Effects of Diamond on Microstructure, Fracture Toughness, and Tribological Properties of TiO2-Diamond Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Microstructure Characterization
2.3. Mechanical Properties and Tribological Properties Test
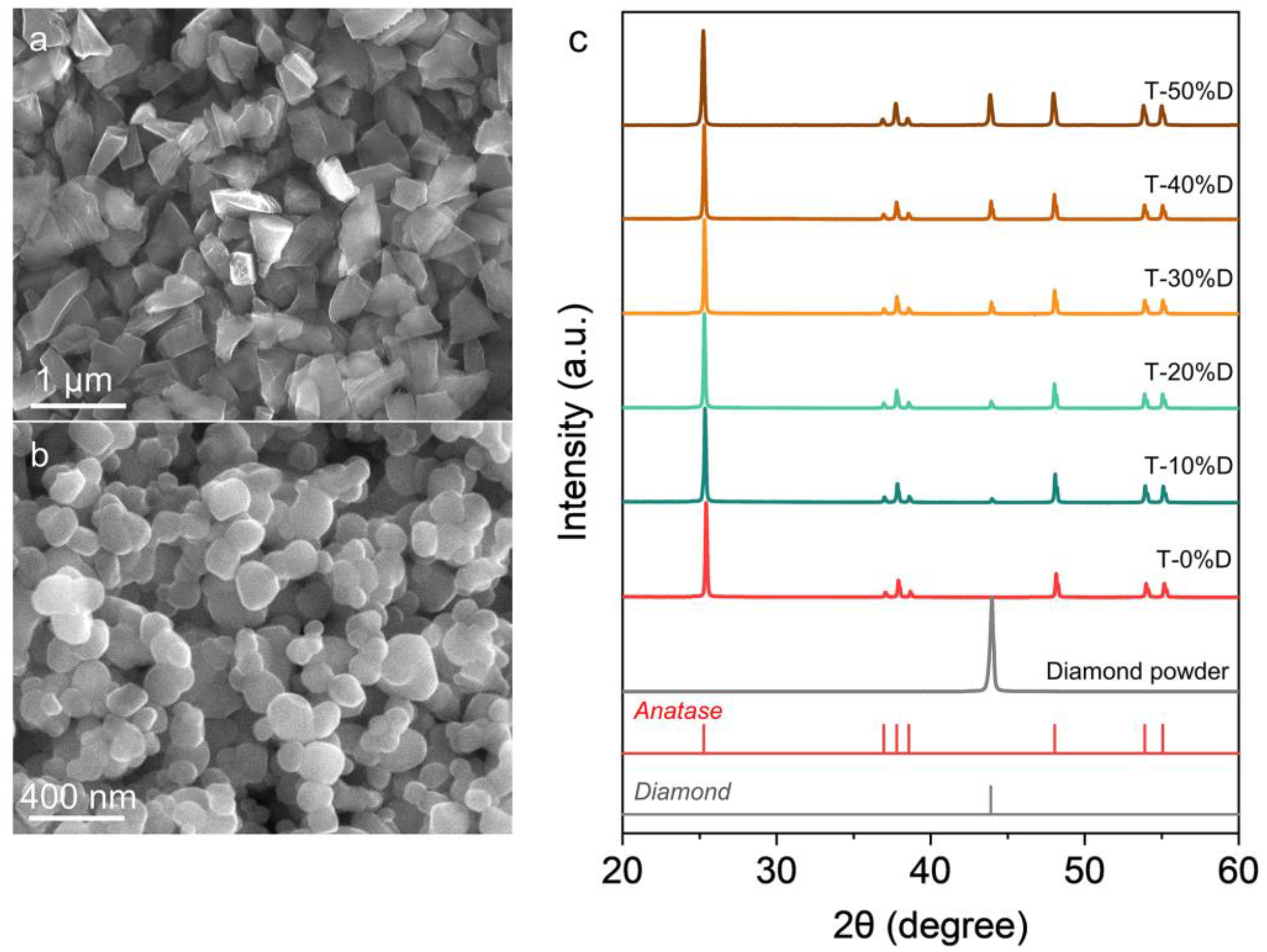
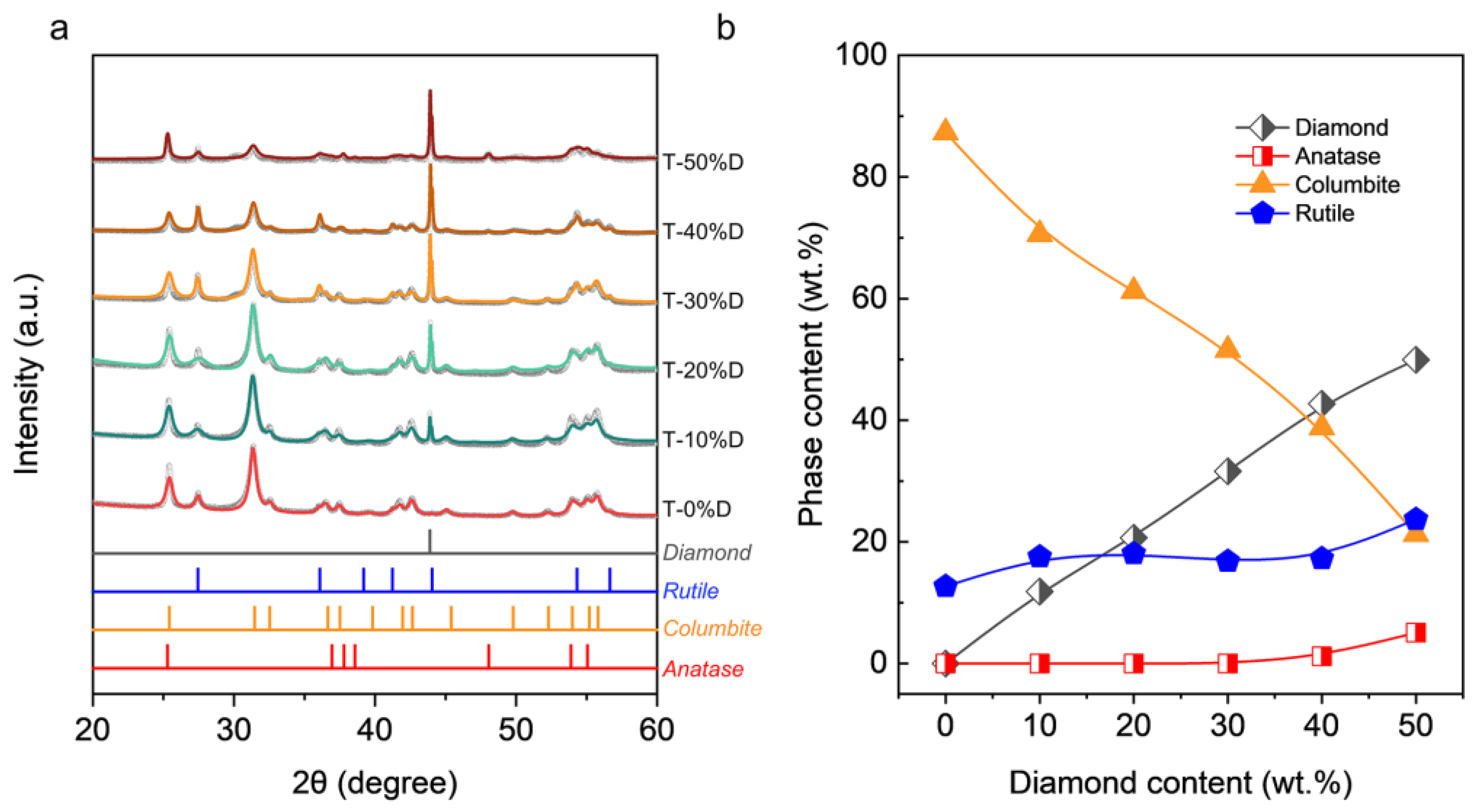
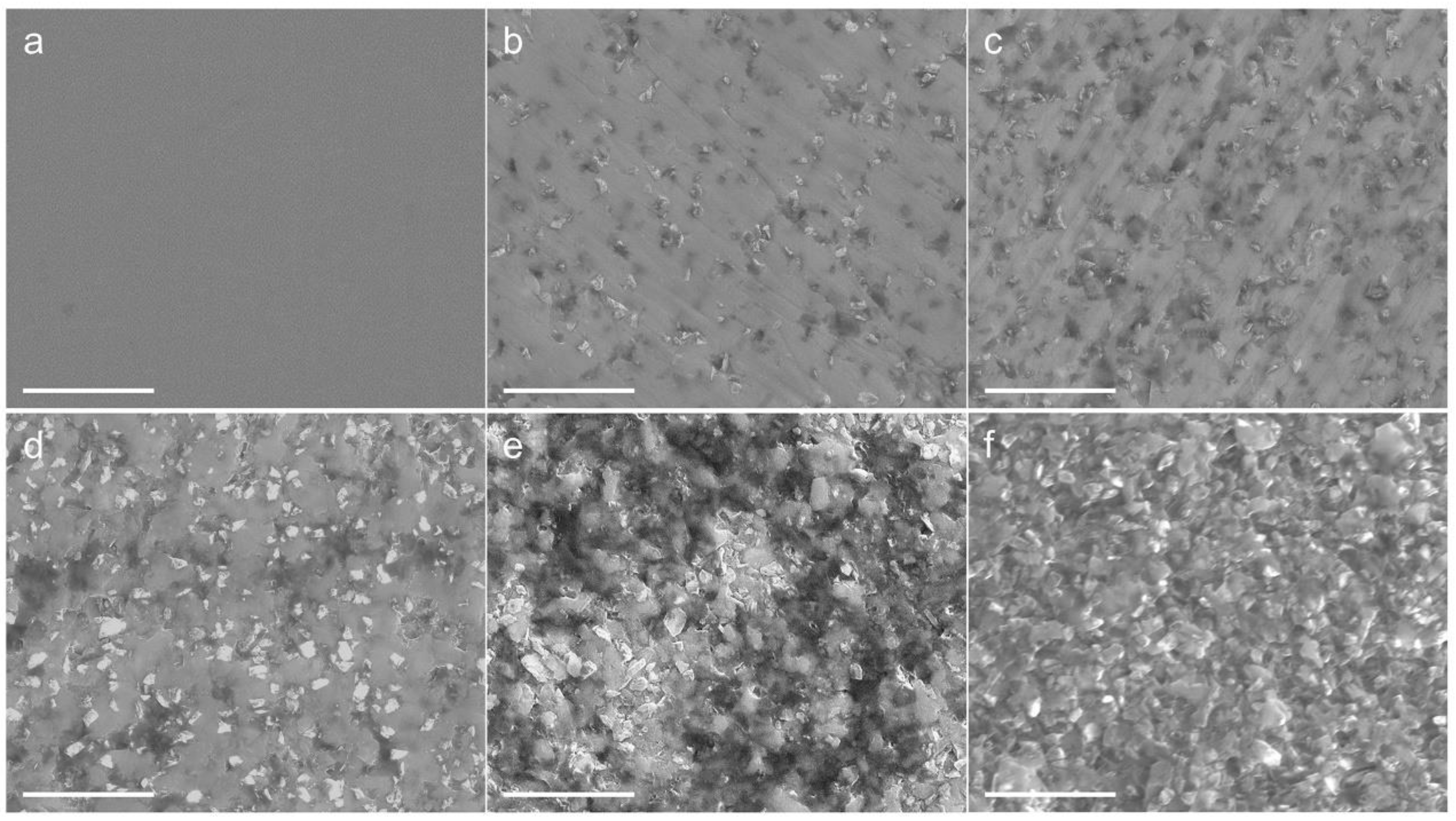
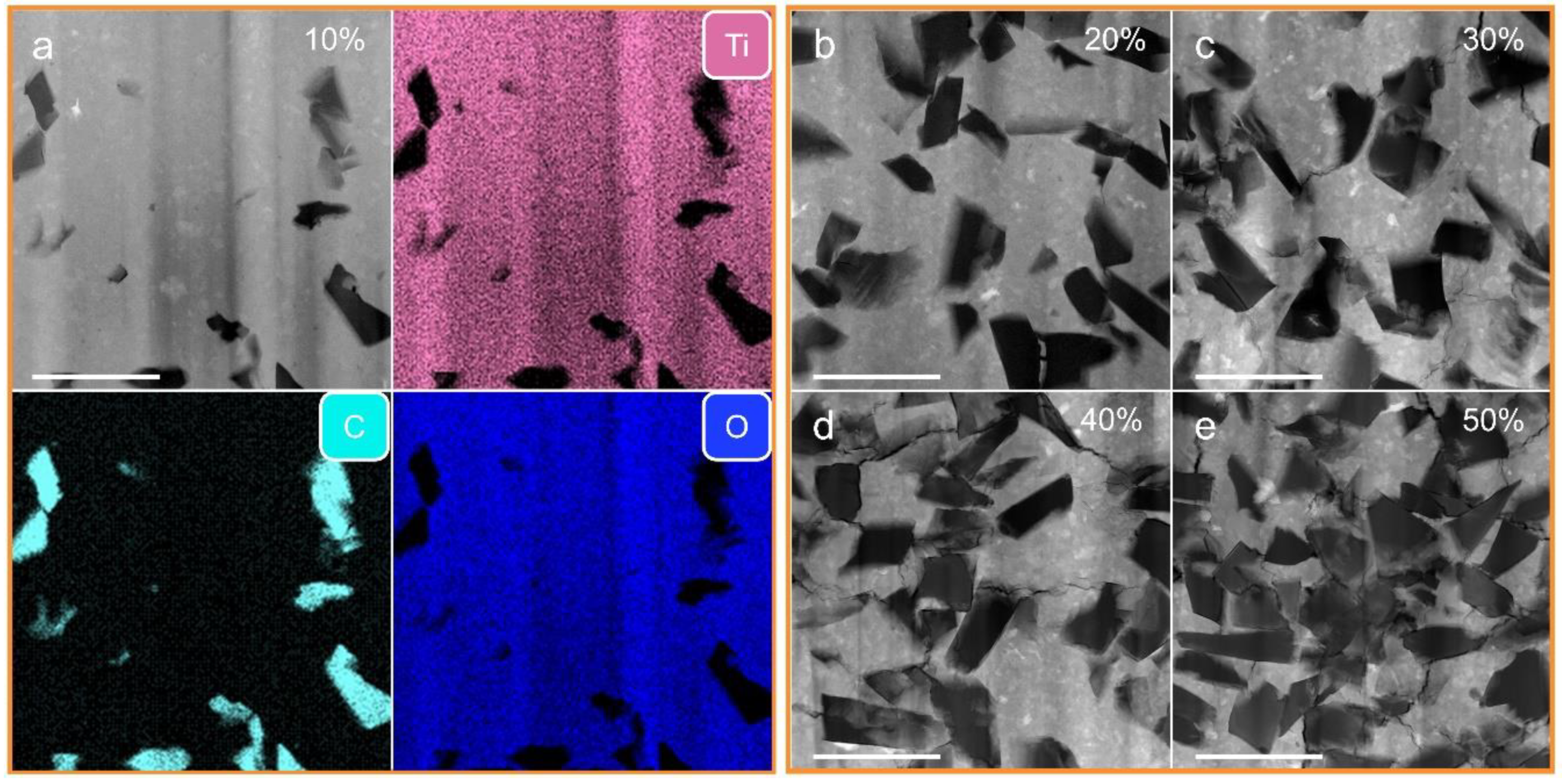
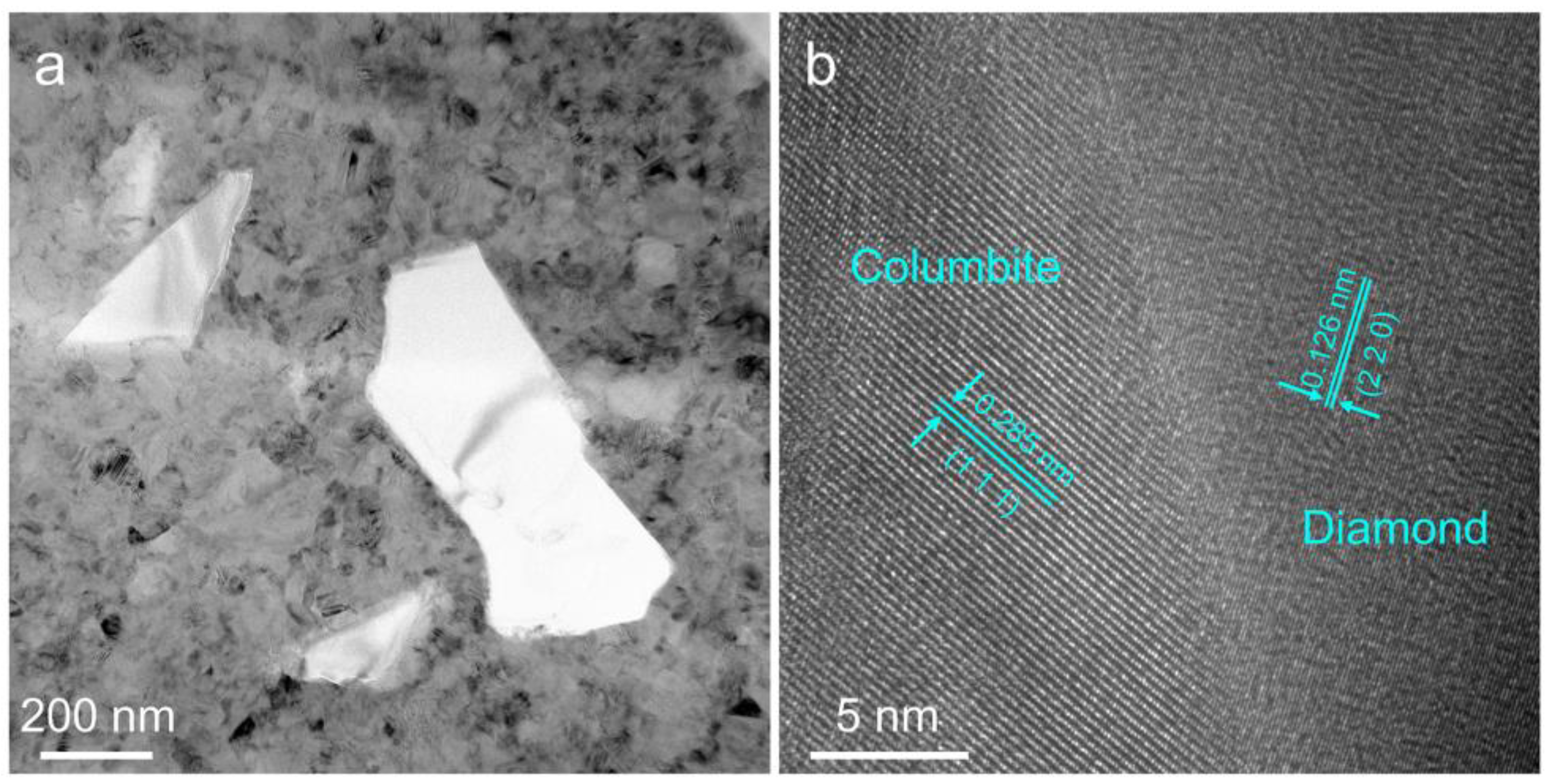
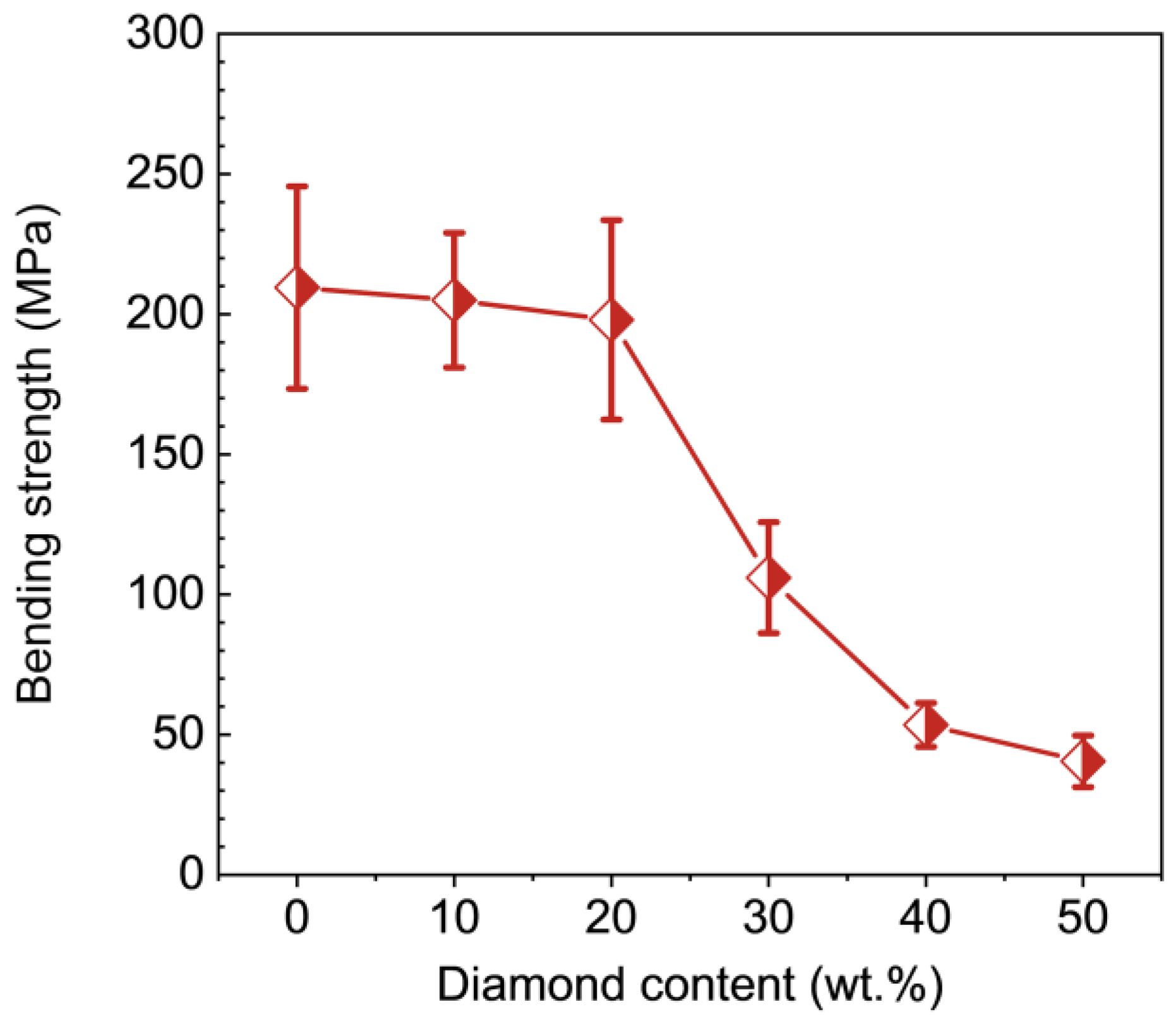
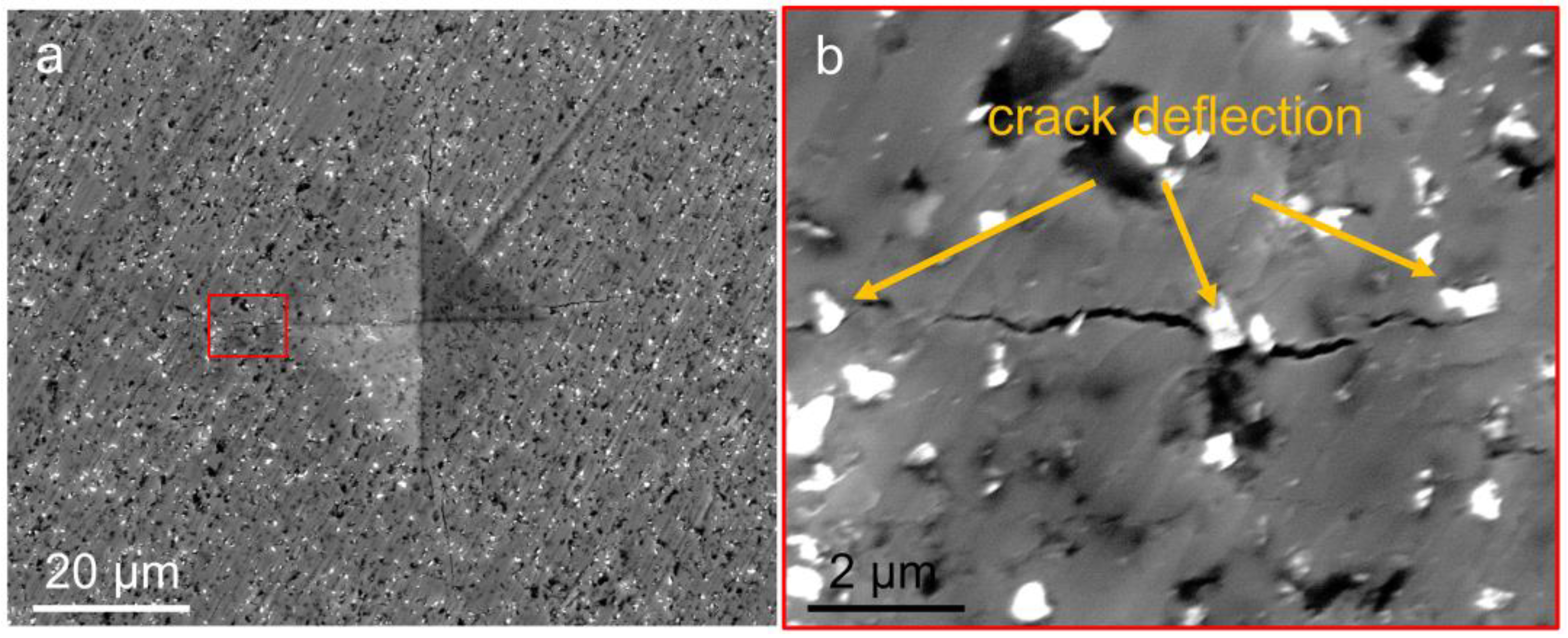
3. Results
4. Conclusions
- (1)
- Diamond can promote the phase transformation from anatase to rutile and hinder the phase transformation from anatase to columbite.
- (2)
- Hardness and bending strength decrease with the increase of diamond content.
- (3)
- Diamond can improve the fracture toughness of TiO2 by the crack deflection mechanism.
- (4)
- The addition of diamond reduces the friction coefficient by up to 66.7%.
- (5)
- To enhance the mechanical properties of the TiO2 matrix, the diamond content needs to be limited in range, i.e., less than or equal to 20 wt.%. The composite composed of 10 wt.% diamond exhibits optimum mechanical and tribological properties, with a hardness of 14.5 GPa, bending strength of 205.2 MPa, fracture toughness of 3.5 MPa∙m1/2, and a friction coefficient of 0.3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, G.D.; Singh, S.P.; Kurchania, R.; Ball, R.J. Cosensitization of dye sensitized solar cells with a thiocyanate free Ru dye and a metal free dye containing thienylfluorene conjugation. RSC Adv. 2013, 3, 6036–6043. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, W.; Yang, C.; Wei, X.; Hao, J. Colossal Permittivity Materials as Superior Dielectrics for Diverse Applications. Adv. Funct. Mater. 2019, 29, 1808118. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjinés, R.; Lévy, F. TiO2 anatase thin films as gas sensors. Sens. Actuators B Chem. 1995, 26, 71–75. [Google Scholar] [CrossRef]
- Lima, R.S.; Marple, B.R. Thermal spray coatings engineered from nanostructured ceramic agglomerated powders for structural, thermal barrier and biomedical applications: A review. J. Therm. Spray Technol. 2007, 16, 40–63. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.Y.; Liu, G.P.; Liu, S.N.; Shen, J.; Jin, W.Q. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Bao, N.; Zhu, X.W.; Ma, D.; Xin, Y.J. Preparation and photocatalytic properties of graphene/TiO2 nanotube arrays photoelectrodes. J. Alloy. Compd. 2015, 618, 761–767. [Google Scholar] [CrossRef]
- Bai, T.; Fang, Y.J.; Wang, J.L. Preparation and tribological properties of graphene/TiO2 ceramic films. Ceram. Int. 2017, 43, 13299–13307. [Google Scholar] [CrossRef]
- Sun, S.; Song, P.; Cui, J.; Liang, S. Amorphous TiO2 nanostructures: Synthesis, fundamental properties and photocatalytic applications. Catal. Sci. Technol. 2019, 9, 4198–4215. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, X.; Liu, W. The synthesis strategies and photocatalytic performances of TiO2/MOFs composites: A state-of-the-art review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Miao, X.G.; Sun, D.D.; Hoo, P.W. Effect of Y-TZP addition on the microstructure and properties of titania-based composites. Ceram. Int. 2009, 35, 281–288. [Google Scholar] [CrossRef]
- Shon, I.; Lee, G.; Doh, J.; Yoon, J. Effect of Milling on Properties and Consolidation of TiO2 by High-Frequency Induction Heated Sintering. Electron. Mater. Lett. 2013, 9, 219–225. [Google Scholar] [CrossRef]
- Shon, I.J.; Yoon, J.K.; Hong, K.T. Enhanced properties of nanostructured TiO2-graphene composites by rapid sintering. Met. Mater. Int. 2018, 24, 130–135. [Google Scholar] [CrossRef]
- Nadaraia, L.; Jalabadze, N.; Khundadze, L.; Rurua, L.; Japaridze, M.; Chedia, R. Effects of graphene on morphology, fracture toughness, and electrical conductivity of titanium dioxide. Diam. Relat. Mater. 2021, 114, 108319. [Google Scholar] [CrossRef]
- Mu, M.; Zhou, X.J.; Xiao, Q.; Liang, J.; Huo, X.D. Preparation and tribological properties of self-lubricating TiO2/graphite composite coating on Ti6Al4V alloy. Appl. Surf. Sci. 2012, 258, 8570–8576. [Google Scholar] [CrossRef]
- He, P.F.; Ma, G.Z.; Wang, H.D.; Yong, Q.S.; Chen, S.Y. Microstructure and mechanical properties of a novel plasma-spray TiO2 coating reinforced by CNTs. Ceram. Int. 2016, 42, 13319–13325. [Google Scholar] [CrossRef]
- Qian, J.; Pantea, C.; Voronin, G.; Zerda, T.W. Partial graphitization of diamond crystals under high-pressure and high-temperature conditions. J. Appl. Phys. 2001, 90, 1632–1637. [Google Scholar] [CrossRef]
- Qian, J.; Pantea, C.; Huang, J.; Zerda, T.W.; Zhao, Y. Graphitization of diamond powders of different sizes at high pressure-high temperature. Carbon 2004, 42, 2691–2697. [Google Scholar] [CrossRef]
- Qiao, Z.J.; Li, J.J.; Zhao, N.Q.; Shi, C.S.; Nash, P. Graphitization and microstructure transformation of nanodiamond to onion-like carbon. Scr. Mater. 2006, 54, 225–229. [Google Scholar] [CrossRef]
- Hanada, K.; Shoji, T.; Mayuzumi, M.; Sano, T. Development of self-lubricating titania/diamond nanoparticle composite. Mater. Sci. Technol. 2004, 20, 1103–1108. [Google Scholar] [CrossRef]
- Gallas, M.R.; Rosa, A.R.; Costa, T.H.; Jornada, J.A.H.D. High pressure compaction of nanosized ceramic powders. J. Mater. Res. 1997, 12, 764–768. [Google Scholar] [CrossRef]
- Andrade, M.J.D.; Lima, M.D.; Bergmann, C.P.; Ramminger, G.D.O.; Balzaretti, N.M.; Costa, T.M.H.; Gallas, M.R. Carbon nanotube/silica composites obtained by sol-gel and high-pressure technique. Nanotechnology 2018, 19, 265607. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.; Bernardi, M.I.B.; Mastelaro, V.R.; Lente, M.H.; Eiras, J.A.; Gallas, M.R.; Costa, T.M.H. Nanograined ferroelectric ceramics prepared by high-pressure densification technique. J. Am. Ceram. Soc. 2009, 92, 1679–1683. [Google Scholar] [CrossRef]
- Lahiri, D.; Singh, V.; Rodrigues, G.R.; Costa, T.M.H.; Gallas, M.R.; Bakshi, S.R.; Seal, S.; Agarwal, A. Ultrahigh-pressure consolidation and deformation of tantalum carbide at ambient and high temperatures. Acta Mater. 2013, 61, 4001–4009. [Google Scholar] [CrossRef]
- Liu, B.; Sun, L.; Wu, Y.J.; Zhang, Y.; Li, Z.H.; Chen, J.Y.; Xie, C.L.; Huang, Q.; Ma, M.D.; Luo, K.; et al. Columbite-rich multiphase TiO2 nanoceramic with superior mechanical and dielectric properties. J. Eur. Ceram. 2021, 41, 4951–4957. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Ouyang, Q.; Okada, K. Friction properties of aluminium-based composites containing cluster diamond. J. Vac. Sci. Technol. A 1994, 12, 2577–2580. [Google Scholar] [CrossRef]
- Ouyang, Q.; Okada, K. Nano-ball bearing effect of ultra-fine particles of cluster diamond. Appl. Surf. Sci. 1994, 78, 309–313. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, J.Z.; Xu, K.; Xue, Q.J. Study on the tribological properties of ultradispersed diamond containing soot as an oil additive. Tribol. 1997, 40, 178–182. [Google Scholar] [CrossRef]
- Hanada, K.; Mayuzumi, M.; Nakayama, N.; San, T. Processing and characterization of cluster diamond dispersed Al-Si-Cu-Mg composite. J. Mater. Process. Tech. 2001, 119, 216–221. [Google Scholar] [CrossRef]
- Wolfrum, A.K.; Quitzke, C.; Matthey, B.; Herrmann, M. Wear behavior of diamond-silicon nitride composites sintered with FAST/SPS. Wear 2018, 396, 172–181. [Google Scholar] [CrossRef]
- Habig, K.H. Fundamentals of the tribological behavior of diamond, diamond-like carbon and cubic boron nitride coatings. Surf. Coat. Tech. 1995, 76–77, 540–547. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhuge, Z.; Zhao, S.; Zou, Y.; Tong, K.; Sun, L.; Wang, X.; Liang, Z.; Li, B.; Jin, T.; et al. Effects of Diamond on Microstructure, Fracture Toughness, and Tribological Properties of TiO2-Diamond Composites. Nanomaterials 2022, 12, 3733. https://doi.org/10.3390/nano12213733
Liu B, Zhuge Z, Zhao S, Zou Y, Tong K, Sun L, Wang X, Liang Z, Li B, Jin T, et al. Effects of Diamond on Microstructure, Fracture Toughness, and Tribological Properties of TiO2-Diamond Composites. Nanomaterials. 2022; 12(21):3733. https://doi.org/10.3390/nano12213733
Chicago/Turabian StyleLiu, Bing, Zewen Zhuge, Song Zhao, Yitong Zou, Ke Tong, Lei Sun, Xiaoyu Wang, Zitai Liang, Baozhong Li, Tianye Jin, and et al. 2022. "Effects of Diamond on Microstructure, Fracture Toughness, and Tribological Properties of TiO2-Diamond Composites" Nanomaterials 12, no. 21: 3733. https://doi.org/10.3390/nano12213733
APA StyleLiu, B., Zhuge, Z., Zhao, S., Zou, Y., Tong, K., Sun, L., Wang, X., Liang, Z., Li, B., Jin, T., Chen, J., & Zhao, Z. (2022). Effects of Diamond on Microstructure, Fracture Toughness, and Tribological Properties of TiO2-Diamond Composites. Nanomaterials, 12(21), 3733. https://doi.org/10.3390/nano12213733





