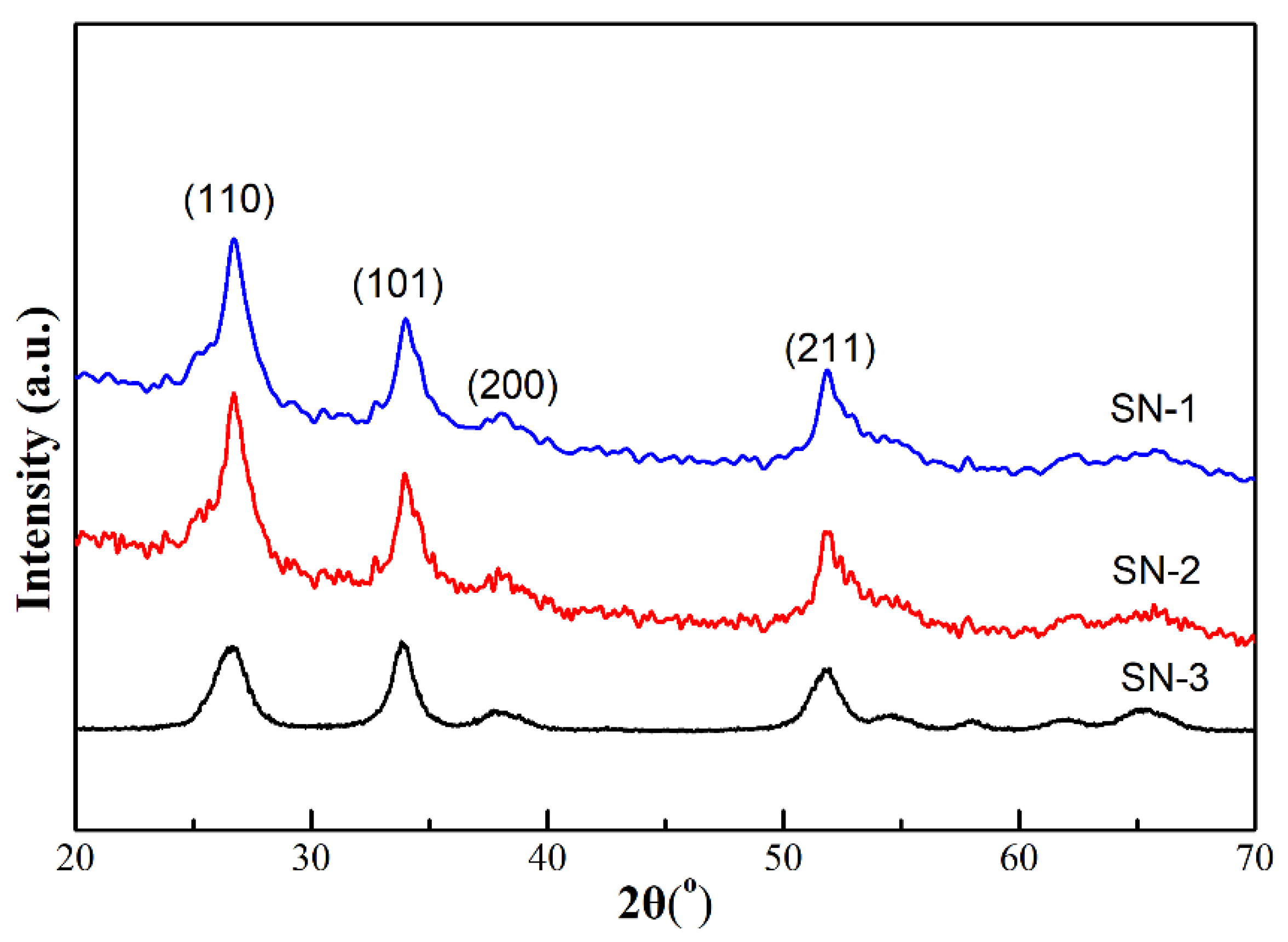
3.1. Sample Characterization
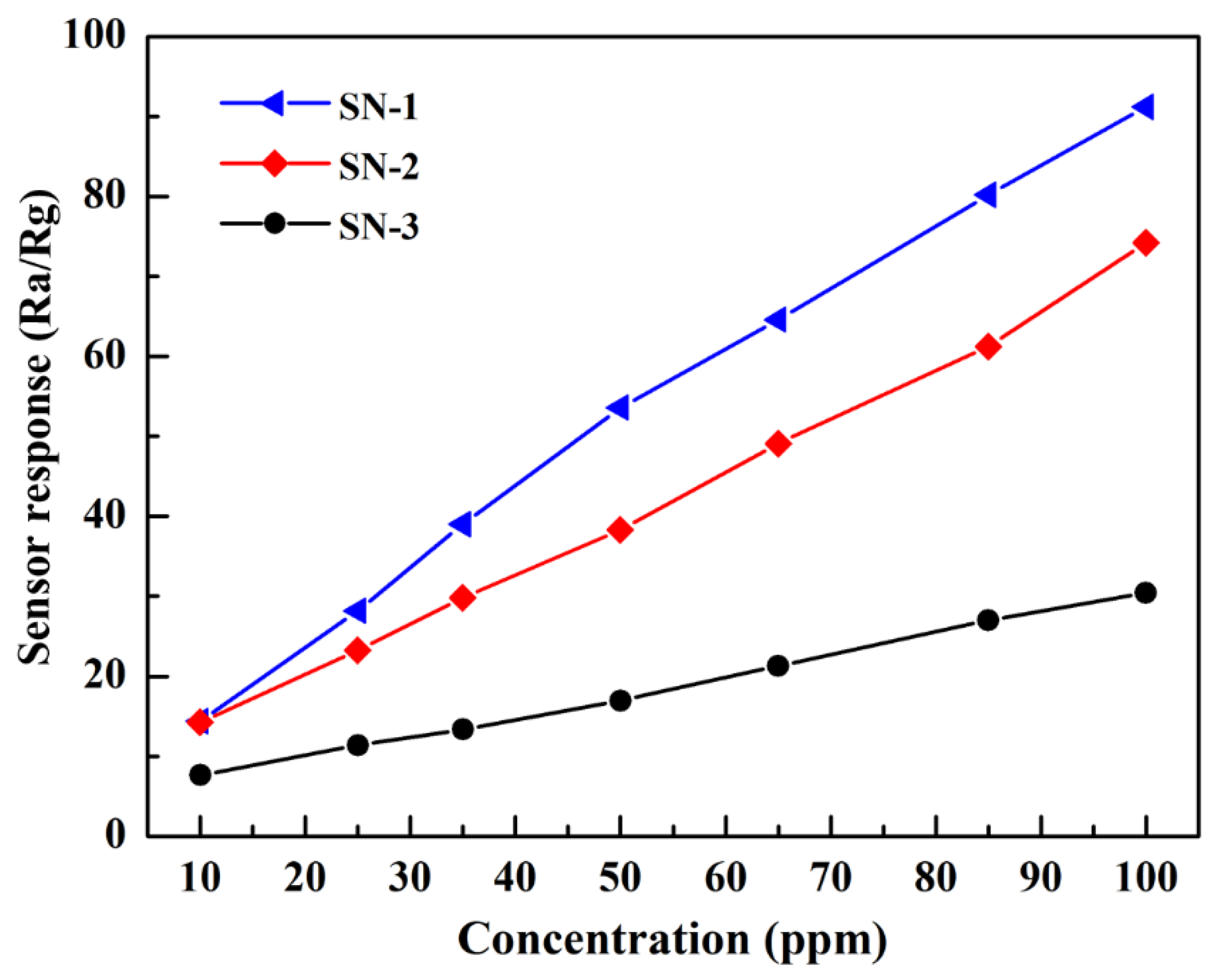
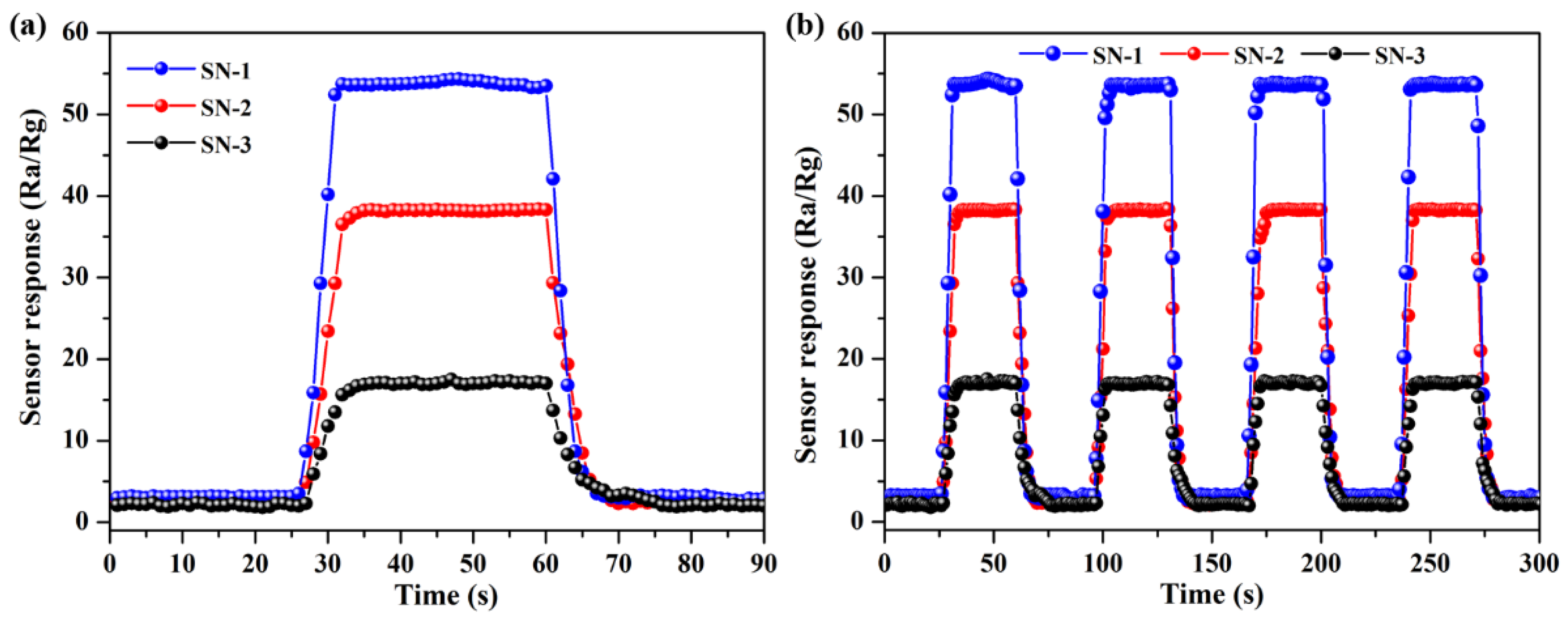
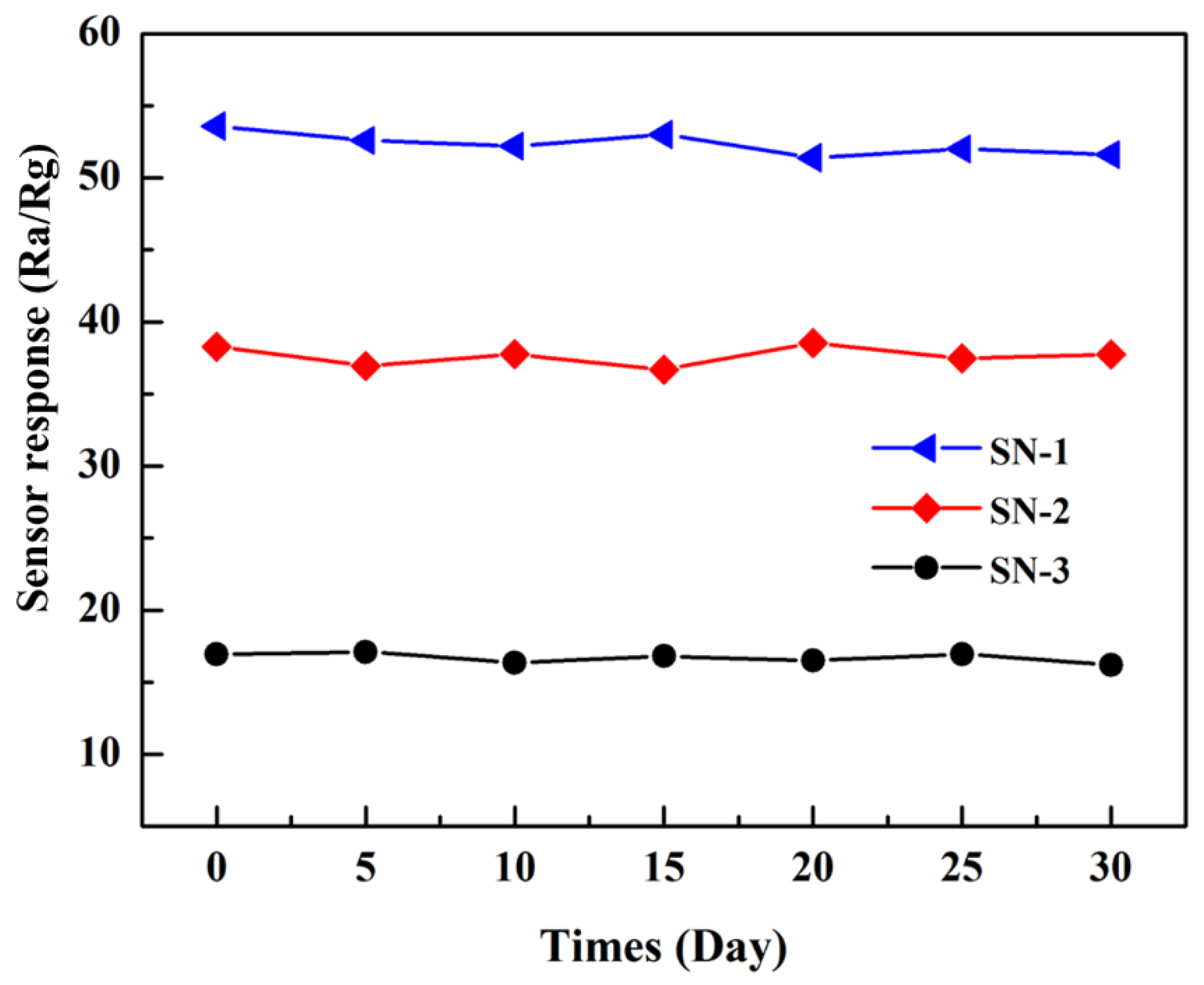
3.2. Gas-Sensing Properties
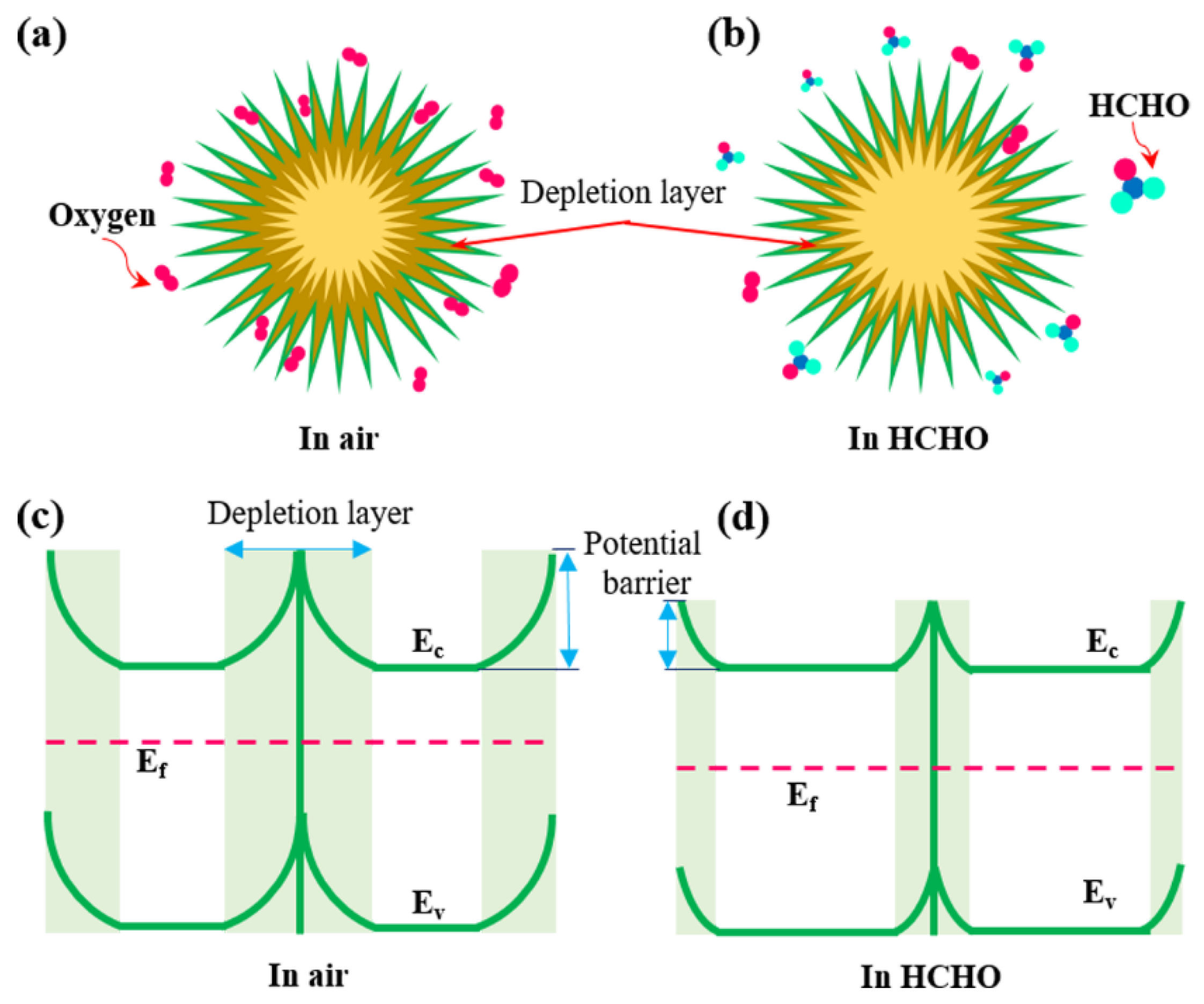
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Wu, H.; Yu, J.; Li, Z.; Yao, G.; Cao, R.; Li, X.; Zhu, H.; He, A.; Tang, Z. Microhotplate gas sensors incorporated with Al electrodes and 3D hierarchical structured PdO/PdO2-SnO2:Sb materials for sensitive VOC detection. Sens. Actuators B Chem. 2021, 329, 128984. [Google Scholar] [CrossRef]
- Pargoletti, E.; Cappelletti, G. Breakthroughs in the design of novel carbon-based metal oxides nanocomposites for VOCs Gas Sensing. Nanomaterials 2020, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M. Efficient formaldehyde sensor development based on Cu-codoped ZnO nanomaterial by an electrochemical approach. Sens. Actuators B Chem. 2020, 305, 127541. [Google Scholar] [CrossRef]
- Huang, X.; Tang, Z.; Tan, Z.; Sheng, S.; Zhao, Q. Hierarchical In2O3 nanostructures for improved formaldehyde: Sensing performance. J. Mater. Sci. Mater. Electron. 2021, 32, 11857–11864. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Xu, J. QCM formaldehyde sensing materials: Design and sensing mechanism. Sens. Actuators B Chem. 2019, 293, 71–82. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Formaldehyde gas sensing properties of transition metal-doped graphene: A first-principles study. J. Mater. Sci. 2021, 56, 12256–12269. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Ye, H.; Zhang, K.; Chen, X.; Zhang, G. Promoting sensitivity and selectivity of HCHO sensor based on strained InP3 monolayer: A DFT study. Appl. Surf. Sci. 2018, 459, 554–561. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal oxide based heterojunctions for gas sensors: A review. Nanomaterials. 2021, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shi, L.; Xie, T.; Wang, D.; Lin, Y. UV-light illumination room temperature HCHO gas-sensing mechanism of ZnO with different nanostructures. Sens. Actuators B Chem. 2016, 227, 220–226. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Peng, S.; Liao, Y. Recent advances of SnO2-based sensors for detecting volatile organic compounds. Front. Chem. 2020, 8, 321. [Google Scholar] [CrossRef]
- Li, N.; Fan, Y.; Shi, Y.; Xiang, Q.; Wang, X.; Xu, J. A low temperature formaldehyde gas sensor based on hirarchical SnO/SnO2 nano-flowers assembled from ultrathin nanosheets: Synthesis, sensing performance and mechanism. Sens. Actuators B Chem. 2019, 294, 106–115. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, P.; Gupta, G. Dimension dependency of tungsten oxide for efficient gas sensing. Environ. Sci. Nano 2021. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Ghule, B.G.; Ekar, S.; Al-Osta, A.; Jadhav, V.V.; Mane, R.S.; Kale, S.N.; Naushad, M.; O’Dwyer, C. Gold sensitized sprayed SnO2 nanostructured film for enhanced LPG sensing. J. Anal. Appl. Pyrol. 2017, 124, 362–368. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Chang, X.; Li, J.; Pan, L.; Jiang, Y.; Gao, W.; Gao, C.; Sun, S. Metal-organic framework-derived porous SnO2 nanosheets with grain sizes comparable to Debye length for formaldehyde detection with high response and low detection limit. Sens. Actuators B Chem. 2021, 347, 130599. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Z.; Zhou, T.; Fei, T.; Wang, R.; Zhang, T. Improvement of gas sensing performance for tin dioxide sensor through construction of nanostructures. J. Colloid Interf. Sci. 2019, 557, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, B.; Ju, X.; Liu, L.; Gong, Y.; Chen, W.; Lu, B. Comparative study of methanol gas sensing performance for SnO2 nanostructures by changing their morphology. Mater. Sci. Semicon. Proc. 2020, 111, 104986. [Google Scholar] [CrossRef]
- Patil, P.; Nakate, Y.T.; Ambare, R.C.; Ingole, R.S.; Kadam, S.L.; Nakate, U.T. 2-D NiO nanostructured material for high reponse acetaldehyde sensing application. Mater. Lett. 2021, 293, 129757. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Zhang, P.; Sui, N.; Cao, S.; Zhou, T.; Zhang, T. Sb/Pd co-doped SnO2 nanoparticles for methane detection: Resistance reduction and sensing performance studies. Nanotechnology 2021, 32, 47. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Sui, N.; Zhang, P.; Zhou, T.; Tu, J.; Zhang, T. TiO2 nanostructures with different crystal phases for sensitive acetone gas sensors. J. Colloid Interf. Sci. 2019, 236, 491–494. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Choudhury, S.P.; Kale, S.N. Microwave assisted synthesis of Co3O4 and NiO nanoplates and structural, optical, magetic characterizations. Nano Struct. Nano Objects 2018, 14, 66–72. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, S. Preparations of Bi-doped SnO2 hierarchical flower-shapled nanostructures with highly sensitive HCHO sensing properties. Mater. Lett. 2019, 607, 357–366. [Google Scholar]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B Chem. 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Wei, Q.; Song, P.; Li, Z.; Yang, Z.; Wang, Q. Herarchical peony-like Sb-doped SnO2 nanostructures: Synthesis, characterization and HCHO sensing properties. Mater. Lett. 2017, 191, 173–177. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Chen, Z.; Li, H.; Chen, A.; Wang, X.; Yang, J. Enhanced formaldehyde sensing properties of hollow SnO2 nanofibers by graphene oxide. Sens. Actuators B Chem. 2016, 250, 533–542. [Google Scholar] [CrossRef]



| Material | Surface Area (m2/g) | Working Temperature (°C) | Gas Concentration (ppm) | Sensor Response towards HCHO | Ref. |
|---|---|---|---|---|---|
| SN-1 | 29.367 | 275 | 50 | 53.6 | This work |
| SN-2 | 24.543 | 275 | 50 | 38.3 | |
| SN-3 | 13.446 | 275 | 50 | 17.0 | |
| petal-like SnO2 | / | 180 | 100 | 12.1 | [22] |
| Bi-doped SnO2 flowers | 170 | 100 | 36.2 | ||
| SnO2 microspheres | 200 | 100 | 38.3 | [23] | |
| Sb-doped SnO2 nanoflowers | 280 | 100 | 45 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, P.; Qi, L.; You, K.; Shi, Q. Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved Formaldehyde Gas Sensing. Nanomaterials 2022, 12, 228. https://doi.org/10.3390/nano12020228
Ren P, Qi L, You K, Shi Q. Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved Formaldehyde Gas Sensing. Nanomaterials. 2022; 12(2):228. https://doi.org/10.3390/nano12020228
Chicago/Turabian StyleRen, Pengyu, Lingling Qi, Kairui You, and Qingwei Shi. 2022. "Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved Formaldehyde Gas Sensing" Nanomaterials 12, no. 2: 228. https://doi.org/10.3390/nano12020228
APA StyleRen, P., Qi, L., You, K., & Shi, Q. (2022). Hydrothermal Synthesis of Hierarchical SnO2 Nanostructures for Improved Formaldehyde Gas Sensing. Nanomaterials, 12(2), 228. https://doi.org/10.3390/nano12020228






