Biopolymeric Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
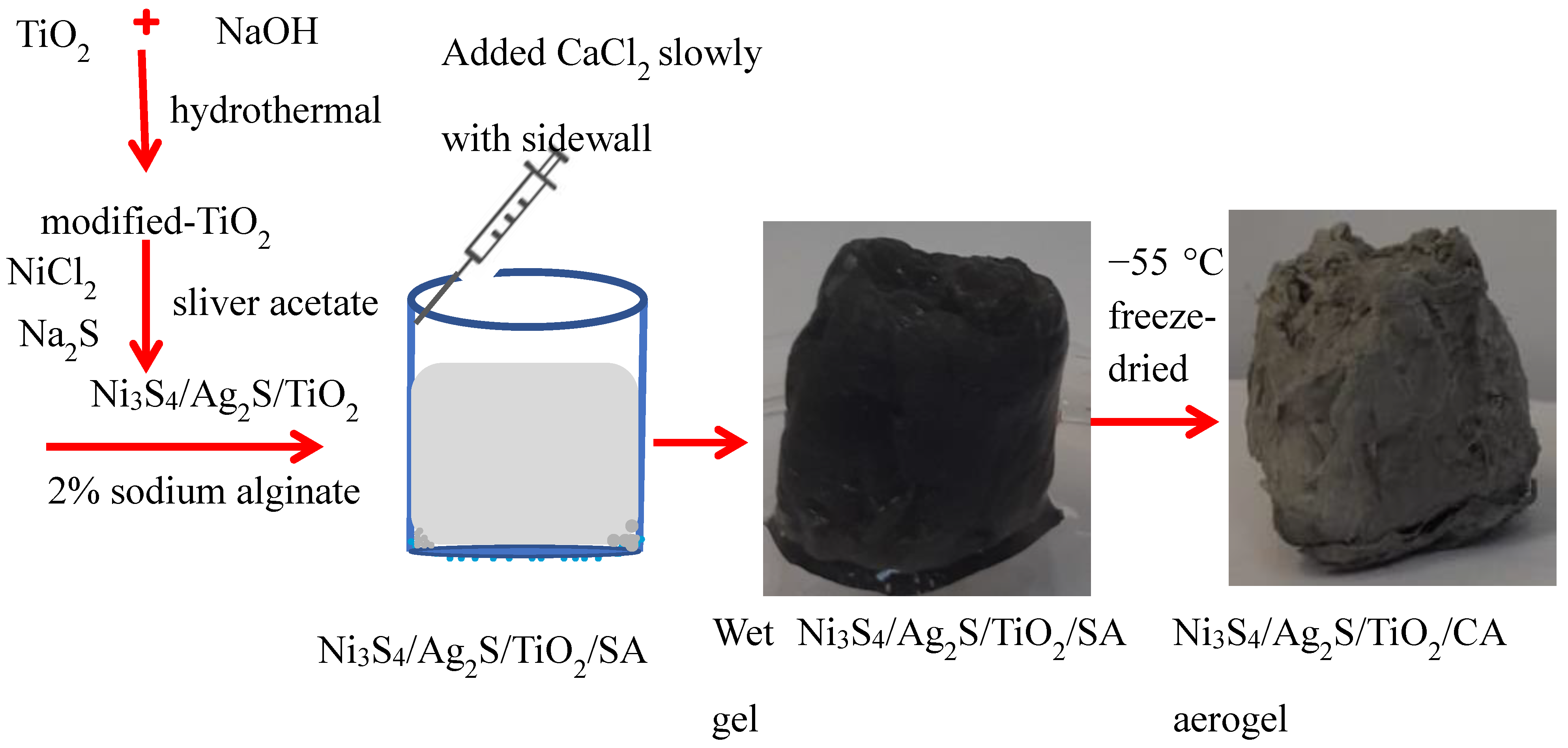
2.2. Synthesis
2.2.1. Modification of TiO2
2.2.2. Synthesis of Ni3S4/Ag2S/TiO2 Nanocomposite
2.2.3. Synthesis of Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel
2.3. Adsorption Studies
2.4. Antimicrobial Studies
2.4.1. MIC/MBC Values of Aerogels
2.4.2. Bacterial Zone Inhibition Analysis
2.5. Characterization
3. Results and Discussion
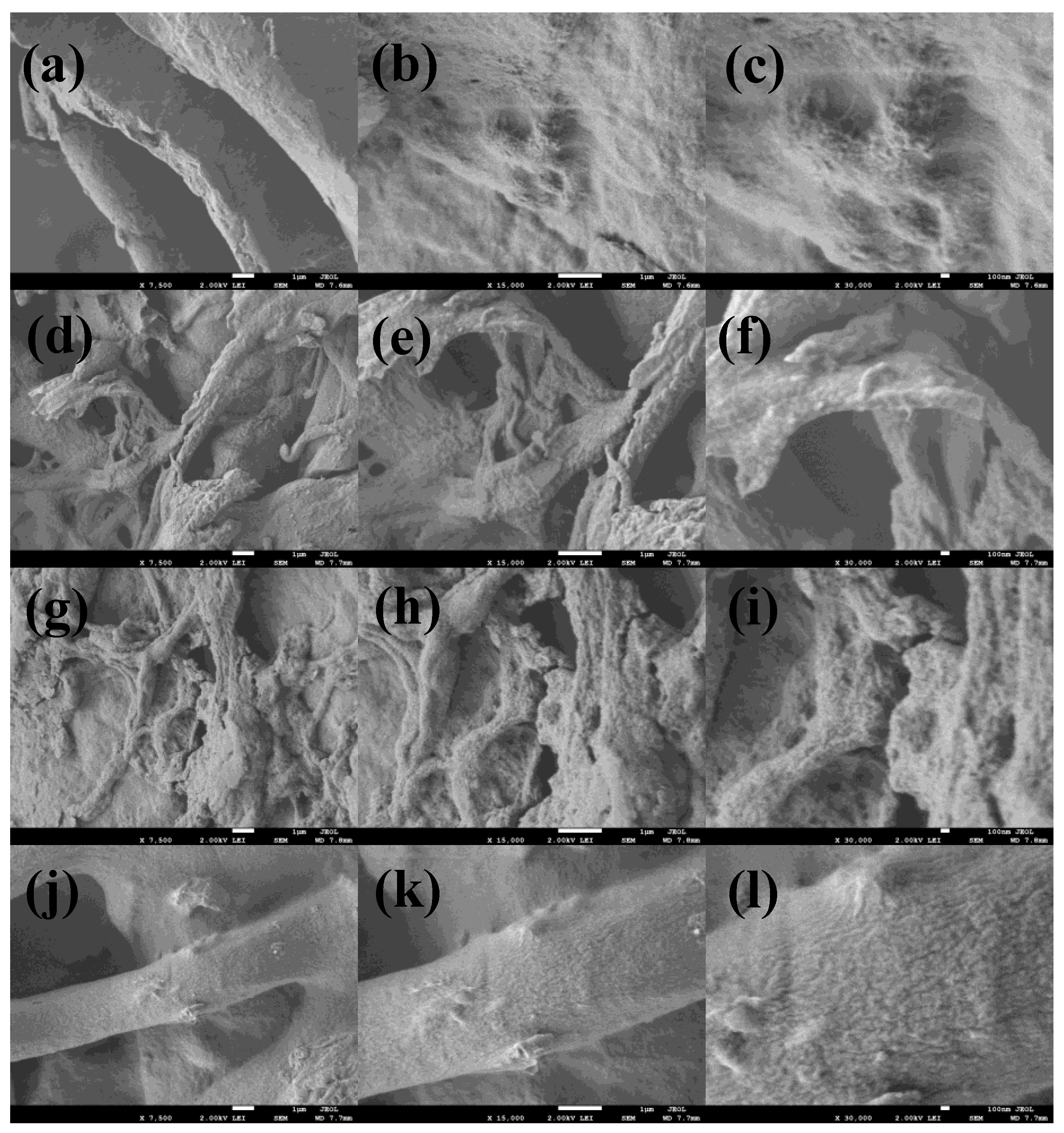
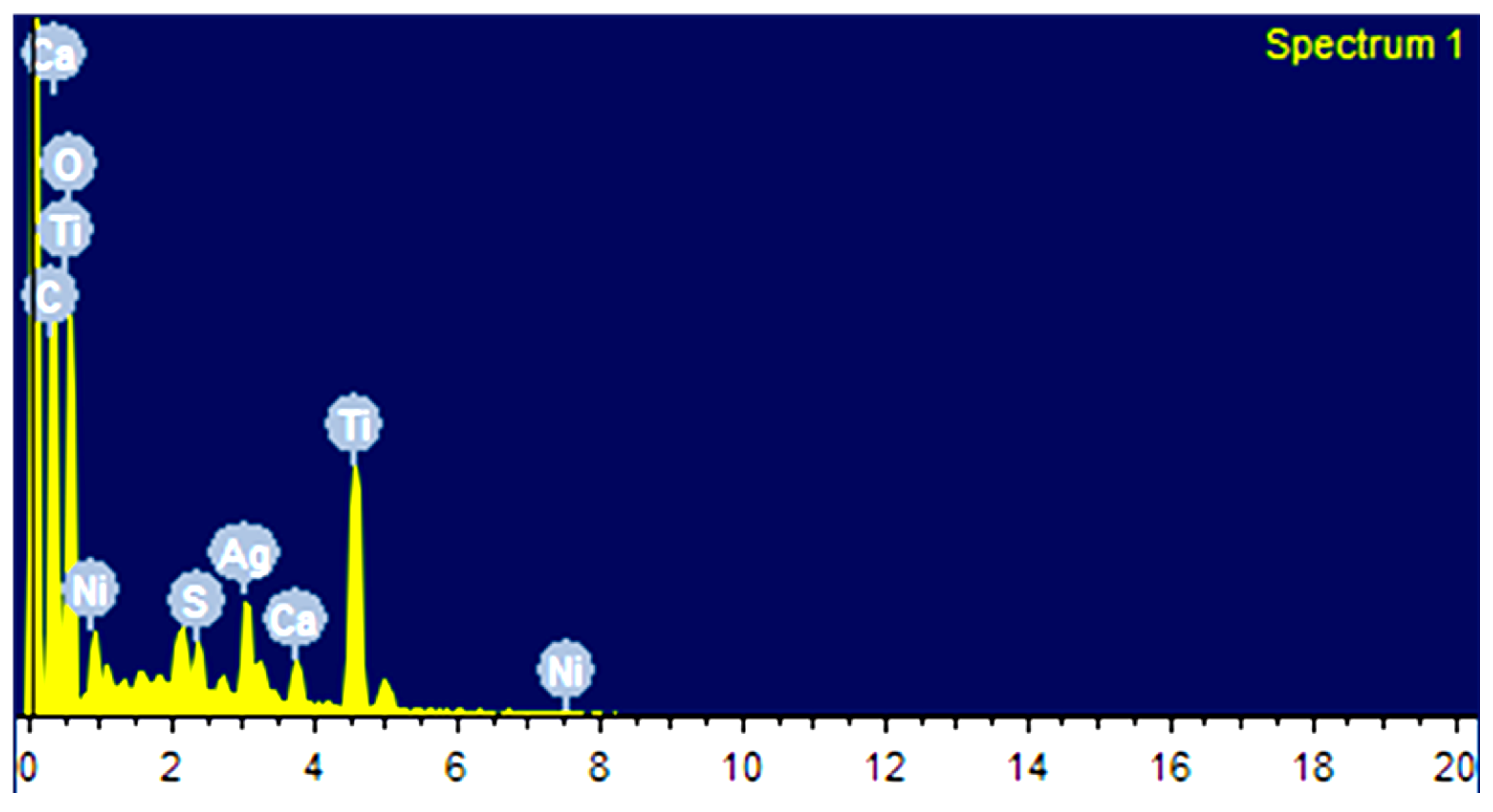
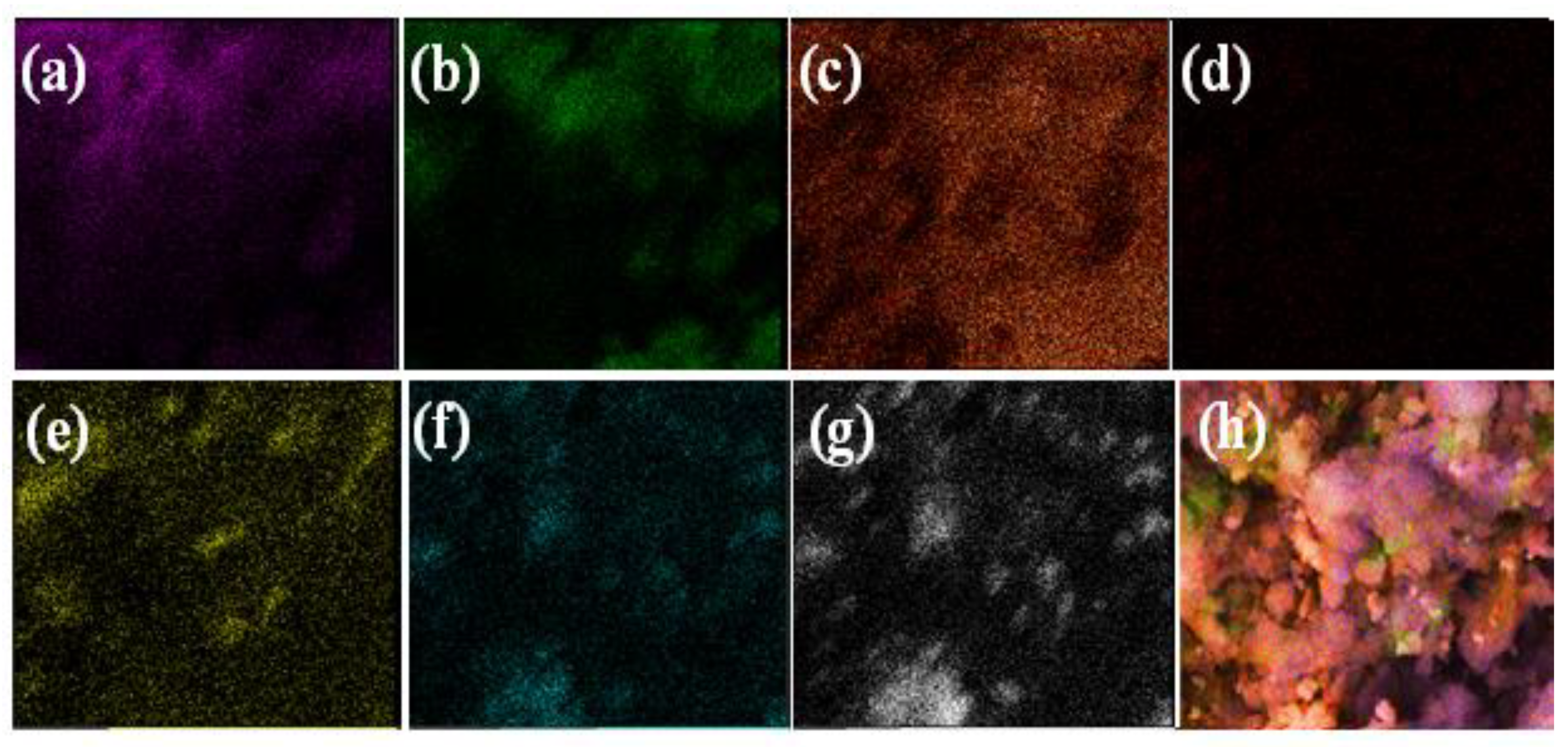
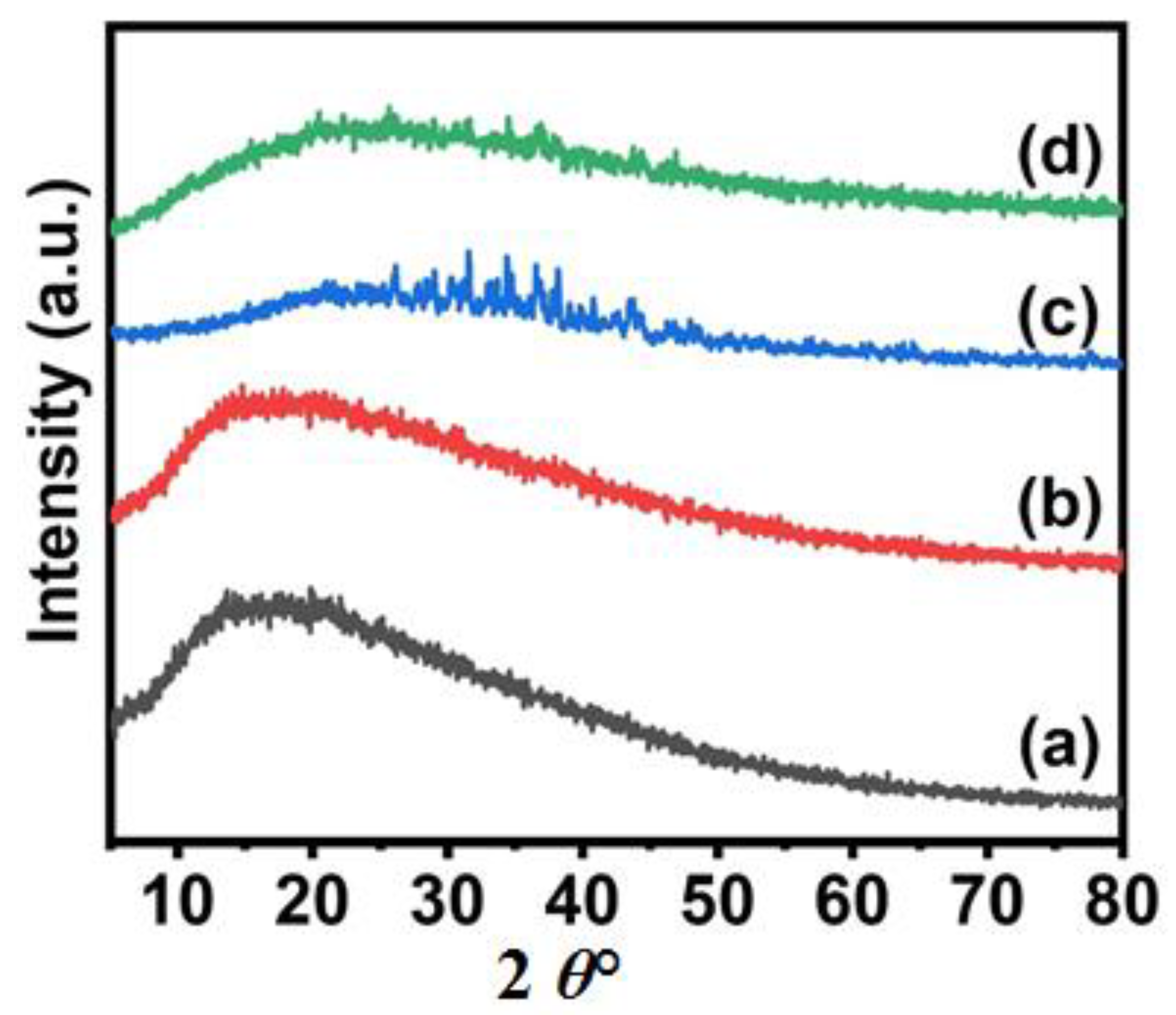
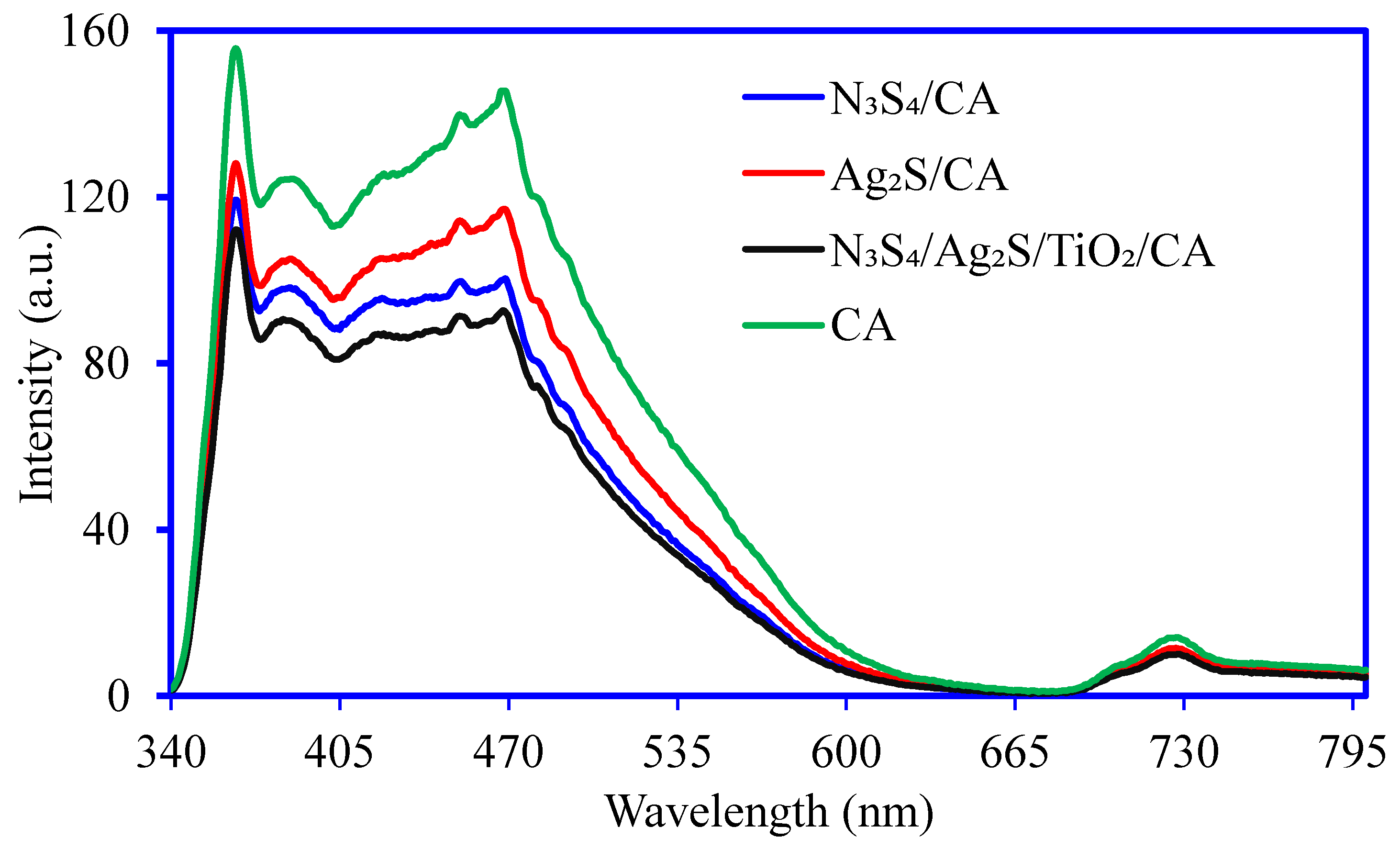
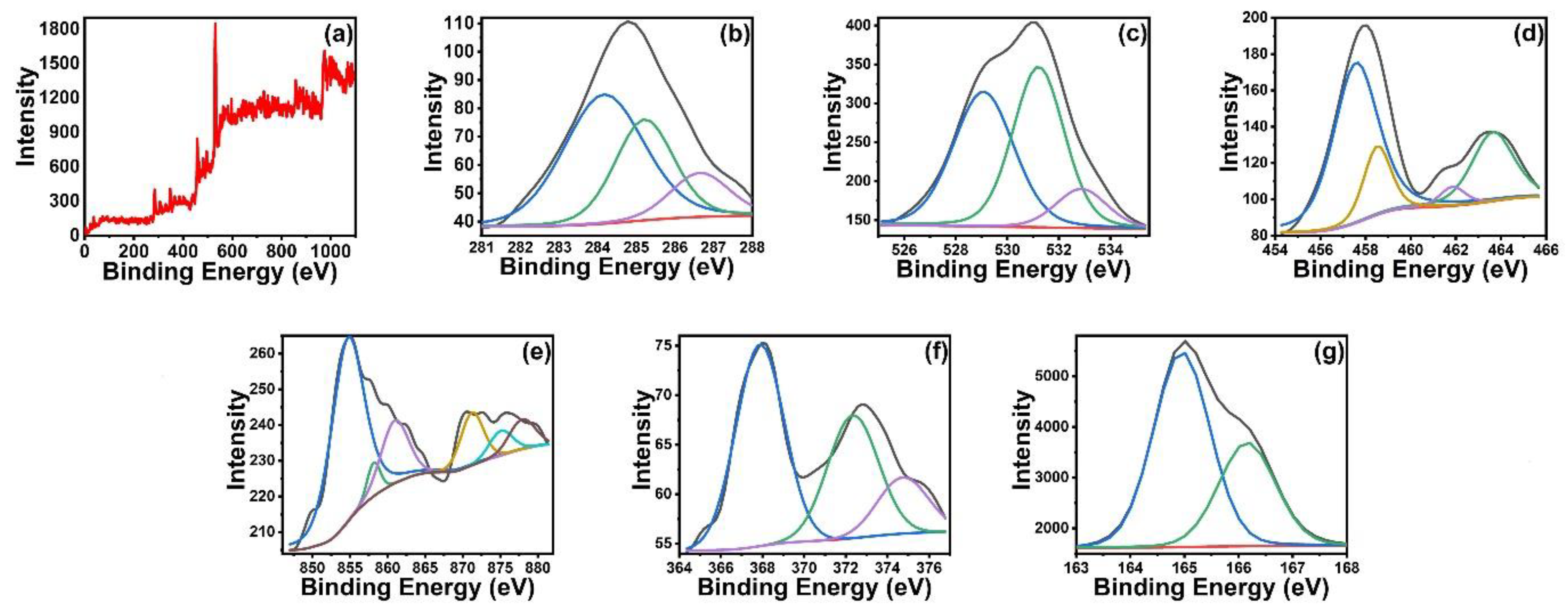
3.1. Synthesis and Characterization
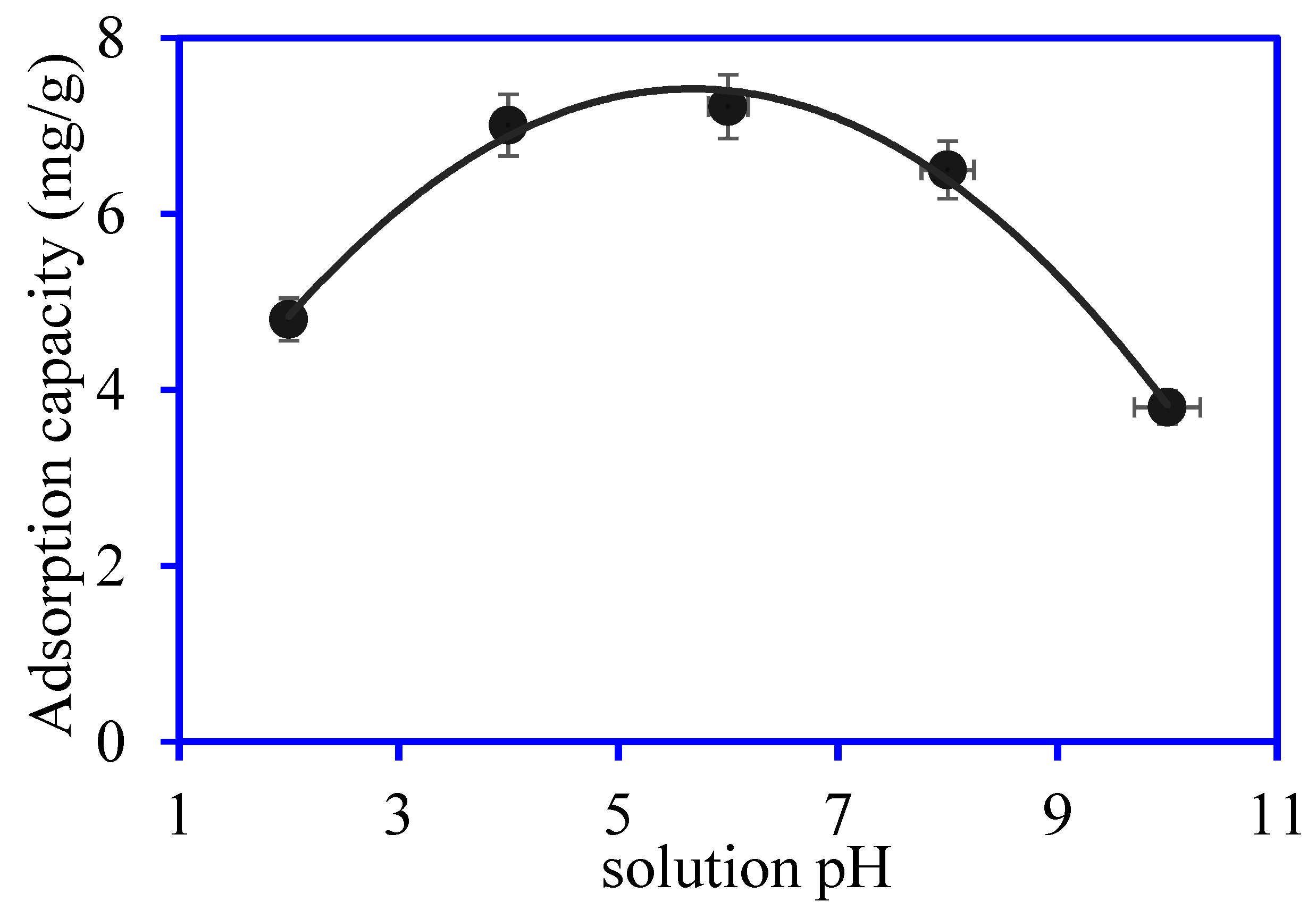
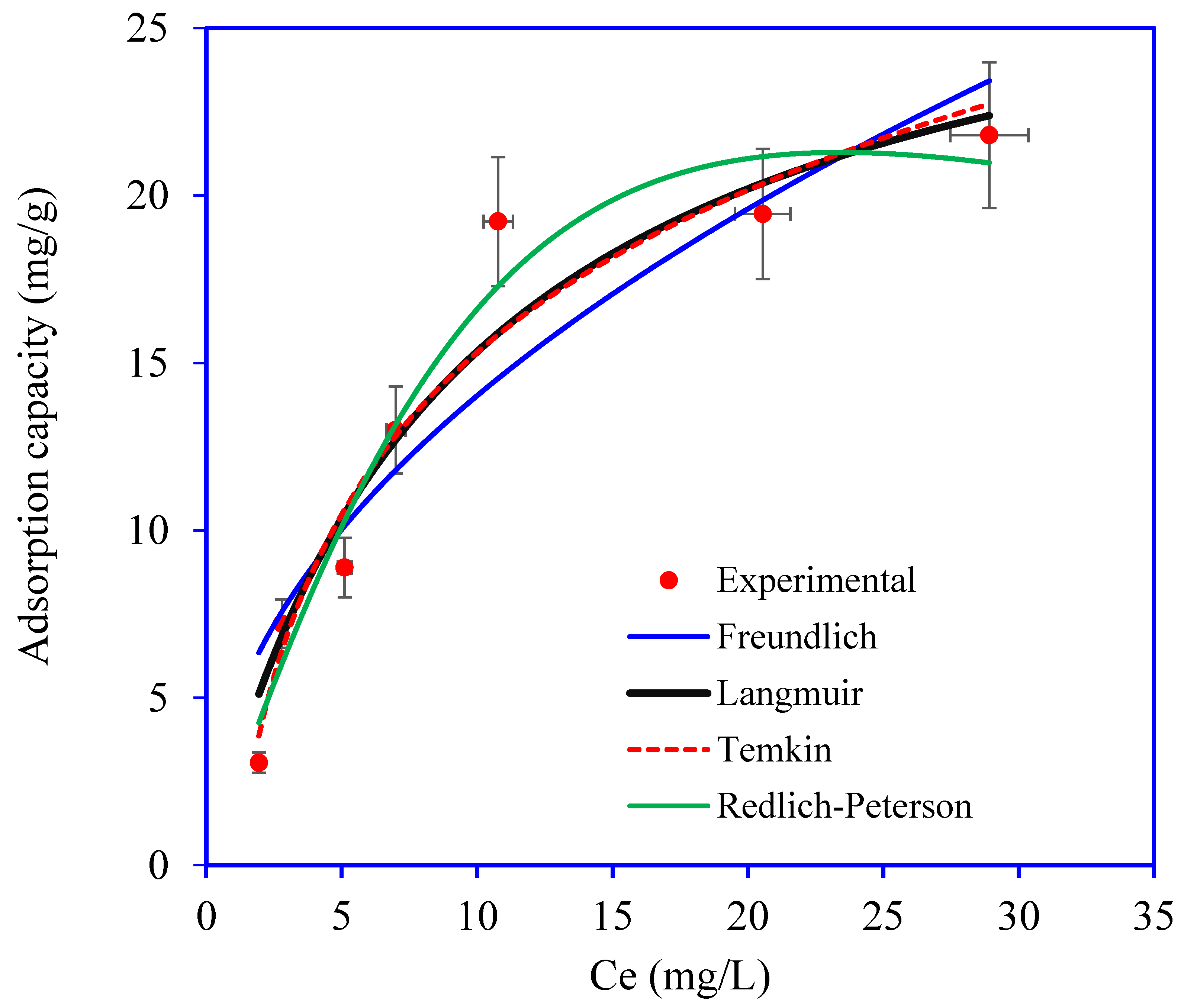
3.2. OTC Removal Studies
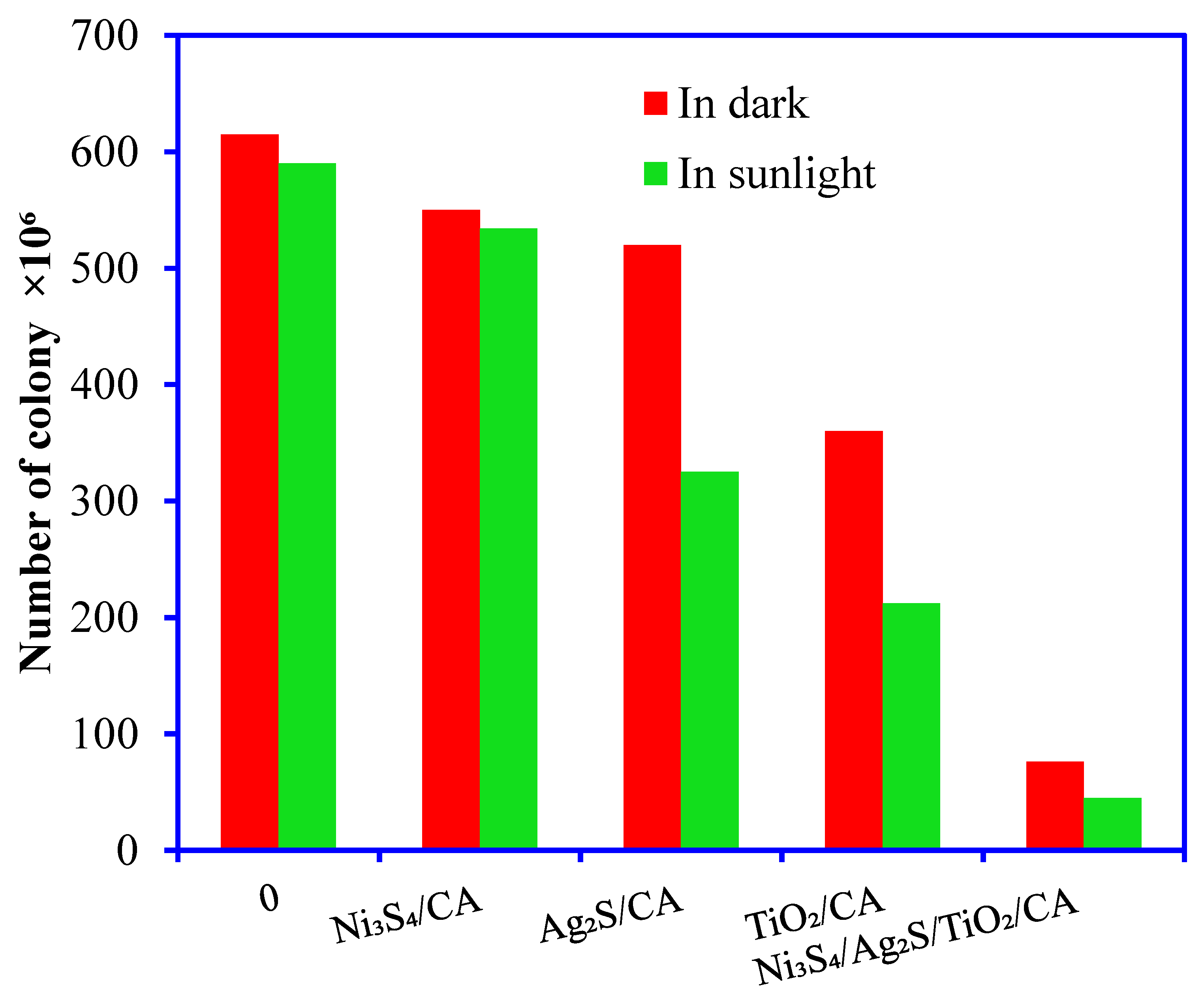
3.3. Antimicrobial Studies
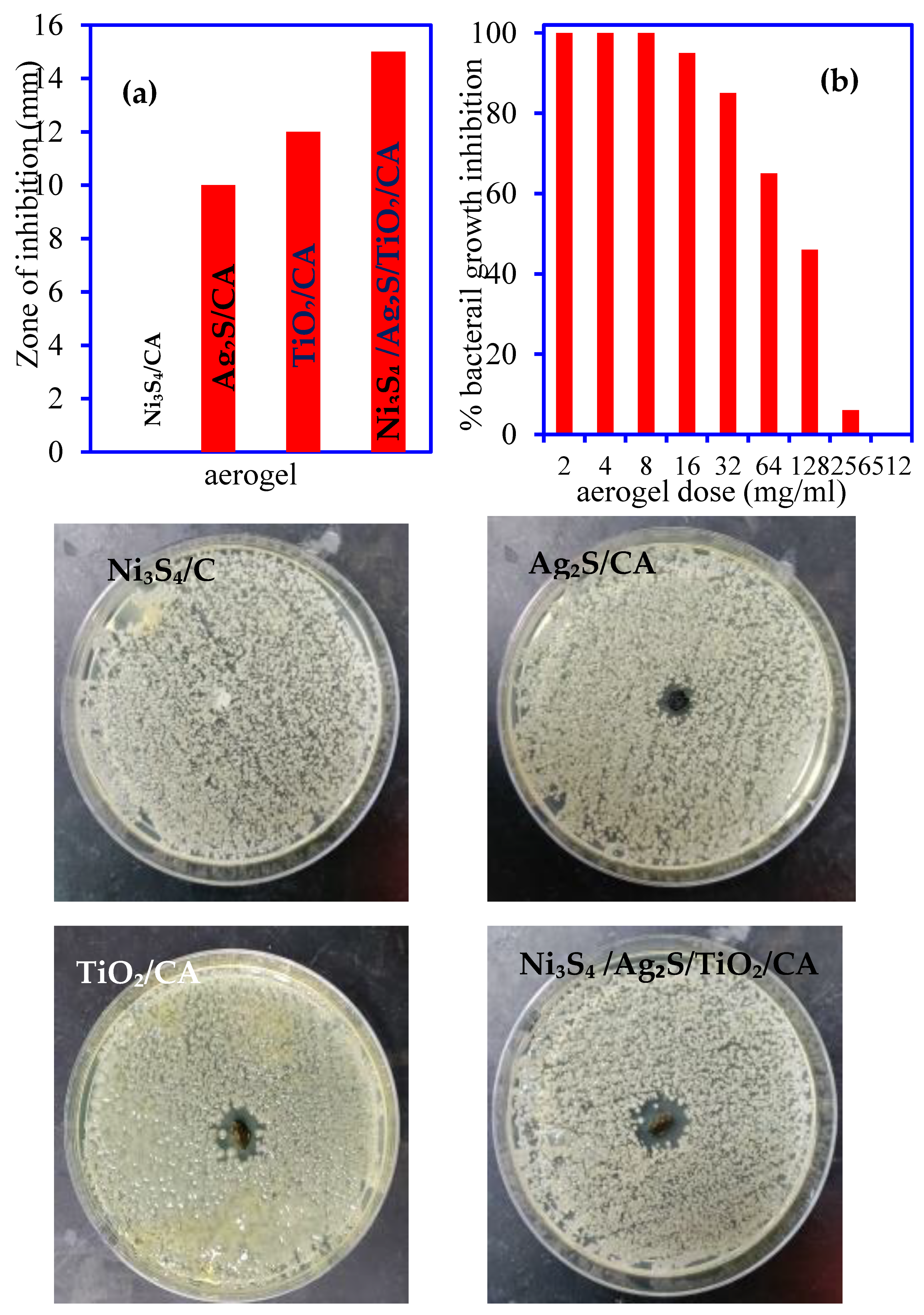
3.3.1. Bacterial Zone Inhibition
3.3.2. MIC/MBC Analysis
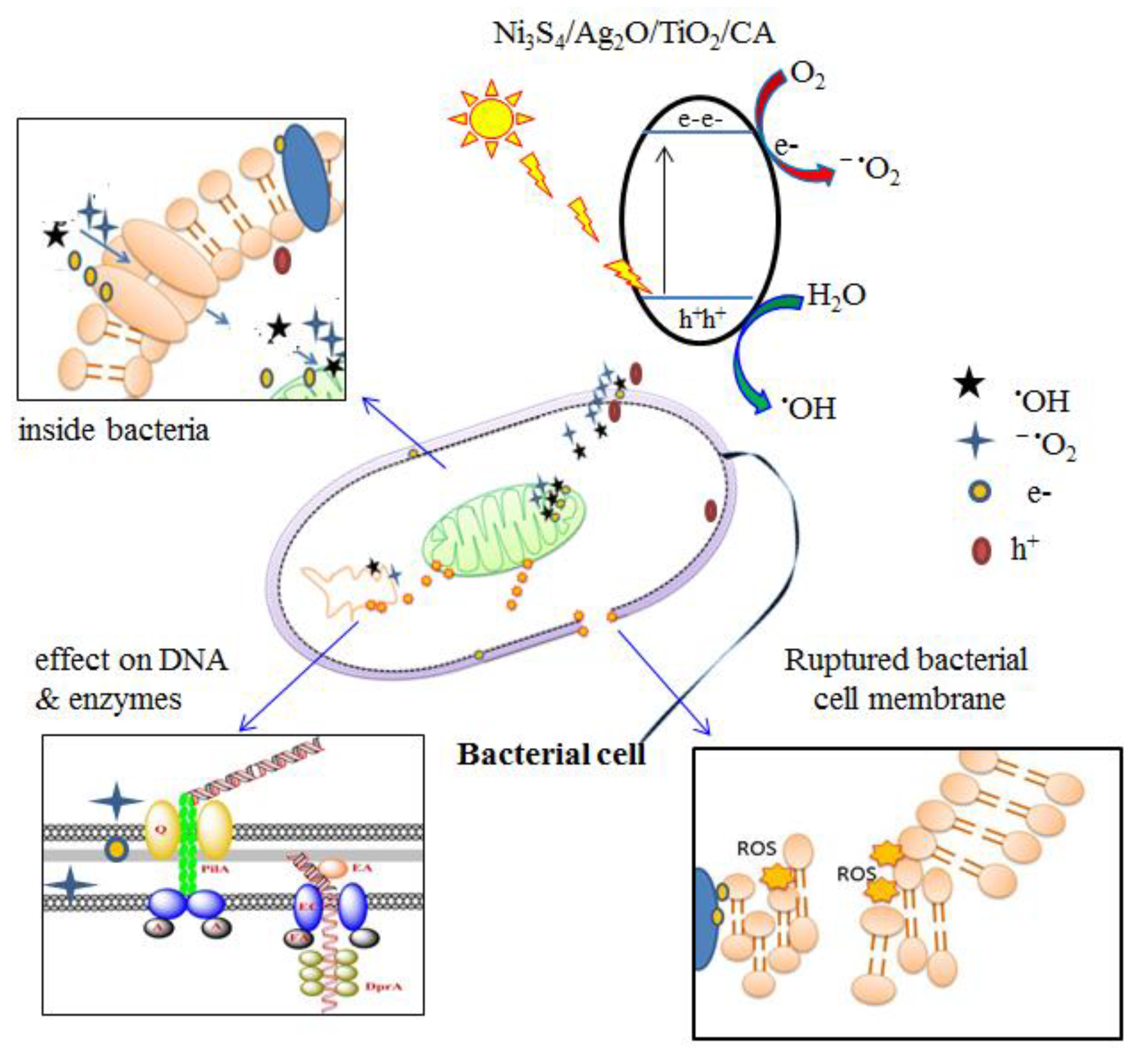
3.3.3. Antimicrobial Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Jana, T.; Iqbal, J.; Ismail, M.; Mansoor, Q.; Mahmood, A.; Ahmad, A. Eradication of multi-drug resistant bacteria by Ni doped ZnO nanorods: Structural, raman and optical characteristics. Appl. Surf. Sci. 2014, 308, 75–81. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Carbonaceous anode materials for nonaqueous sodium- and potassium-ion hybrid capacitors. ACS Energy Lett. 2021, 6, 4127–4154. [Google Scholar] [CrossRef]
- Chattacharjee, S. Iron sulfide nanoparticles prepared using date seed extract: Green synthesis, characterization and potential application for removal of ciprofloxacin and chromium. Powder Technol. 2021, 380, 219–228. [Google Scholar] [CrossRef]
- Kromah, V.; Zhang, G. Aqueous adsorption of heavy metals on metal sulfide nanomaterials: Synthesis and application. Water 2021, 13, 1843. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yuan, X.; Wu, Y.; Wang, H.; Tan, Y.Z.; Chew, J.W. Roles of sulfur-edge sites, metal-edge sites, terrace sites, and defects in metal sulfides for photocatalysis. Chem. Catal. 2021, 1, 44–68. [Google Scholar] [CrossRef]
- Kumar, N.; Mittal, H.; Parashar, V.; Sinha Ray, S.; Ngila, J.C. Efficient removal of rhodamine 6G dye from aqueous solution using nickel sulphide incorporated polyacrylamide grafted gum karaya bionanocomposite hydrogel. RSC Adv. 2016, 6, 21929–21939. [Google Scholar] [CrossRef]
- Hosseini, M.; Fazelian, N.; Fakhri, A.; Kamyab, H.; Yadav, K.K.; Chelliapan, S. Preparation, and structural of new NiS-SiO2 and Cr2S3-TiO2 nano-catalyst: Photocatalytic and antimicrobial studies. J. Photochem. Photobiol. B Biol. 2019, 194, 128–134. [Google Scholar] [CrossRef]
- Pang, M.; Hu, J.; Zeng, H.C. Synthesis, Morphological Control, and Antibacterial Properties of Hollow/Solid Ag2S/Ag Heterodimers. J. Am. Chem. Soc. 2010, 132, 10771–10785. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.; Wang, D.; Xu, L.; Liu, X. Laser induced fabrication of mono-dispersed Ag2S@Ag nano-particles and their superior adsorption performance for dye removal. Opt. Mater. Express 2016, 6, 2573–2583. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fakhri, A.; Agarwal, S.; Azad, M. Synthesis and characterization of Ag2S decorated chitosan nanocomposites and chitosan nanofibers for removal of Lincosamides antibiotic. Int. J. Biol. Macromol. 2017, 103, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Sun, Q.; Banis, M.N.; Sun, X.; Sham, T.-K. Tracking the effect of sodium insertion/extraction in amorphous and anatase TiO2 nanotubes. J. Phys. Chem. C 2017, 121, 11773–11782. [Google Scholar] [CrossRef]
- Kumar, R. Mixed phase lamellar titania-titanate anchored with Ag2O and polypyrrole for enhanced adsorption and photocatalytic activity. J. Colloid Interf. Sci. 2016, 477, 83–93. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Enhanced thermoelectric behaviour and visible light activity of Ag@TiO2/polyaniline nanocomposite synthesized by biogenic-chemical route. RSC Adv. 2014, 4, 23713–23719. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.Y.; Lee, D.S. Reduced graphene oxide −TiO2/sodium alginate 3-dimensional structure aerogel for enhanced photocatalytic degradation of ibuprofen and sulfamethoxazole. Chemosphere 2020, 261, 127702. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 11712. [Google Scholar] [CrossRef]
- Peng, L.; Doménech-Carbó, A.; Primo, A.; García, H. 3D defective graphenes with subnanometric porosity obtained by soft-templating following zeolite procedures. Nanoscale Adv. 2019, 1, 4827–4833. [Google Scholar] [CrossRef]
- Kao, C.W.; Wu, P.T.; Liao, M.Y.; Chung, I.J.; Yang, K.C.; Tseng, W.Y.; Yu, J. Magnetic nanoparticles conjugated with peptides derived from monocyte chemoattractant protein-1 as a tool for targeting atherosclerosis. Pharmaceutics 2018, 10, 62. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Thangaraju, D.; Prakash, N.; Hayakawa, Y. Single-step synthesis and catalytic activity of structure-controlled nickel sulfide nanoparticles. Cryst. Eng. Comm. 2015, 17, 5431–5439. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Kang, Y.C. Sodium-ion storage properties of nickel sulfide hollow nanospheres/reduced graphene oxide composite powders prepared by a spray drying process and the nanoscale Kirkendall effect. Nanoscale 2015, 7, 16781–16788. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wan, Y.; Tian, R.; Wang, S.; Han, T.; Wang, G. In situ cation exchange generated ZnS–Ag2S nanoparticles for photothermal detection of transcription factor. ACS Appl. Bio Mater. 2020, 3, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Lach, J.; Ociepa-Kubicka, A.; Mrowiec, M. Oxytetracycline Adsorption from Aqueous Solutions on Commercial and High-Temperature Modified Activated Carbons. Energies 2021, 14, 3481. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Bi, W.; Qin, J.; Wang, G.; Wang, Z.; Fu, P.; Liu, F. Schwertmannite catalyze persulfate to remove oxytetracycline from wastewater under solar light or UV-254. J. Clean. Prod. 2022, 364, 132572. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; He, Y.; Chen, Y.; Luo, X.; Shang, R. Study on adsorption of tetracycline by Cu-immobilized alginate adsorbent from water environment. Int. J. Biol. Macromol. 2019, 124, 418–428. [Google Scholar] [CrossRef]
- Liu, W.; Shen, X.; Han, Y.; Liu, Z.; Dai, W.; Dutta, A.; Kumar, A.; Liu, J. Selective adsorption and removal of drug contaminants by using an extremely stable Cu(II)-based 3D metal-organic framework. Chemosphere 2019, 215, 524–531. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Lu, H.; Fang, Q.; Rong, H. Efficient Adsorption of Tetracycline from Aqueous Solutions by Modified Alginate Beads after the Removal of Cu(II) Ions. ACS Omega 2021, 6, 6240–6251. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Al-Rashdi, A.A.; Barakat, M.A. Hydrothermal synthesis of structurally variable binary CuAl, MnAl and ternary CuMnAl hydroxides for oxytetracycline antibiotic adsorption. J. Environ. Chem. Eng. 2020, 8, 103535. [Google Scholar] [CrossRef]
- Fideles, R.A.; Ferreira, G.M.D.; Teodoro, F.S.; Adarme, O.F.H.; da Silva, L.H.M.; Gil, L.F.; Gurgel, L.V.A. Trimellitated sugarcane bagasse: A versatile adsorbent for removal of cationic dyes from aqueous solution. Part I: Batch adsorption in a monocomponent system. J. Colloid Interface Sci. 2018, 515, 172–188. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. In Advanced Sorption Process Applications; Edebali, S., Ed.; Intech Open: London, UK, 2018. [Google Scholar]
- Yang, Z.; Chen, C.; Li, B.; Zheng, Y.; Liu, X.; Shen, J.; Zhang, Y.; Wu, S. A core-shell 2DMoS2@MOF hetero-structure for rapid therapy of bacteria-infected wounds by enhanced photocatalysis. Chem. Eng. J. 2022, 451, 139127. [Google Scholar] [CrossRef]
- Kiani, M.; Bagherzadeh, M.; Kaveh, R.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Novel Pt-Ag3PO4/CdS/chitosan nanocomposite with enhanced photocatalytic and biological activities. Nanomaterials 2020, 10, 2320. [Google Scholar] [CrossRef]
- Packirisamy, R.G.; Govindasamy, C.; Sanmugam, A.; Karuppasamy, K.; Kim, H.S.; Vikraman, D. Synthesis and antibacterial properties of novel ZnMn2O4–chitosan nanocomposites. Nanomaterials 2019, 9, 1589. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol Sci. 2019, 20, 2468. [Google Scholar] [CrossRef]
- Geraldo, D.A.; Arancibia-Miranda, N.; Villagra, N.A.; Mora, G.C.; Arratia-Perez, R. Synthesis of CdTe QDs/single-walled aluminosilicate nanotubes hybrid compound and their antimicrobial activity on bacteria. J. Nanoparticle Res. 2012, 14, 1–9. [Google Scholar] [CrossRef]
- Shahri, N.N.M.; Taha, H.S.A.; Hamid, M.H.; Kusrini, E.; Lim, J.W.; Hobley, J.; Usman, A. Antimicrobial activity of silver sulfide quantum dots functionalized with highly conjugated Schiff bases in a one-step synthesis. RSC Adv. 2022, 12, 3136–3146. [Google Scholar] [CrossRef]
- Rui, J.; Deng, N.; Zhao, Y.; Tao, C.; Zhou, J.; Zhao, Z.; Huang, X. Activation of persulfate via Mn doped Mg/Al layered double hydroxide for effective degradation of organics: Insights from chemical and structural variability of catalyst. Chemosphere 2022, 302, 134849. [Google Scholar] [CrossRef]
- Patra, P.; Roy, S.; Sarkar, S.; Mitra, S.; Pradhan, S.; Debnath, N.; Goswami, A. Damage of lipopolysaccharides in outer cell membrane and production of ROS-mediated stress within bacteria makes nano zinc oxide a bactericidal agent. Appl. Nanosci. 2015, 5, 857–866. [Google Scholar] [CrossRef]



| Kinetic Model | Parameters | 10 mg/L | 50 mg/L |
|---|---|---|---|
| Pseudo-first order: | qe (exp) (mg g−1): | 7.1 | 21 |
| qe (cal) (mg g−1): | 7.074 | 21.043 | |
| k1 (min−1): | 0.0266 | 0.022 | |
| R2: | 0.939 | 0.983 | |
| RMSE: | 0.437 | 0.651 | |
| χ2 | 1.469 | 0.412 | |
| Pseudo-second order: | qe (cal) (mg g−1): | 8.216 | 25.454 |
| k2 (g mg−1 min−1): | 0.0042 | 0.0010 | |
| R2: | 0.934 | 0.991 | |
| RMSE: | 0.385 | 0.454 | |
| χ2 | 0.629 | 0.091 | |
| Elovich model: | a (mg g−1 min−1): | 0.635 | 1.054 |
| β (mg g−1): | 0.587 | 0.163 | |
| R2: | 0.944 | 0.993 | |
| RMSE: | 0.353 | 0.691 | |
| χ2 | 0.219 | 0.339 |
| Isotherm Model | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg g−1): | 29.571 |
| KL (L mg−1): | 0.107 | |
| R2: | 0.9370 | |
| RMSE: | 1.660 | |
| χ2: | 1.852 | |
| Freundlich | n: | 2.069 |
| Kf (mg g−1) (mg L−1)−1/nF: | 4.609 | |
| R2: | 0.8739 | |
| RMSE: | 2.349 | |
| χ2: | 3.622 | |
| Temkin | Bt (J mg−1) | 553.70 |
| Kt (L mg−1): | 0.895 | |
| R2: | 0.9431 | |
| RMSE: | 1.577 | |
| χ2: | 1.354 | |
| Redlich–Peterson | (L/g): | 2.242 |
| × 10−3 (L/mg): | 7.309 | |
| β: | 1.681 | |
| R2: | 0.9602 | |
| RMSE: | 1.319 | |
| χ2: | 1.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Oves, M.; Ansari, M.O.; Taleb, M.A.; Barakat, M.A.; Alghamdi, M.A.; Makishah, N.H.A. Biopolymeric Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater. Nanomaterials 2022, 12, 3642. https://doi.org/10.3390/nano12203642
Kumar R, Oves M, Ansari MO, Taleb MA, Barakat MA, Alghamdi MA, Makishah NHA. Biopolymeric Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater. Nanomaterials. 2022; 12(20):3642. https://doi.org/10.3390/nano12203642
Chicago/Turabian StyleKumar, Rajeev, Mohammad Oves, Mohammad Omaish Ansari, Md. Abu Taleb, Mohamed A. Barakat, Mansour A. Alghamdi, and Naief Hamoud Al Makishah. 2022. "Biopolymeric Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater" Nanomaterials 12, no. 20: 3642. https://doi.org/10.3390/nano12203642
APA StyleKumar, R., Oves, M., Ansari, M. O., Taleb, M. A., Barakat, M. A., Alghamdi, M. A., & Makishah, N. H. A. (2022). Biopolymeric Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater. Nanomaterials, 12(20), 3642. https://doi.org/10.3390/nano12203642






