Curcumin Nanodiscs Improve Solubility and Serve as Radiological Protectants against Ionizing Radiation Exposures in a Cell-Cycle Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
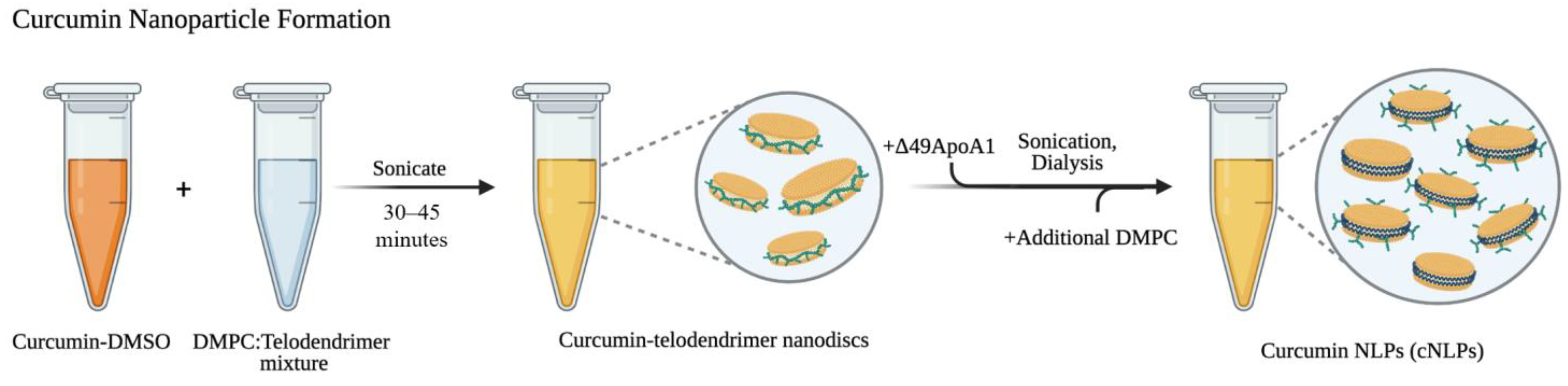
2.1. Formulating Curcumin-Telodiscs (Cur-Telodiscs)
2.2. Formulating Curcumin Nanolipoprotein Particles (cNLPs)
2.3. UV-Vis Spectroscopy
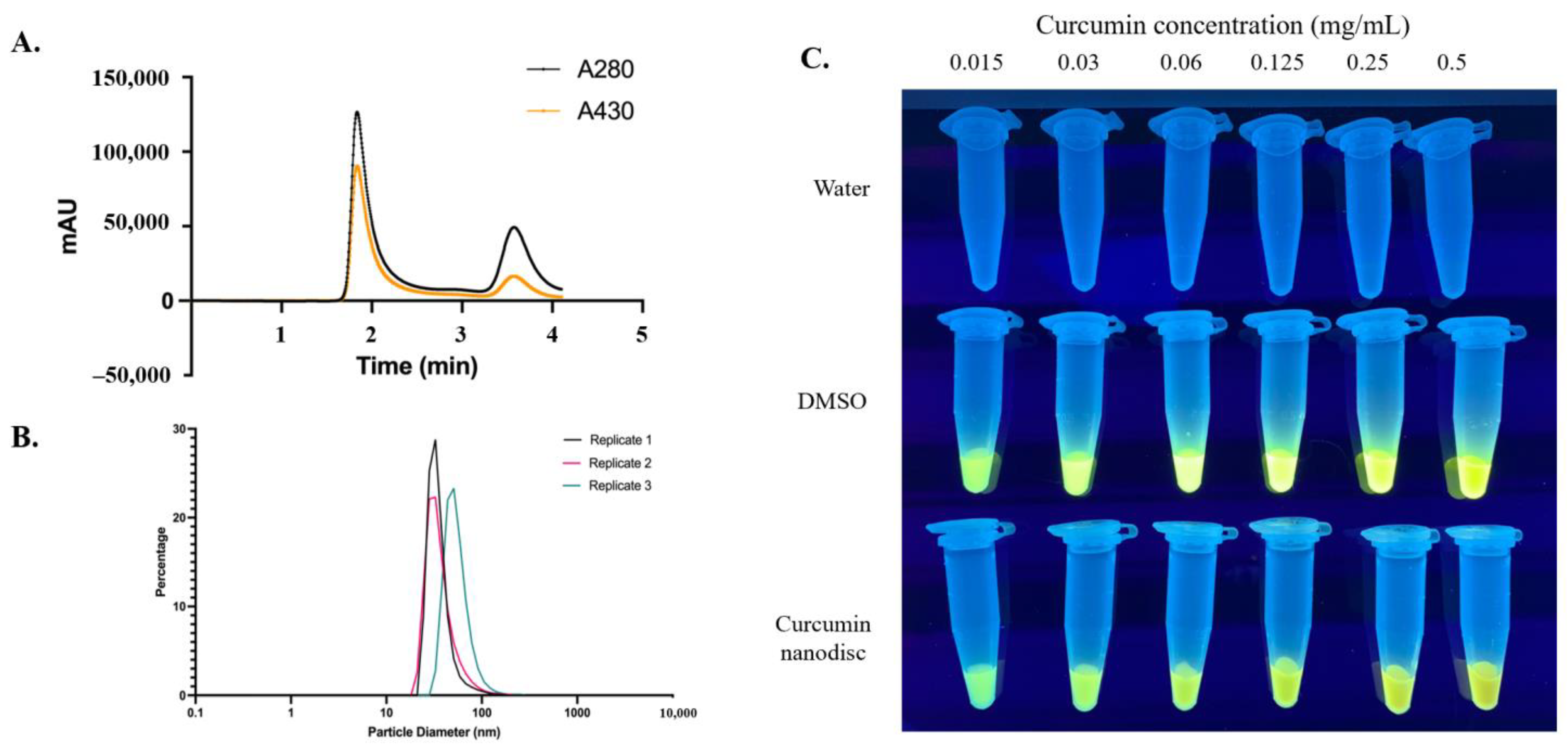
2.4. Size Exclusion Chromatography (SEC), Dynamic Light Scattering (DLS), and UV Transluminescence
2.5. Cell Culture
2.6. Mitotic Index Assay
2.7. Cell Proliferation (MTS) Assay
2.8. DNA Damage Immunohistochemistry
2.9. Survival Curves
2.10. Statistical Analysis
3. Results
3.1. Nanolipoprotein Particles Support Curcumin Loading
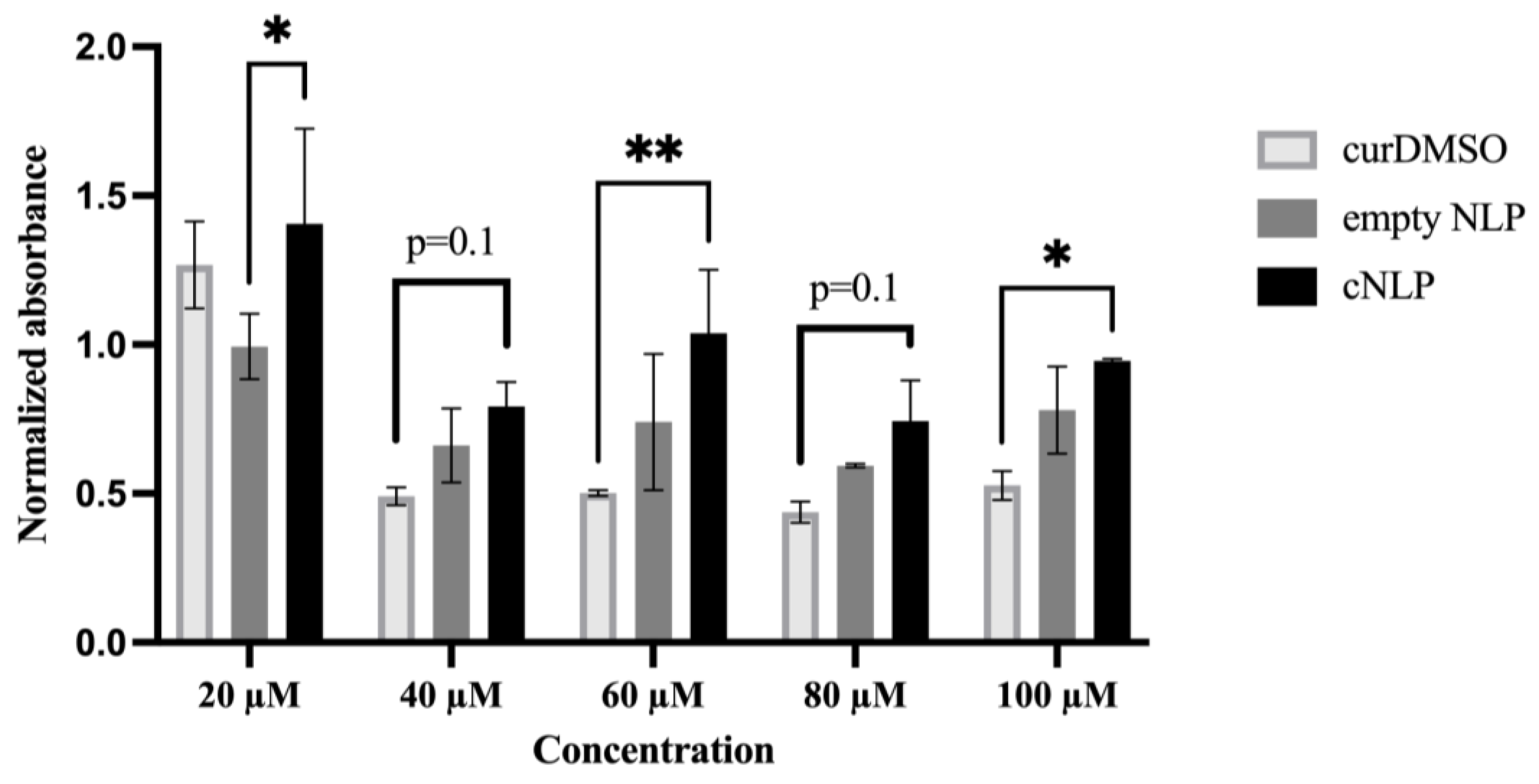
3.2. Normal Human Fibroblasts Tolerate cNLPs Better than DMSO-Solubilized Curcumin
3.3. cNLP Pre-Treatment Alters Foci Persistence Following Gamma Irradiation
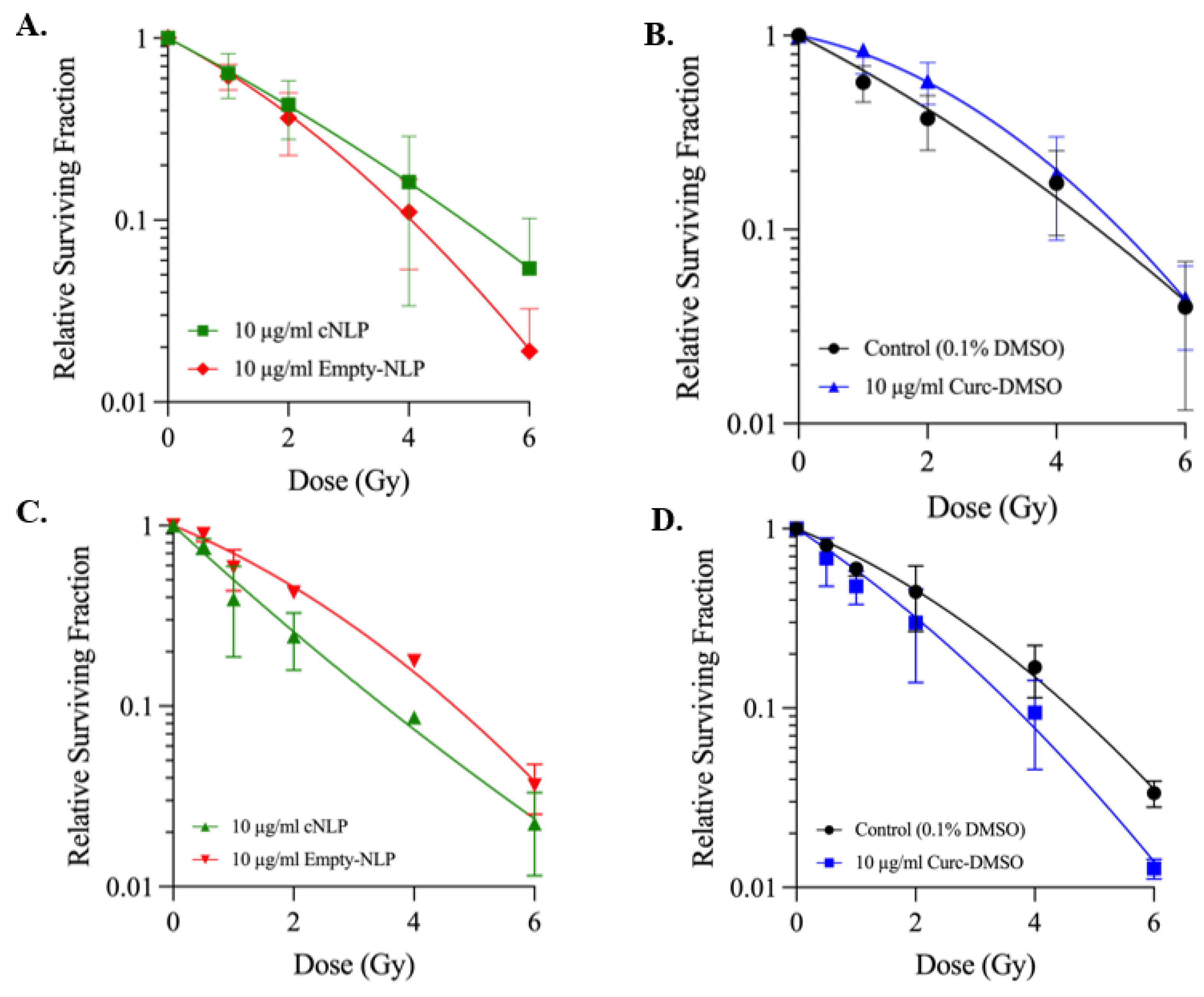
3.4. cNLP Effects Following 137Cs Irradiation Cell Survival Are Cell-Cycle Dependent
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, H.; Karthikeyan, K. Turmeric: A condiment, cosmetic and cure. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H.B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.; Raju, Y.S.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D 2008, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Kalpravidh, R.W.; Siritanaratkul, N.; Insain, P.; Charoensakdi, R.; Panichkul, N.; Hatairaktham, S. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin. Biochem. 2010, 43, 424–429. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin MTt Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastia, N.; Montoro, A.; Hervas, D.; Pantelias, G.; Hatzi, V.I.; Soriano, J.M. Curcumin and trans-resveratrol exert cell cycle-dependent radioprotective or radiosensitizing effects as elucidated by the PCC and G2-assay. Mutat. Res. 2014, 766–767, 49–55. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007. [Google Scholar]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Srinivasan, M.; Rajendra Prasad, N.; Menon, V.P. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat. Res. 2006, 611, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Rajanikant, G.K. Acceleration of wound repair by curcumin in the excision wound of mice exposed to different doses of fractionated gamma radiation. Int. Wound. J. 2012, 9, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.K.; Sarma, L.; Kesavan, P.C. Protective effects of chlorogenic acid, curcumin and beta-carotene against gamma-radiation-induced in vivo chromosomal damage. Mutat. Res. 1993, 303, 109–112. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Kumar, A. Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res. 2012, 1488, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta. Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (nanocurcumin): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, N.O.; Weilhammer, D.R.; Dunkle, A.; Thomas, C.; Hwang, M.; Corzett, M. Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform. PLoS ONE 2014, 9, e93342. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Evans, A.C.; Rasley, A.; Bourguet, F.; Peters, S.; Kamrud, K.I. Cationic HDL mimetics enhance in vivo delivery of self-replicating, mRNA. Nanomedicine 2020, 24, 102154. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Felderman, M.; Evans, A.C.; Geng, J.; Homan, D.; Bourguet, F. Cell-free production of a functional oligomeric form of a Chlamydia major outer-membrane protein (MOMP) for vaccine development. J. Biol. Chem. 2017, 292, 15121–15132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Scharadin, T.M.; Saldana, M.; Gellner, C.; Hoang-Phou, S.; Takanishi, C. Cell-free expression of functional receptor tyrosine kinases. Sci. Rep. 2015, 5, 12896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, M.; Ryan, R.O. ApoE enhances nanodisk-mediated curcumin delivery to glioblastoma multiforme cells. Nanomedicine (Lond) 2014, 9, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, M.; Singh, A.T.; Xu, W.; Sulchek, T.; Gordon, L.I.; Ryan, R.O. Curcumin nanodisks: Formulation and characterization. Nanomedicine 2011, 7, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamoorthy, A.; Tavoosi, N.; Chan, G.K.L.; Liu, J.; Ren, G.; Cavigiolio, G. Effect of curcumin on amyloid-like aggregates generated from methionine-oxidized apolipoprotein A-I. FEBS Open Bio 2018, 8, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.F.; Nham, P.B.; Urbin, S.S.; Hinz, J.M.; Jones, I.M.; Thompson, L.H. Inter-individual variation in DNA double-strand break repair in human fibroblasts before and after exposure to low doses of ionizing radiation. Mutat. Res. 2010, 683, 91–97. [Google Scholar] [CrossRef]
- He, W.; Luo, J.; Bourguet, F.; Xing, L.; Yi, S.K.; Gao, T. Controlling the diameter, monodispersity, and solubility of ApoA1 nanolipoprotein particles using telodendrimer chemistry. Protein. Sci. 2013, 22, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudevan, S.; Prabhune, A.A. Photophysical studies on curcumin-sophorolipid nanostructures: Applications in quorum quenching and imaging. R. Soc. Open Sci. 2018, 5, 170865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortezaee, K.; Salehi, E.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Aggarwal, B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, S.; Zhang, Q.; Wu, J.; Tang, Q.; Zhou, J. Combination of curcumin bicalutamide enhanced the growth inhibition, o.f.; rogen-independent prostate cancer cells through, SAPK/JNK.; MEK/ERK1/2-mediated targeting, NF-kappaB/p65.; MUC1-C. J. Exp. Clin. Cancer Res. 2015, 34, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell Physiol. 2018, 233, 4634–4642. [Google Scholar] [CrossRef]
- Rana, C.; Piplani, H.; Vaish, V.; Nehru, B.; Sanyal, S.N. Downregulation of PI3-K/Akt/PTEN pathway and activation of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in colon cancer. Mol. Cell Biochem. 2015, 402, 225–241. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch. Biochem. Biophys. 2020, 689, 108412. [Google Scholar] [CrossRef]
- Qiang, Z.; Meng, L.; Yi, C.; Yu, L.; Chen, W.; Sha, W. Curcumin regulates the miR-21/PTEN/Akt pathway and acts in synergy with PD98059 to induce apoptosis of human gastric cancer MGC-803 cells. J. Int. Med. Res. 2019, 47, 1288–1297. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hang, Y.; Liu, J.; Hou, Y.; Wang, N.; Wang, M. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol. Lett. 2017, 13, 4825–4831. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiao, H.; Wu, D.; Lu, H.; Liu, L.; Sang, X. Curcumin potentiates the galbanic acid-induced anti-tumor effect in non-small cell lung cancer cells through inhibiting Akt/mTOR signaling pathway. Life Sci. 2019, 239, 117044. [Google Scholar] [CrossRef]
- Mosieniak, G.; Adamowicz, M.; Alster, O.; Jaskowiak, H.; Szczepankiewicz, A.A.; Wilczynski, G.M. Curcumin induces permanent growth arrest of human colon cancer cells: Link between senescence and autophagy. Mech. Ageing Dev. 2012, 133, 444–455. [Google Scholar] [CrossRef]
- Deng, L.; Wu, X.; Zhu, X.; Yu, Z.; Liu, Z.; Wang, J. Combination effect of curcumin with docetaxel on the PI3K/AKT/mTOR pathway to induce autophagy and apoptosis in esophageal squamous cell carcinoma. Am. J. Transl. Res. 2021, 13, 57–72. [Google Scholar] [PubMed]
- Lv, X.A.; Wang, B.; Xu, X.H.; Pan, L.; Wang, B.; Dong, X.X.; Zheng, C.H.; Du, Q.W. Curcumin re-sensitizes multidrug resistant (MDR) breast cancer to cisplatin through inducing autophagy by decreasing CCAT1 expression. RSC Adv. 2017, 7, 33572–33579. [Google Scholar]
- Deng, X.Z.; Geng, S.S.; Luo, M.; Chai, J.J.; Xu, Y.; Chen, C.L. Curcumin potentiates laryngeal squamous carcinoma radiosensitivity via NF-KappaB inhibition by suppressing IKKgamma expression. J. Recept. Signal. Transduct. Res. 2020, 40, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Xu, J.; Hu, D.; Liu, W.; Zhang, L.; Morrow, G. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 890–898. [Google Scholar] [CrossRef]
- Sordillo, P.P.; Helson, L. Curcumin and cancer stem cells: Curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer. Res. 2015, 35, 599–614. [Google Scholar]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [Green Version]
- Dolati, S.; Aghebati-Maleki, L.; Ahmadi, M.; Marofi, F.; Babaloo, Z.; Ayramloo, H. Nanocurcumin restores aberrant miRNA expression profile in multiple sclerosis, randomized, double-blind, placebo-controlled trial. J. Cell Physiol. 2018, 233, 5222–5230. [Google Scholar] [CrossRef]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, L.H. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutat. Res. 2012, 751, 158–246. [Google Scholar] [CrossRef] [PubMed]
- Redon, C.E.; Dickey, J.S.; Bonner, W.M.; Sedelnikova, O.A. Gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv. Space. Res. 2009, 43, 1171–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FitzGerald, J.E.; Grenon, M.; Lowndes, N.F. 53BP1: Function and mechanisms of focal recruitment. Biochem. Soc. Trans. 2009, 37 Pt 4, 897–904. [Google Scholar] [CrossRef]
- Ogiwara, H.; Ui, A.; Shiotani, B.; Zou, L.; Yasui, A.; Kohno, T. Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis 2013, 34, 2486–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Yang, B.; Najafi, M. Targeting of cancer cell death mechanisms by curcumin; implications to cancer therapy. Basic. Clin. Pharmacol. Toxicol. 2021, 129, 397–415. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Villegas-Sepulveda, N.; Meraz-Rios, M.A.; Hernandez-Zavala, A.; Berumen, J.; Coleman, M.A. Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells. Oncol. Lett. 2018, 15, 6777–6783. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, A.C.; Martin, K.A.; Saxena, M.; Bicher, S.; Wheeler, E.; Cordova, E.J.; Porada, C.D.; Almeida-Porada, G.; Kato, T.A.; Wilson, P.F.; et al. Curcumin Nanodiscs Improve Solubility and Serve as Radiological Protectants against Ionizing Radiation Exposures in a Cell-Cycle Dependent Manner. Nanomaterials 2022, 12, 3619. https://doi.org/10.3390/nano12203619
Evans AC, Martin KA, Saxena M, Bicher S, Wheeler E, Cordova EJ, Porada CD, Almeida-Porada G, Kato TA, Wilson PF, et al. Curcumin Nanodiscs Improve Solubility and Serve as Radiological Protectants against Ionizing Radiation Exposures in a Cell-Cycle Dependent Manner. Nanomaterials. 2022; 12(20):3619. https://doi.org/10.3390/nano12203619
Chicago/Turabian StyleEvans, Angela C., Kelly A. Martin, Manoj Saxena, Sandra Bicher, Elizabeth Wheeler, Emilio J. Cordova, Christopher D. Porada, Graça Almeida-Porada, Takamitsu A. Kato, Paul F. Wilson, and et al. 2022. "Curcumin Nanodiscs Improve Solubility and Serve as Radiological Protectants against Ionizing Radiation Exposures in a Cell-Cycle Dependent Manner" Nanomaterials 12, no. 20: 3619. https://doi.org/10.3390/nano12203619
APA StyleEvans, A. C., Martin, K. A., Saxena, M., Bicher, S., Wheeler, E., Cordova, E. J., Porada, C. D., Almeida-Porada, G., Kato, T. A., Wilson, P. F., & Coleman, M. A. (2022). Curcumin Nanodiscs Improve Solubility and Serve as Radiological Protectants against Ionizing Radiation Exposures in a Cell-Cycle Dependent Manner. Nanomaterials, 12(20), 3619. https://doi.org/10.3390/nano12203619






