Changes in Mechanical Properties of Vesicles by Mucin in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Morphology
3.2. Force Measurements
3.3. Theoretical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2007, 80, 209–276. [Google Scholar]
- Strous, G.J.; Dekker, J. Mucin-type glycoproteins. Crit. Rev. Biochem. Mol. 1992, 27, 57–92. [Google Scholar]
- Shi, L.; Caldwell, K.D. Mucin adsorption to hydrophobic surface. J. Colloid Interface Sci. 2000, 224, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Maill, A.S. Analysis of mucins: Role in laboratory diagnosis. J. Clin. Pathol. 2008, 61, 10118–11024. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Lev, R. The mucin histochemistry of normal and neoplastic gastric mucosa. Lab. Investig. J. Tech. Methods Pathol. 1965, 14, 2080–2100. [Google Scholar]
- Goldenberg, D.M.; Pegram, C.A.; Vazquez, J.J. Identification of a colon-specific antigen (CSA) in normal and neoplastic tissues. J. Immunol. 1975, 114, 1008–1013. [Google Scholar]
- Wiedmann, T.A.; Herrington, H.; Deyea, C.; Kallick, D. Analysis of the diffusion of bile salt/phospholipid micelles in rat intestinal mucin. Chem. Phys. Lipids 2001, 112, 81–92. [Google Scholar] [CrossRef]
- Millar, T.J.; Tragoulias, S.T.; Anderton, P.J.; Ball, M.S.; Miano, F.; Dennis, G.R.; Mudgil, P. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Corea 2006, 25, 91–100. [Google Scholar] [CrossRef]
- Rogunova, M.A.; Blackwell, J.; Jamieson, A.M.; Pasumar-Thy, M.; Gerken, T.A. Effect of lipids on the structure and rheology of gels formed by canine submaxillary mucin. Biorheology 1997, 34, 295–308. [Google Scholar] [CrossRef]
- Korytowski, A.; Abuillan, W.; Amadei, F.; Makkya, A.; Gumiero, A.; Sinning, I.; Gauss, A.; Stremmel, W.; Tanak., M. Accumulation of phosphatidylcholine on gut mucosal surface is not dominated by electrostatic interactions. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Alp, G.; Aydogan, N. Lipid-based mucus penetrating nanoparticles and their biophysical interactions with pulmonary mucus layer. Eur. J. Pharm. Biopharm. 2020, 149, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K.; Latal, A.; Degovics, G.; Garidel, P. Packing characteristics of a model system mimicking cytoplasmic bacterial membranes. Chem. Phys. Lipids 2001, 111, 177–192. [Google Scholar] [CrossRef]
- Zhu, Y.; Stevens, C.F. Probing synaptic vesicle fusion by altering mechanical properties of the neuronal surface membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 18018–18022. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hernandez, I.C.; López-Ortega, O.; Acevedo-Ochoa, E.; Cervantes-Díaz, R.; Romero-Ramírez, S.; Sosa-Hernández, V.A.; Meza-Sánchez, D.E.; Juárez-Vega, G.; Pérez-Martínez, C.A.; Chávez-Munguía, B.; et al. Tetraspanin 33 (TSPAN33) regulates endocytosis and migration of human B lymphocytes by affecting the tension of the plasma membrane. FEBS J. 2020, 287, 3449–3471. [Google Scholar] [CrossRef]
- Khadka, N.K.; Teng, P.; Cai, J.; Pan, J. Modulation of lipid membrane structural and mechanical properties by a peptidomimetic derived from reduced amide scaffold. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 734–744. [Google Scholar]
- Engler, A.J.; Richert, L.; Wong, J.Y.; Picart, C.; Discher, D.E. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: Correlations between substrate stiffness and cell adhesion. Surf. Sci. 2004, 570, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Wright, C.J.; Shah, M.K.; Powell, L.C.; Armstrong, I. Application of AFM from microbial cell to biofilm. Scanning 2010, 32, 134–149. [Google Scholar] [CrossRef]
- Alam, F.; Kumar, S.; Varadarajan, K.M. Quantification of Adhesion Force of Bacteria on the Surface of Biomaterials: Techniques and Assays. ACS Biomater. Sci. Eng. 2019, 5, 2093–2110. [Google Scholar] [CrossRef]
- Park, J.-W. Probe chemistry effect on surface properties of asymmetric-phase lipid bilayers. Colloids Surf. Biointerfaces 2010, 75, 290–293. [Google Scholar] [CrossRef]
- Ruiz-Cabello, F.J.M.; Trefalt, G.; Maroni, P.; Borkovec, M. Electric double-layer potentials and surface regulation properties measured by colloidal-probe atomic force microscopy. Phys. Rev. E 2014, 90, 012301. [Google Scholar]
- Black, J.M.; Zhu, M.; Zhang, P.; Unocic, R.R.; Guo, D.; Okatan, M.B.; Dai, S.; Cummings, P.T.; Kalinin, S.V.; Feng, G.; et al. Fundamental aspects of electric double layer force-distance measurements at liquid-solid interfaces using atomic force microscopy. Sci. Rep. 2016, 6, 32389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturri, J.; Toca-Herrera, J.L. Characterization of Cell Scaffolds by Atomic Force Microscopy. Polymers 2017, 9, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Qian, X.; Guan, L.; Zhang, M.; Li, Q.; Wu, A.; Dong, M. Studying the Adhesion Force and Glass Transition of Thin Polystyrene Films by Atomic Force Microscopy. Nanoscale Res. Lett. 2018, 13, 5. [Google Scholar] [CrossRef]
- Kelly, E.G.; Butler, P.D.; Ashkar, R.; Nagao, M. Scaling relationships for the elastic moduli and viscosity of mixed lipid membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 23365–23373. [Google Scholar] [CrossRef]
- Tytgat, K.M.; Opdam, F.J.; Einerhand, A.W.; Büller, H.A.; Dekker, J. MUC2 is the prominent colonic mucin expressed in ulcerative colitis. Gut 1996, 38, 554–563. [Google Scholar] [CrossRef]
- New, R.R.C. Liposomes: A Practical Approach; Academic Press: New York, NY, USA, 1990; pp. 20–45. [Google Scholar]
- Laney, D.E.; Garcia, R.A.; Parsons, S.M.; Hansma, H.G. Changes in the elastic properties of cholinergic synaptic vesicles as measured by atomic force microscopy. Biophys. J. 1997, 72, 806–813. [Google Scholar]
- Radmacher, M.; Fritz, M.; Kacher, C.M.; Walters, D.A.; Hansma, P.K. Imaging adhesion forces and elasticity of lysozyme adsorbed on mica with the atomic-force microscope. Langmuir 1994, 10, 3809–3814. [Google Scholar] [CrossRef]
- Solletti, J.M.; Botreau, M.; Sommer, F.; Brunat, W.L.; Kasas, S.; Duc, T.M.; Celio, M.R. Elaboration and Characterization of Phospholipid Langmuir−Blodgett Films. Langmuir 1996, 12, 5379. [Google Scholar] [CrossRef]
- Duarte, A.A.; Marquês, J.T.; Brasil, F.; Viana, A.S.; Tavares, P.; Raposo, M. In Situ AFM Imaging of Adsorption Kinetics of DPPG Liposomes: A Quantitative Analysis of Surface Roughness. Microsc. Microanal. 2019, 25, 798–809. [Google Scholar] [CrossRef]
- Weisenhorn, A.L.; Khorsandi, M.; Kasas, S.; Gotzos, V.; Butt, H.-J. Deformation and height anomaly of soft surfaces studied with an AFM. Nanotechnology 1993, 4, 106–113. [Google Scholar] [CrossRef]
- Jadidi, T.; Seyyed-Allaei, H.; Tabar, M.R.R.; Mashaghi, A. Poisson’s ratio and Young’s modulus of lipid bilayers in different phases. Front. Bioeng. Biotechnol. 2014, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, J.; Park, J.-W. Trehalose-Induced Variation in Mechanical Properties of Vesicles in Aqueous Solution. J. Membr. Biol. 2015, 248, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Lee, G.U. Properties of mixed lipid monolayers assembled on hydrophobic surfaces through vesicle adsorption. Langmuir 2006, 22, 5057–5063. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Rojewska, M.; Prochaska, K. Study of mucin interaction with model phospholipid membrane at the air–water interface. Colloids Surf. A Physicochem. Eng. 2019, 578, 123587. [Google Scholar] [CrossRef]
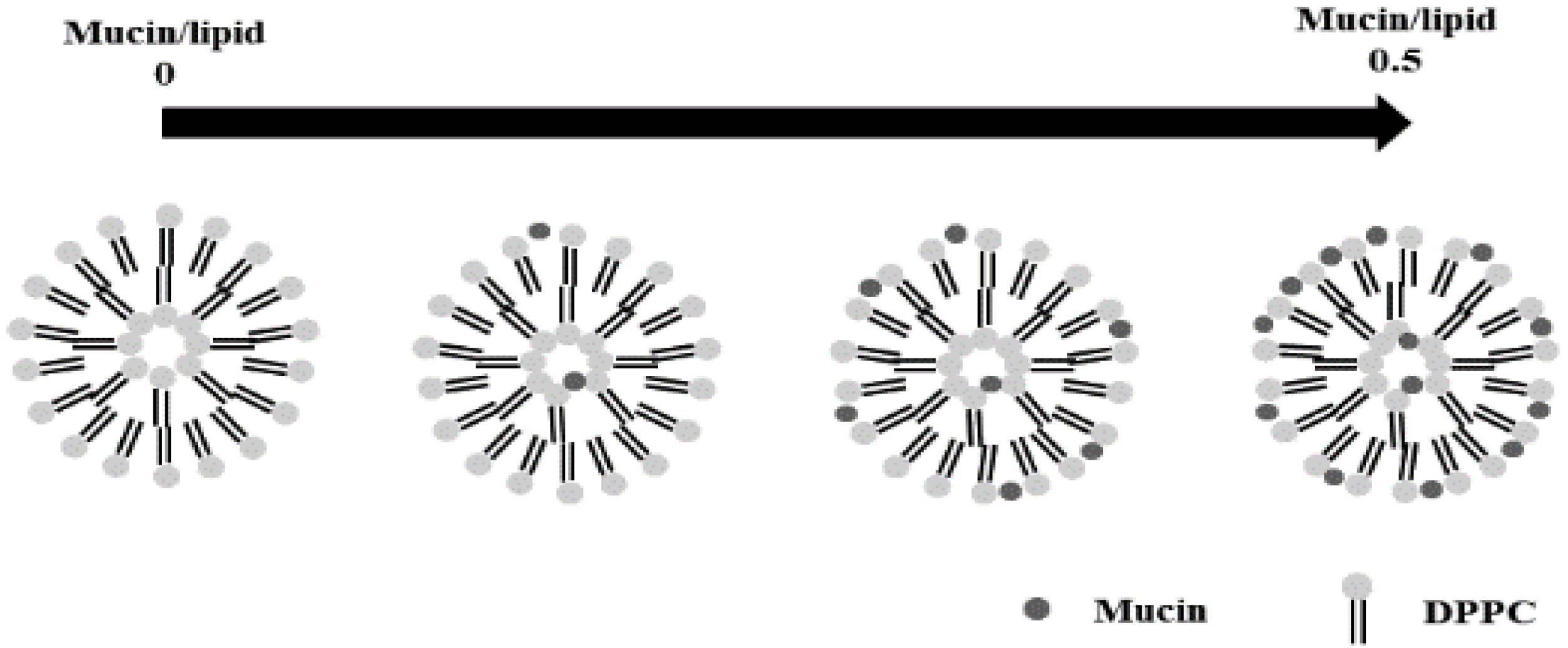

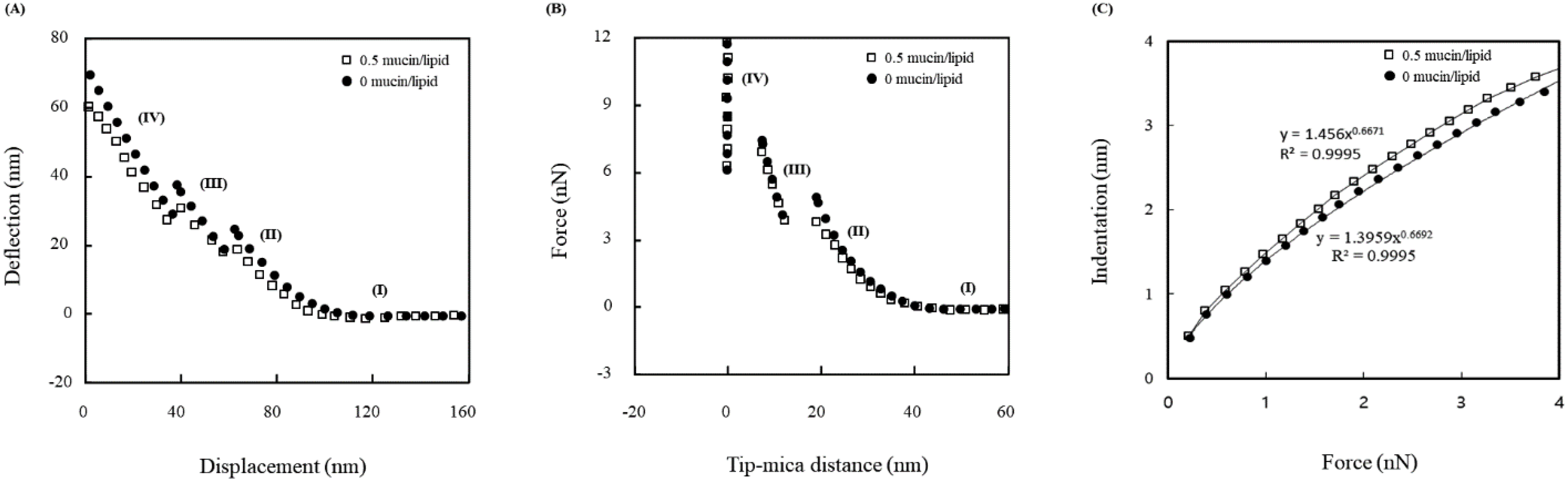

| Ratio of Mucin to Lipid | 0 | 0.1 | 0.3 | 0.5 | 0.7 | 1.0 |
|---|---|---|---|---|---|---|
| Root-mean-square roughness (nm) | 9.3 ± 0.7 | 9.2 ± 0.6 | 9.3 ± 0.6 | 9.3 ± 0.7 | 9.2 ± 0.7 | 9.3 ± 0.6 |
| Change rate of 1st steric force (N/m) | 0.7 ± 0.01 | 0.69 ± 0.01 | 0.67 ± 0.01 | 0.65 ± 0.01 | 0.65 ± 0.01 | 0.65 ± 0.01 |
| Change rate of 2nd steric force (N/m) | 3.6 ± 0.01 | 3.5 ± 0.01 | 3.4 ± 0.01 | 3.3 ± 0.01 | 3.3 ± 0.01 | 3.3 ± 0.01 |
| Ratio of Mucin/Lipid | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.3 | 0.5 | 0.7 | 1.0 | |
| Eves × 106 (Pa) | 81 ± 2 | 80 ± 2 | 78 ± 2 | 76 ± 2 | 76 ± 2 | 76 ± 2 |
| kc × 10−19 (J) | 11.3 ± 0.3 | 11.2 ± 0.3 | 10.8 ± 0.3 | 10.5 ± 0.3 | 10.5 ± 0.3 | 10.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Hadinoto, K.; Park, J.-W. Changes in Mechanical Properties of Vesicles by Mucin in Aqueous Solution. Nanomaterials 2022, 12, 3683. https://doi.org/10.3390/nano12203683
Lee G, Hadinoto K, Park J-W. Changes in Mechanical Properties of Vesicles by Mucin in Aqueous Solution. Nanomaterials. 2022; 12(20):3683. https://doi.org/10.3390/nano12203683
Chicago/Turabian StyleLee, Gaeul, Kunn Hadinoto, and Jin-Won Park. 2022. "Changes in Mechanical Properties of Vesicles by Mucin in Aqueous Solution" Nanomaterials 12, no. 20: 3683. https://doi.org/10.3390/nano12203683
APA StyleLee, G., Hadinoto, K., & Park, J.-W. (2022). Changes in Mechanical Properties of Vesicles by Mucin in Aqueous Solution. Nanomaterials, 12(20), 3683. https://doi.org/10.3390/nano12203683







