Multifunctional Loblolly Pine-Derived Superactivated Hydrochar: Effect of Hydrothermal Carbonization on Hydrogen and Electron Storage with Carbon Dioxide and Dye Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Acquired
2.2. Synthesis of SAH from Loblolly Pine
2.3. Characterization of SAH
2.4. Gas Adsorption in SAH
2.5. Electrode Preparation and Testing Using SAH
2.6. Methylene Blue Adsorption Using SAH
2.7. Adsorption Isotherm Models
3. Results
3.1. Alteration in SAH Morphology and Functionality with HTC Temperature
3.2. HTC Affecting SAH Applications
3.2.1. H2 Storage in SAH at High Pressure and Cryogenic Temperature
3.2.2. Carbon Capture on SAH at Ambient Condition
3.2.3. Adsorption of Methylene Blue (MB) Dye from Water by SAH
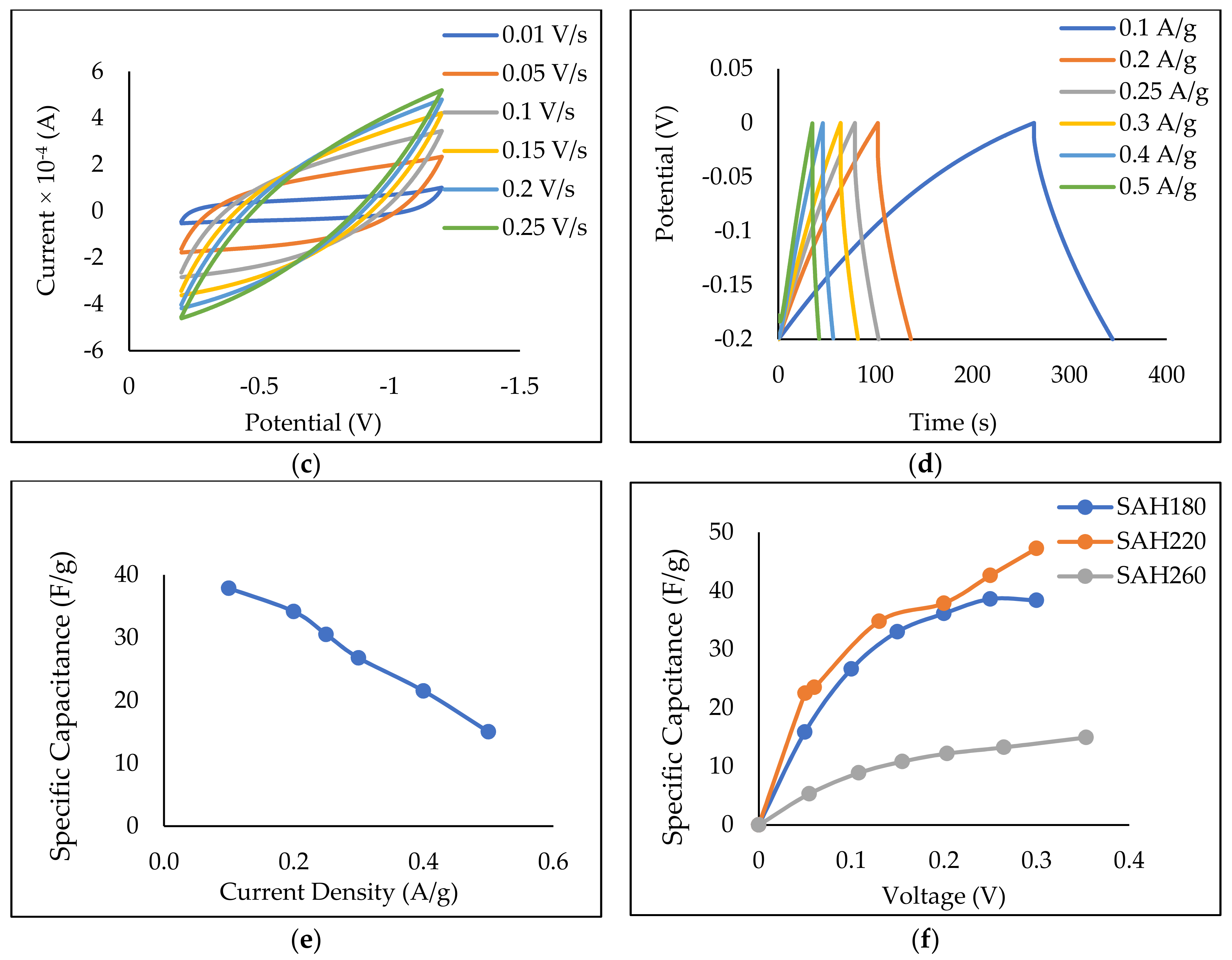
3.2.4. Electron Storage on SAH
3.2.5. Comparing Adsorption Isotherms of Various Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perlack, R.D. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2005. [Google Scholar]
- U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry. Available online: https://www.energy.gov/eere/bioenergy/downloads/us-billion-ton-update-biomass-supply-bioenergy-and-bioproducts-industry (accessed on 6 April 2022).
- Vlaskin, M.S.; Kostyukevich, Y.I.; Grigorenko, A.V.; Kiseleva, E.A.; Vladimirov, G.N.; Yakovlev, P.V.; Nikolaev, E.N. Hydrothermal Treatment of Organic Waste. Russ. J. Appl. Chem. 2017, 90, 1285–1292. [Google Scholar] [CrossRef]
- Islam, M.T.; Sultana, A.I.; Saha, N.; Klinger, J.L.; Reza, M.T. Pretreatment of Biomass by Selected Type-III Deep Eutectic Solvents and Evaluation of the Pretreatment Effects on Hydrothermal Carbonization. Ind. Eng. Chem. Res. 2021, 60, 15479–15491. [Google Scholar] [CrossRef]
- Ischia, G.; Cutillo, M.; Guella, G.; Bazzanella, N.; Cazzanelli, M.; Orlandi, M.; Miotello, A.; Fiori, L. Hydrothermal Carbonization of Glucose: Secondary Char Properties, Reaction Pathways, and Kinetics. Chem. Eng. J. 2022, 449, 137827. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Zhang, Q.; Wang, C.; Zhan, H.; Ma, L. A Review on the Utilization of Industrial Biowaste via Hydrothermal Carbonization. Renew. Sustain. Energy Rev. 2022, 154, 111877. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Cui, D.; Zhou, H.; Wu, D.; Pan, S.; Xu, F.; Wang, Z. Co-Hydrothermal Carbonization of Organic Solid Wastes to Hydrochar as Potential Fuel: A Review. Sci. Total Environ. 2022, 850, 158034. [Google Scholar] [CrossRef] [PubMed]
- Tahmid Islam, M.; Klinger, J.L.; Toufiq Reza, M. Evaluating Combustion Characteristics and Combustion Kinetics of Corn Stover-Derived Hydrochars by Cone Calorimeter. Chem. Eng. J. 2023, 452, 139419. [Google Scholar] [CrossRef]
- Hammud, H.H.; Shmait, A.; Hourani, N. Removal of Malachite Green from Water Using Hydrothermally Carbonized Pine Needles. RSC Adv. 2015, 5, 7909–7920. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Chen, J.; Zimmerman, A.R. Hydrochars Derived from Plant Biomass under Various Conditions: Characterization and Potential Applications and Impacts. Chem. Eng. J. 2015, 267, 253–259. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Hui, T.S.; Zaini, M.A.A. Potassium Hydroxide Activation of Activated Carbon: A Commentary. Carbon Lett. 2015, 16, 275–280. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black Perspectives for a Green Future: Hydrothermal Carbons for Environment Protection and Energy Storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Sinan, N.; Unur, E. Hydrothermal Conversion of Lignocellulosic Biomass into High-Value Energy Storage Materials. J. Energy Chem. 2017, 26, 783–789. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and Its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Sultana, A.I.; Reza, M.T. Techno-Economic Assessment of Superactivated Hydrochar Production by KOH Impregnation Compared to Direct Chemical Activation. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Giri, A.K.; Saha, A.; Mondal, A.; Ghosh, S.C.; Kundu, S.; Panda, A.B. Rectangular ZnO Porous Nano-Plate Assembly with Excellent Acetone Sensing Performance and Catalytic Activity. RSC Adv. 2015, 5, 102134–102142. [Google Scholar] [CrossRef]
- Sultana, A.I.; Reza, M.T. Investigation of Hydrothermal Carbonization and Chemical Activation Process Conditions on Hydrogen Storage in Loblolly Pine-Derived Superactivated Hydrochars. Int. J. Hydrog. Energy 2022, 47, 26422–26434. [Google Scholar] [CrossRef]
- Song, T.; Liao, J.; Xiao, J.; Shen, L. Effect of Micropore and Mesopore Structure on CO2 Adsorption by Activated Carbons from Biomass. New Carbon Mater. 2015, 30, 156–166. [Google Scholar] [CrossRef]
- Blankenship, L.S.; Mokaya, R. Cigarette Butt-Derived Carbons Have Ultra-High Surface Area and Unprecedented Hydrogen Storage Capacity. Energy Environ. Sci. 2017, 10, 2552–2562. [Google Scholar] [CrossRef]
- Sevilla, M.; Falco, C.; Titirici, M.-M.; Fuertes, A.B. High-Performance CO2 Sorbents from Algae. RSC Adv. 2012, 2, 12792–12797. [Google Scholar] [CrossRef]
- Fu, R.; Li, Z.; Liang, Y.; Li, F.; Xu, F.; Wu, D. Hierarchical Porous Carbons: Design, Preparation, and Performance in Energy Storage. New Carbon Mater. 2011, 26, 171–179. [Google Scholar] [CrossRef]
- Hor, A.A.; Hashmi, S.A. Optimization of Hierarchical Porous Carbon Derived from a Biomass Pollen-Cone as High-Performance Electrodes for Supercapacitors. Electrochim. Acta 2020, 356, 136826. [Google Scholar] [CrossRef]
- Liu, X.; Song, P.; Wang, B.; Wu, Y.-Y.; Jiang, Y.; Xu, F.; Zhang, X. Lignosulfonate-Directed Synthesis of Consubstantial Yolk-Shell Carbon Microspheres with Pollen-Like Surface from Sugar Biomass. ACS Sustain. Chem. Eng. 2018, 6, 6315–16322. [Google Scholar] [CrossRef]
- Li, H.; Shi, F.; An, Q.; Zhai, S.; Wang, K.; Tong, Y. Three-Dimensional Hierarchical Porous Carbon Derived from Lignin for Supercapacitors: Insight into the Hydrothermal Carbonization and Activation. Int. J. Biol. Macromol. 2021, 166, 923–933. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on Recent Advances of Carbon Based Adsorbent for Methylene Blue Removal from Waste Water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Chaudhary, R.G.; Potbhare, A.K.; Aziz, S.K.T.; Umekar, M.S.; Bhuyar, S.S.; Mondal, A. Phytochemically Fabricated Reduced Graphene Oxide-ZnO NCs by Sesbania Bispinosa for Photocatalytic Performances. Mater. Today Proc. 2021, 36, 756–762. [Google Scholar] [CrossRef]
- Jin, Q.; Li, Y.; Yang, D.; Cui, J. Chitosan-Derived Three-Dimensional Porous Carbon for Fast Removal of Methylene Blue from Wastewater. RSC Adv. 2018, 8, 1255–1264. [Google Scholar] [CrossRef]
- Mishra, R.; Prasad, P.R.; Panda, P.; Barman, S. Highly Porous Activated N-Doped Carbon as an Ideal Electrode Material for Capacitive Energy Storage and Physisorption of H2, CO2, and CH4. Energy Fuels 2021, 35, 14177–14187. [Google Scholar] [CrossRef]
- Falco, C.; Marco-Lozar, J.P.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M.; Lozano-Castelló, D. Tailoring the Porosity of Chemically Activated Hydrothermal Carbons: Influence of the Precursor and Hydrothermal Carbonization Temperature. Carbon 2013, 62, 346–355. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding Chemical Reactions between Carbons and NaOH and KOH: An Insight into the Chemical Activation Mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Sultana, A.I.; Saha, N.; Reza, M.T. Upcycling Simulated Food Wastes into Superactivated Hydrochar for Remarkable Hydrogen Storage. J. Anal. Appl. Pyrolysis 2021, 159, 105322. [Google Scholar] [CrossRef]
- Analysis of Oxygen, Oxygen Content of Biomass—Celignis Biomass Analysis Laboratory. Available online: https://www.celignis.com/analyte.php?value=26 (accessed on 8 March 2021).
- Islam, M.T.; Chambers, C.; Toufiq Reza, M. Effects of Process Liquid Recirculation on Material Properties of Hydrochar and Corresponding Adsorption of Cationic Dye. J. Anal. Appl. Pyrolysis 2022, 161, 105418. [Google Scholar] [CrossRef]
- Schaefer, S.; Jeder, A.; Sdanghi, G.; Gadonneix, P.; Abdedayem, A.; Izquierdo, M.T.; Maranzana, G.; Ouederni, A.; Celzard, A.; Fierro, V. Oxygen-Promoted Hydrogen Adsorption on Activated and Hybrid Carbon Materials. Int. J. Hydrog. Energy 2020, 45, 30767–30782. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Exploring the Use of Rachis of Chicken Feathers for Hydrogen Storage. J. Anal. Appl. Pyrolysis 2013, 104, 243–248. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Hadj, E. A Comparative Study of the Linear and Non-Linear Methods for Determination of the Optimum Equilibrium Isotherm for Adsorption of Pb2+ Ions onto Algerian Treated Clay. Iran. J. Chem. Chem. Eng. 2020, 39, 19. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Sangchoom, W.; Mokaya, R. Valorization of Lignin Waste: Carbons from Hydrothermal Carbonization of Renewable Lignin as Superior Sorbents for CO2 and Hydrogen Storage. ACS Sustain. Chem. Eng. 2015, 3, 1658–1667. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Maisano, S.; Urbani, F.; Mondello, N.; Chiodo, V. Catalytic Pyrolysis of Mediterranean Sea Plant for Bio-Oil Production. Int. J. Hydrog. Energy 2017, 42, 28082–28092. [Google Scholar] [CrossRef]
- Cataldo, S.; Chiodo, V.; Crea, F.; Maisano, S.; Milea, D.; Pettignano, A. Biochar from Byproduct to High Value Added Material—A New Adsorbent for Toxic Metal Ions Removal from Aqueous Solutions. J. Mol. Liq. 2018, 271, 481–489. [Google Scholar] [CrossRef]
- Pedicini, R.; Maisano, S.; Chiodo, V.; Conte, G.; Policicchio, A.; Agostino, R.G. Posidonia Oceanica and Wood Chips Activated Carbon as Interesting Materials for Hydrogen Storage. Int. J. Hydrog. Energy 2020, 45, 14038–14047. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, D.; Wang, Y.; Wei, S.; Yang, W.; Kuang, M.; Ma, L.; Fang, D.; Xu, S.; Du, S. Bioethanol Production from Cotton Stalk: A Comparative Study of Various Pretreatments. Fuel 2016, 184, 527–532. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Sun, F.; Wang, L.; Peng, Y.; Gao, J.; Pi, X.; Qu, Z.; Zhao, G.; Qin, Y. Converting Biomass Waste into Microporous Carbon with Simultaneously High Surface Area and Carbon Purity as Advanced Electrochemical Energy Storage Materials. Appl. Surf. Sci. 2018, 436, 486–494. [Google Scholar] [CrossRef]
- Han, M.; Jiang, K.; Jiao, P.; Ji, Y.; Zhou, J.; Zhuang, W.; Chen, Y.; Liu, D.; Zhu, C.; Chen, X.; et al. Bio-Butanol Sorption Performance on Novel Porous-Carbon Adsorbents from Corncob Prepared via Hydrothermal Carbonization and Post-Pyrolysis Method. Sci. Rep. 2017, 7, 11753. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and Structural Differences between Glucose, Cellulose and Lignocellulosic Biomass Derived Hydrothermal Carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of High Surface Area Sludge-Based Activated Hydrochar via Hydrothermal Carbonization and Application in the Removal of Basic Dye. Environ. Res. 2019, 175, 457–467. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Saha, N.; McGaughy, K.; Reza, M.T. Elucidating Hydrochar Morphology and Oxygen Functionality Change with Hydrothermal Treatment Temperature Ranging from Subcritical to Supercritical Conditions. J. Anal. Appl. Pyrolysis 2020, 152, 104965. [Google Scholar] [CrossRef]
- Akasaka, H.; Takahata, T.; Toda, I.; Ono, H.; Ohshio, S.; Himeno, S.; Kokubu, T.; Saitoh, H. Hydrogen Storage Ability of Porous Carbon Material Fabricated from Coffee Bean Wastes. Int. J. Hydrog. Energy 2011, 36, 580–585. [Google Scholar] [CrossRef]
- Sun, Y.; Webley, P.A. Preparation of Activated Carbons from Corncob with Large Specific Surface Area by a Variety of Chemical Activators and Their Application in Gas Storage. Chem. Eng. J. 2010, 162, 883–892. [Google Scholar] [CrossRef]
- Wróbel-Iwaniec, I.; Díez, N.; Gryglewicz, G. Chitosan-Based Highly Activated Carbons for Hydrogen Storage. Int. J. Hydrog. Energy 2015, 40, 5788–5796. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, Q.; Jiang, J. High Hydrogen Uptake Capacity of Mesoporous Nitrogen-Doped Carbons Activated Using Potassium Hydroxide. Carbon 2010, 48, 2968–2973. [Google Scholar] [CrossRef]
- Xiao, Y.; Dong, H.; Long, C.; Zheng, M.; Lei, B.; Zhang, H.; Liu, Y. Melaleuca Bark Based Porous Carbons for Hydrogen Storage. Int. J. Hydrog. Energy 2014, 39, 11661–11667. [Google Scholar] [CrossRef]
- Blankenship Ii, T.S.; Balahmar, N.; Mokaya, R. Oxygen-Rich Microporous Carbons with Exceptional Hydrogen Storage Capacity. Nat. Commun. 2017, 8, 1545. [Google Scholar] [CrossRef]
- Yang, R.; Liu, G.; Li, M.; Zhang, J.; Hao, X. Preparation and N2, CO2 and H2 Adsorption of Super Activated Carbon Derived from Biomass Source Hemp (Cannabis sativa L.) Stem. Microporous Mesoporous Mater. 2012, 158, 108–116. [Google Scholar] [CrossRef]
- Agarwal, R.K.; Noh, J.S.; Schwarz, J.A.; Davini, P. Effect of Surface Acidity of Activated Carbon on Hydrogen Storage. Carbon 1987, 25, 219–226. [Google Scholar] [CrossRef]
- Sultana, A.I.; Saha, N.; Reza, M.T. Synopsis of Factors Affecting Hydrogen Storage in Biomass-Derived Activated Carbons. Sustainability 2021, 13, 1947. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen Adsorption in Different Carbon Nanostructures. Carbon 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Luo, L.; Wu, X.; Li, Z.; Fan, M.; Zhao, W. Preparation and Characterization of Heteroatom Self-Doped Activated Biocarbons as Hydrogen Storage and Supercapacitor Electrode Materials. Electrochim. Acta 2019, 325, 134941. [Google Scholar] [CrossRef]
- Balathanigaimani, M.S.; Haider, M.d.B.; Jha, D.; Kumar, R.; Lee, S.J.; Shim, W.G.; Shon, H.K.; Kim, S.C.; Moon, H. Nanostructured Biomass Based Carbon Materials from Beer Lees for Hydrogen Storage. J. Nanosci. Nanotechnol. 2018, 18, 2196–2199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Luo, L.; Wang, H.; Fan, M. Synthesis of Bamboo-Based Activated Carbons with Super-High Specific Surface Area for Hydrogen Storage. BioResources 2017, 12, 1246–1262. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Pütter, H.; Müller, U. Hydrogen Adsorption in Metal–Organic Frameworks: Cu-MOFs and Zn-MOFs Compared. Adv. Funct. Mater. 2006, 16, 520–524. [Google Scholar] [CrossRef]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 Saturation Uptake in Microporous Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a Dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Wu, D.; Lu, X.; Cao, W.; Yang, T.; Zhu, Y. Molecular Simulation Study of the Adsorption and Diffusion of a Mixture of CO2/CH4 in Activated Carbon: Effect of Textural Properties and Surface Chemistry. J. Chem. Eng. Data 2016, 61, 4139–4147. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Xing, W. CHAPTER 1 Carbon-Based CO2 Adsorbents; Royal Society of Chemistry: London, UK, 2018; pp. 1–75. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of Pore Size on Carbon Dioxide Sorption by Carbide Derived Carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on Molecular Sieves and Activated Carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Kikkinides, E.S.; Yang, R.T.; Cho, S.H. Concentration and Recovery of Carbon Dioxide from Flue Gas by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 1993, 32, 2714–2720. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly Microporous Activated Carbons from Biomass for CO2 Capture and Effective Micropores at Different Conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Magnetite Loaded Multi-Wall Carbon Nanotube: Kinetic, Isotherm and Mechanism Analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-Activated Carbon Prepared from Sucrose Spherical Carbon: Adsorption Equilibrium, Kinetic and Thermodynamic Studies for Methylene Blue Removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption Isotherms and Kinetic Modeling of Methylene Blue Dye onto a Carbonaceous Hydrochar Adsorbent Derived from Coffee Husk Waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cho, H.-H.; Poster, D.L.; Ball, W.P. Evidence for a Pore-Filling Mechanism in the Adsorption of Aromatic Hydrocarbons to a Natural Wood Char. Environ. Sci. Technol. 2007, 41, 1212–1217. [Google Scholar] [CrossRef]
- Islam, A. Mesoporous Activated Coconut Shell-Derived Hydrochar Prepared via Hydrothermal Carbonization-NaOH Activation for Methylene Blue Adsorption. J. Environ. Manag. 2017, 8, 237–244. [Google Scholar] [CrossRef]
- Alshareef, S.A.; Otero, M.; Alanazi, H.S.; Siddiqui, M.R.; Khan, M.A.; Alothman, Z.A. Upcycling Olive Oil Cake through Wet Torrefaction to Produce Hydrochar for Water Decontamination. Chem. Eng. Res. Des. 2021, 170, 13–22. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, H.H.; Pham, T.H.; Nguyen, D.T.; La, D.D.; Chang, S.W.; Lee, S.M.; Chung, W.J.; Nguyen, D.D. Comparative Study on Methylene Blue Adsorption Behavior of Coffee Husk-Derived Activated Carbon Materials Prepared Using Hydrothermal and Soaking Methods. J. Environ. Chem. Eng. 2021, 9, 105362. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Wang, Y.; Sun, X.; Wang, F.; Yang, R. Soybean Root-Derived Hierarchical Porous Carbon as Electrode Material for High-Performance Supercapacitors in Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 33626–33634. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Ren, X.; Wan, J.; Du, Y.; Wu, G.; Ma, F. Biowaste-Based Porous Carbon for Supercapacitor: The Influence of Preparation Processes on Structure and Performance. J. Colloid Interface Sci. 2019, 535, 276–286. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, J.-P.; Zhao, X.-Y.; Zhuang, Q.-Q.; Zhou, Z.; Huang, Y.; Wei, X.-Y. High-Performance Electrode Material for Electric Double-Layer Capacitor Based on Hydrothermal Pre-Treatment of Lignin by ZnCl2. Appl. Surf. Sci. 2020, 508, 144536. [Google Scholar] [CrossRef]
- Gao, F.; Shao, G.; Qu, J.; Lv, S.; Li, Y.; Wu, M. Tailoring of Porous and Nitrogen-Rich Carbons Derived from Hydrochar for High-Performance Supercapacitor Electrodes. Electrochim. Acta 2015, 155, 201–208. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Jia, B.; Wei, J.; Liu, C.; Zhu, J.; Tang, S.; Wu, Z.; Chen, G. Microwave-Assisted Hydrothermal Preparation of Corn Straw Hydrochar as Supercapacitor Electrode Materials. ACS Omega 2020, 5, 26084–26093. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, Y.; Yang, Z.; Yang, F. High-performance Activated Carbons for Electrochemical Double Layer Capacitors: Effects of Morphology and Porous Structures. Int. J. Energy Res. 2020, 44, 1930–1950. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Z.; Zhao, X.; Chen, X.; Liu, Y. Green Needle Coke-Derived Porous Carbon for High-Performance Symmetric Supercapacitor. J. Power Sources 2021, 494, 229770. [Google Scholar] [CrossRef]
- Temkin, M.I. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Yang, C. Statistical Mechanical Aspects of Adsorption Systems Obeying the Temkin Isotherm. J. Phys. Chem. 1993, 97, 7097–7101. [Google Scholar] [CrossRef]
- Smith, J.M. Chemical Engineering Kinetics, 3rd Edition (McGraw-Hill Chemical Engineerin g Series); McGraw-Hill: New York, NY, USA, 1981; ISBN 978-0-07-058710-6. [Google Scholar]
- Kim, S.J.; Chung, J.H.; Kim, T.Y.; Cho, S.Y. Biosorption of Heavy Metals and Cyanide Complexes on Biomass. In Studies in Surface Science and Catalysis; Rhee, H.-K., Nam, I.-S., Park, J.M., Eds.; New Developments and Application in Chemical Reaction Engineering; Elsevier: Amsterdam, The Netherlands, 2006; Volume 159, pp. 141–144. [Google Scholar]
- Huang, C.-C.; Chen, H.-M.; Chen, C.-H.; Huang, J.-C. Effect of Surface Oxides on Hydrogen Storage of Activated Carbon. Sep. Purif. Technol. 2010, 70, 291–295. [Google Scholar] [CrossRef]
- Georgakis, M.; Stavropoulos, G.; Sakellaropoulos, G.P. Molecular Dynamics Study of Hydrogen Adsorption in Carbonaceous Microporous Materials and the Effect of Oxygen Functional Groups. Int. J. Hydrog. Energy 2007, 32, 1999–2004. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Zeldowitsch, J. Adsorption Site Energy Distribution. Acta Phys. Chim. URSS 1934, 1, 961–973. [Google Scholar]
- Haghseresht, F.; Lu, G.Q. Adsorption Characteristics of Phenolic Compounds onto Coal-Reject-Derived Adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Lee, T.B.; Kim, D.; Jung, D.H.; Choi, S.B.; Yoon, J.H.; Kim, J.; Choi, K.; Choi, S.-H. Understanding the Mechanism of Hydrogen Adsorption into Metal Organic Frameworks. Catal. Today 2007, 120, 330–335. [Google Scholar] [CrossRef]
- Martienssen, W.; Warlimont, H. Springer Handbook of Condensed Matter and Materials Data; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-30437-1. [Google Scholar]
- Yaremov, P.S.; Shcherban, N.D.; Aho, A.; Murzin, D.Y. Molecular Insight on Unusually High Specific Hydrogen Adsorption over Silicon Carbide. Int. J. Hydrog. Energy 2019, 44, 6074–6085. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic Adsorption onto Iron Oxide-Coated Cement (IOCC): Regression Analysis of Equilibrium Data with Several Isotherm Models and Their Optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Tu, W.; Liu, Y.; Xie, Z.; Chen, M.; Ma, L.; Du, G.; Zhu, M. A Novel Activation-Hydrochar via Hydrothermal Carbonization and KOH Activation of Sewage Sludge and Coconut Shell for Biomass Wastes: Preparation, Characterization and Adsorption Properties. J. Colloid Interface Sci. 2021, 593, 390–407. [Google Scholar] [CrossRef]
- Jawad, A.H.; Saud Abdulhameed, A.; Wilson, L.D.; Syed-Hassan, S.S.A.; ALOthman, Z.A.; Rizwan Khan, M. High Surface Area and Mesoporous Activated Carbon from KOH-Activated Dragon Fruit Peels for Methylene Blue Dye Adsorption: Optimization and Mechanism Study. Chin. J. Chem. Eng. 2021, 32, 281–290. [Google Scholar] [CrossRef]
- Sips, R. Combined Form of Langmuir and Freundlich Equations. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Potential of Palm Kernel Shell as Activated Carbon Precursors through Single Stage Activation Technique for Carbon Dioxide Adsorption. J. Clean. Prod. 2017, 168, 474–486. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Comparison of Optimized Isotherm Models and Error Functions for Carbon Dioxide Adsorption on Activated Carbon. J. Chem. Eng. Data 2015, 60, 3148–3158. [Google Scholar] [CrossRef]
- Khasri, A.; Bello, O.S.; Ahmad, M.A. Mesoporous Activated Carbon from Pentace Species Sawdust via Microwave-Induced KOH Activation: Optimization and Methylene Blue Adsorption. Res. Chem. Intermed. 2018, 44, 5737–5757. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Statistical Modeling of Methylene Blue Dye Adsorption by High Surface Area Mesoporous Activated Carbon from Bamboo Chip Using KOH-Assisted Thermal Activation. Energ. Ecol. Environ. 2020, 5, 456–469. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Hashim, R.; Said, N.; Akhtar, M.N.; Mohamad-Saleh, J.; Sulaiman, O. Comparison of Surface Properties of Wood Biomass Activated Carbons and Their Application against Rhodamine B and Methylene Blue Dye. Surf. Interfaces 2018, 11, 1–13. [Google Scholar] [CrossRef]
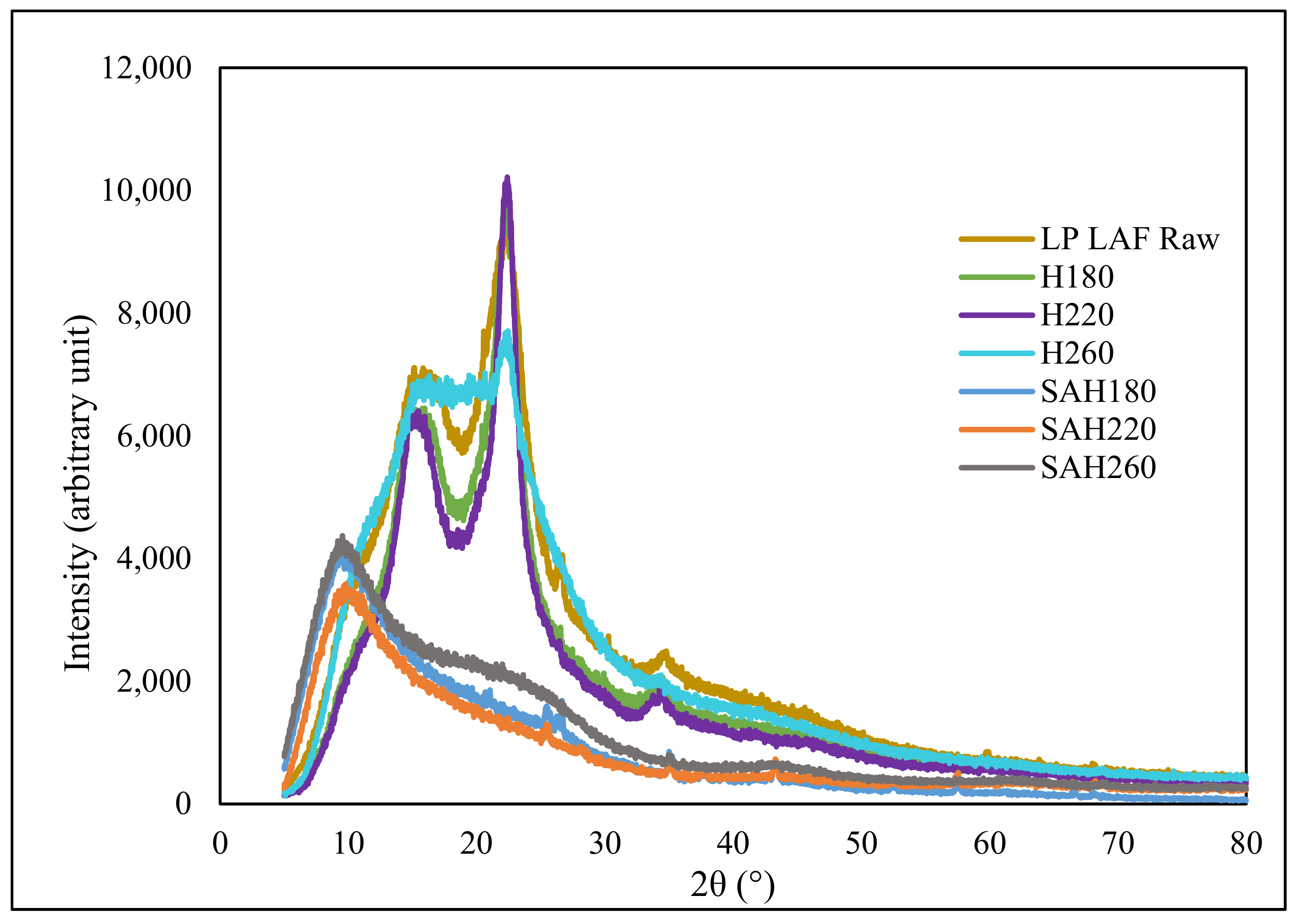
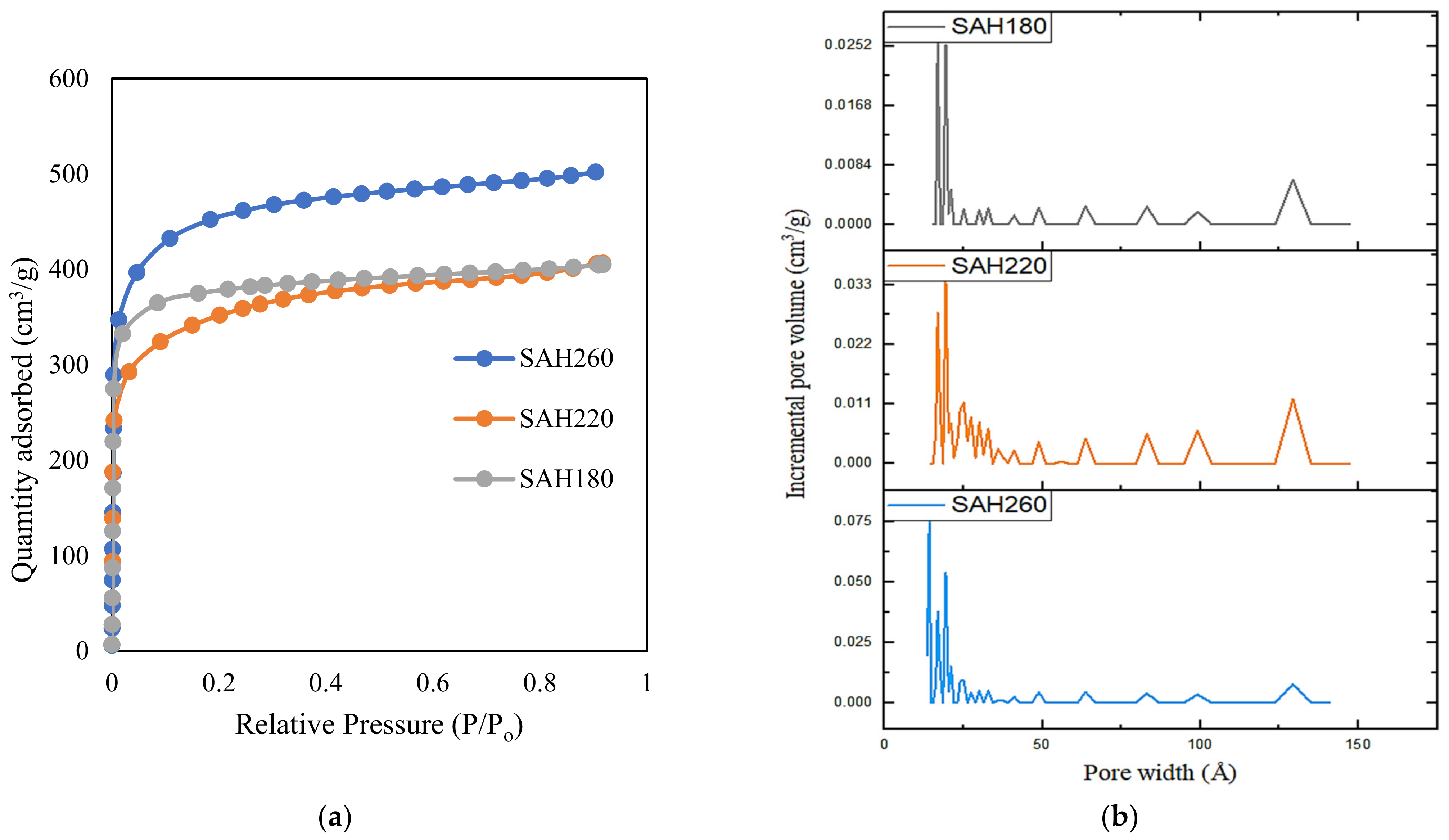



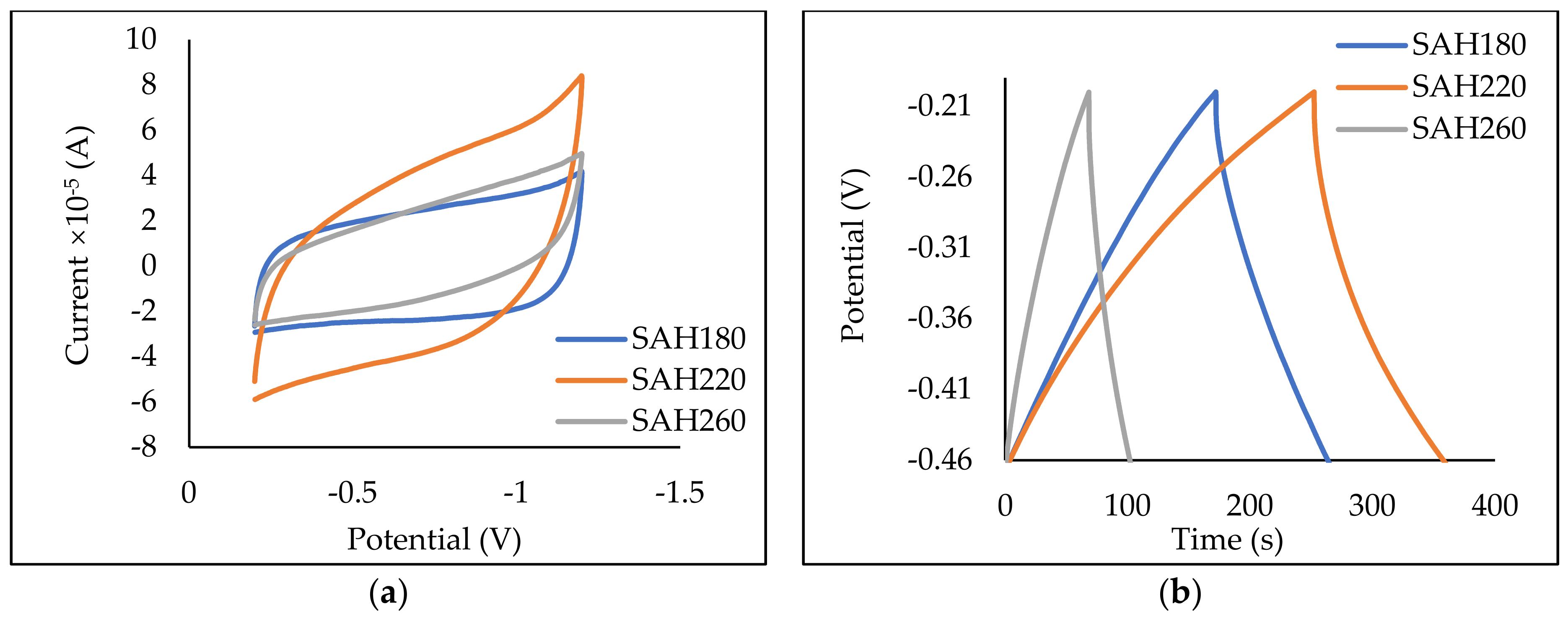
| Sample | LP | H180 | H220 | H260 | SAH180 | SAH220 | SAH260 | |
|---|---|---|---|---|---|---|---|---|
| Property | ||||||||
| BET SSA (m2/g) | 1.6 | 4.8 | 5.2 | 10.9 | 1462 | 1308 | 1703 | |
| Total Pore Volume (cm3/g) | 0.00 | 0.01 | 0.01 | 0.02 | 0.63 | 0.62 | 0.78 | |
| Micropore Area (cm2/g) | BD * | BD * | BD * | BD * | 930 | 625 | 941 | |
| Micropore Volume (m3/g) | BD * | BD * | BD * | BD * | 0.46 | 0.33 | 0.49 | |
| Mesopore + Macropore Volume * (cm3/g) | BD * | BD * | BD * | BD * | 0.17 | 0.29 | 0.29 | |
| Adsorbent | Total Oxygen Functional Groups (Acidic) (mol/g) | Density of Surface Functional Groups (mol/m2) |
|---|---|---|
| SAH180 | ||
| SAH220 | ||
| SAH260 |
| Adsorbate | Model | Temkin | Freundlich | Langmuir | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Model Parameters | R2 | Model Parameters | R2 | Model Parameters | |||||
| Adsorbent | kT | bT | nF | kF | Qmax | kL | ||||
| H2 | H180 | 0.99681 | 42.8 ± 4.67 | 0.113 ± 0.002 | 0.95993 | 4.950 ± 0.0170 | 21.3 ± 0.841 | 0.93979 | 37.1 ± 1.1 | 1.40 ± 0.30 |
| H220 | 0.95838 | 3.91 ± 0.78 | 0.782 ± 0.005 | 0.99873 | 2.618 ± 0.006 | 12.1 ± 0.210 | 0.98904 | 43.4 ± 2.7 | 0.25 ± 0.05 | |
| H260 | 0.96633 | 7.48 ± 2.39 | 0.067 ± 0.006 | 0.99813 | 2.994 ± 0.008 | 18.7 ± 0.353 | 0.93140 | 54.7 ± 4.4 | 0.35 ± 0.10 | |
| CO2 | H180 | 0.96703 | 17.8 ± 3.12 | 0.015 ± 0.001 | 0.99839 | 1.506 ± 0.016 | 137 ± 1.48 | 0.99858 | 255.4 ± 11.6 | 1.08 ± 0.08 |
| H220 | 0.95838 | 16.6 ± 3.13 | 0.020 ± 0.002 | 0.99896 | 1.420 ± 0.014 | 100 ± 0.894 | 0.99871 | 212.0 ± 11.1 | 0.86 ± 0.07 | |
| H260 | 0.96118 | 17.6 ± 3.21 | 0.014 ± 0.001 | 0.99149 | 1.529 ± 0.035 | 138 ± 3.26 | 0.99943 | 269.2 ± 7.1 | 1.03 ± 0.05 | |
| H180 | N/A | N/A | N/A | 0.01300 | N/A | N/A | 0.99800 | 588.3 | 1.42 | |
| MB | H220 | N/A | N/A | N/A | 0.67700 | N/A | N/A | 0.99800 | 666.7 | 0.52 |
| H260 | N/A | N/A | N/A | 0.60300 | N/A | N/A | 0.99900 | 714.3 | 0.82 | |
| H180 | N/A | N/A | N/A | 0.97321 | 2.319 ± 0.334 | 69.2 ± 7.33 | 0.99272 | 54.1 ± 2.9 | 9.50 ± 1.37 | |
| Electron | H220 | N/A | N/A | N/A | 0.99351 | 2.484 ± 0.160 | 75.2 ± 3.46 | 0.98912 | 57.6 ± 3.0 | 11.79 ± 1.79 |
| H260 | N/A | N/A | N/A | 0.98870 | 2.052 ± 0.170 | 25.5 ± 1.65 | 0.99887 | 21.5 ± 0.5 | 6.38 ± 0.36 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, A.I.; Chambers, C.; Ahmed, M.M.N.; Pathirathna, P.; Reza, T. Multifunctional Loblolly Pine-Derived Superactivated Hydrochar: Effect of Hydrothermal Carbonization on Hydrogen and Electron Storage with Carbon Dioxide and Dye Removal. Nanomaterials 2022, 12, 3575. https://doi.org/10.3390/nano12203575
Sultana AI, Chambers C, Ahmed MMN, Pathirathna P, Reza T. Multifunctional Loblolly Pine-Derived Superactivated Hydrochar: Effect of Hydrothermal Carbonization on Hydrogen and Electron Storage with Carbon Dioxide and Dye Removal. Nanomaterials. 2022; 12(20):3575. https://doi.org/10.3390/nano12203575
Chicago/Turabian StyleSultana, Al Ibtida, Cadianne Chambers, Muzammil M. N. Ahmed, Pavithra Pathirathna, and Toufiq Reza. 2022. "Multifunctional Loblolly Pine-Derived Superactivated Hydrochar: Effect of Hydrothermal Carbonization on Hydrogen and Electron Storage with Carbon Dioxide and Dye Removal" Nanomaterials 12, no. 20: 3575. https://doi.org/10.3390/nano12203575
APA StyleSultana, A. I., Chambers, C., Ahmed, M. M. N., Pathirathna, P., & Reza, T. (2022). Multifunctional Loblolly Pine-Derived Superactivated Hydrochar: Effect of Hydrothermal Carbonization on Hydrogen and Electron Storage with Carbon Dioxide and Dye Removal. Nanomaterials, 12(20), 3575. https://doi.org/10.3390/nano12203575