On the Development of Nanocomposite Covalent Associative Networks Based on Polycaprolactone and Reduced Graphite Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PCL-OH
2.3. Preparation of the Crosslinked Systems
2.4. Preparation of Nanocomposites
2.5. Characterization
3. Results
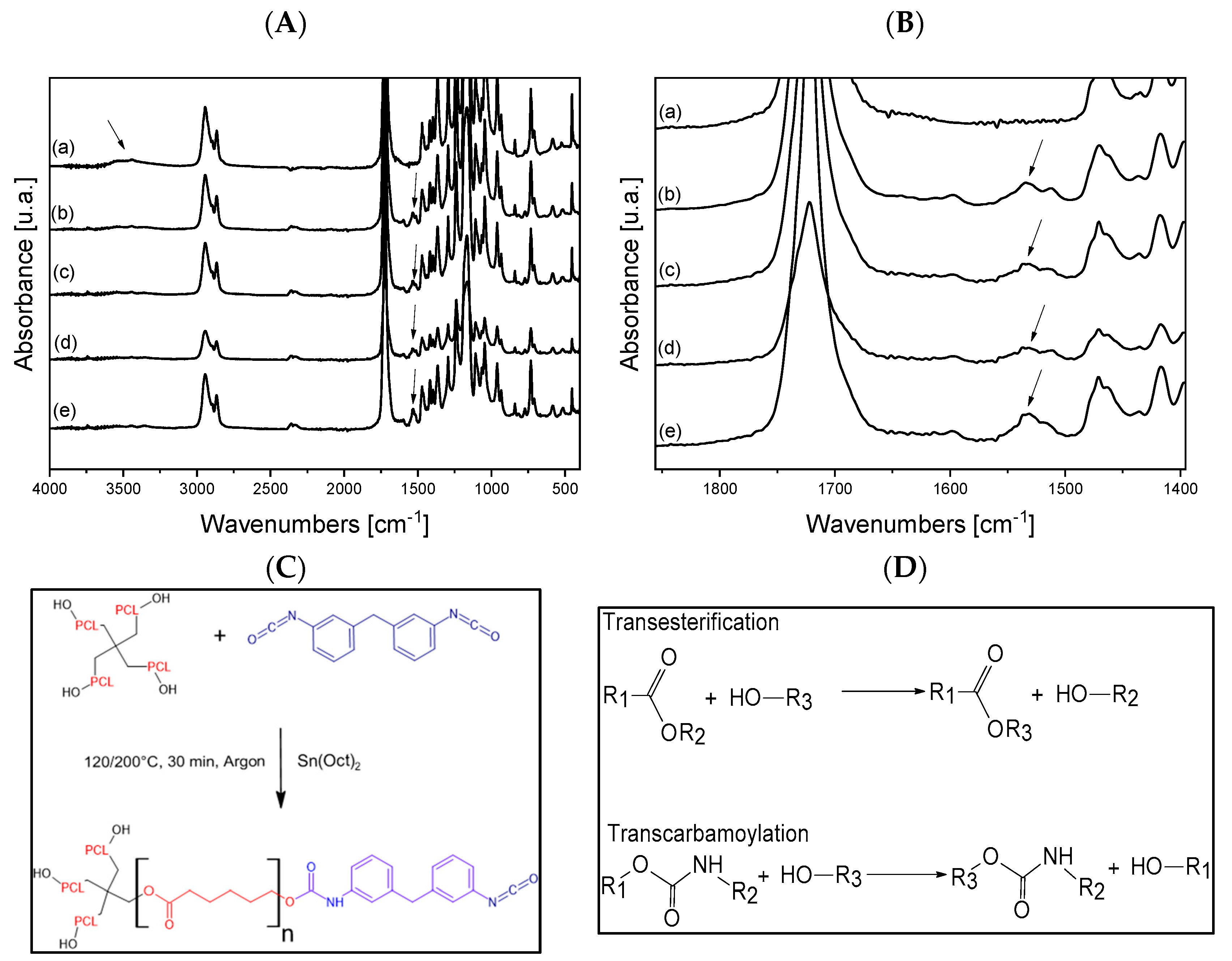
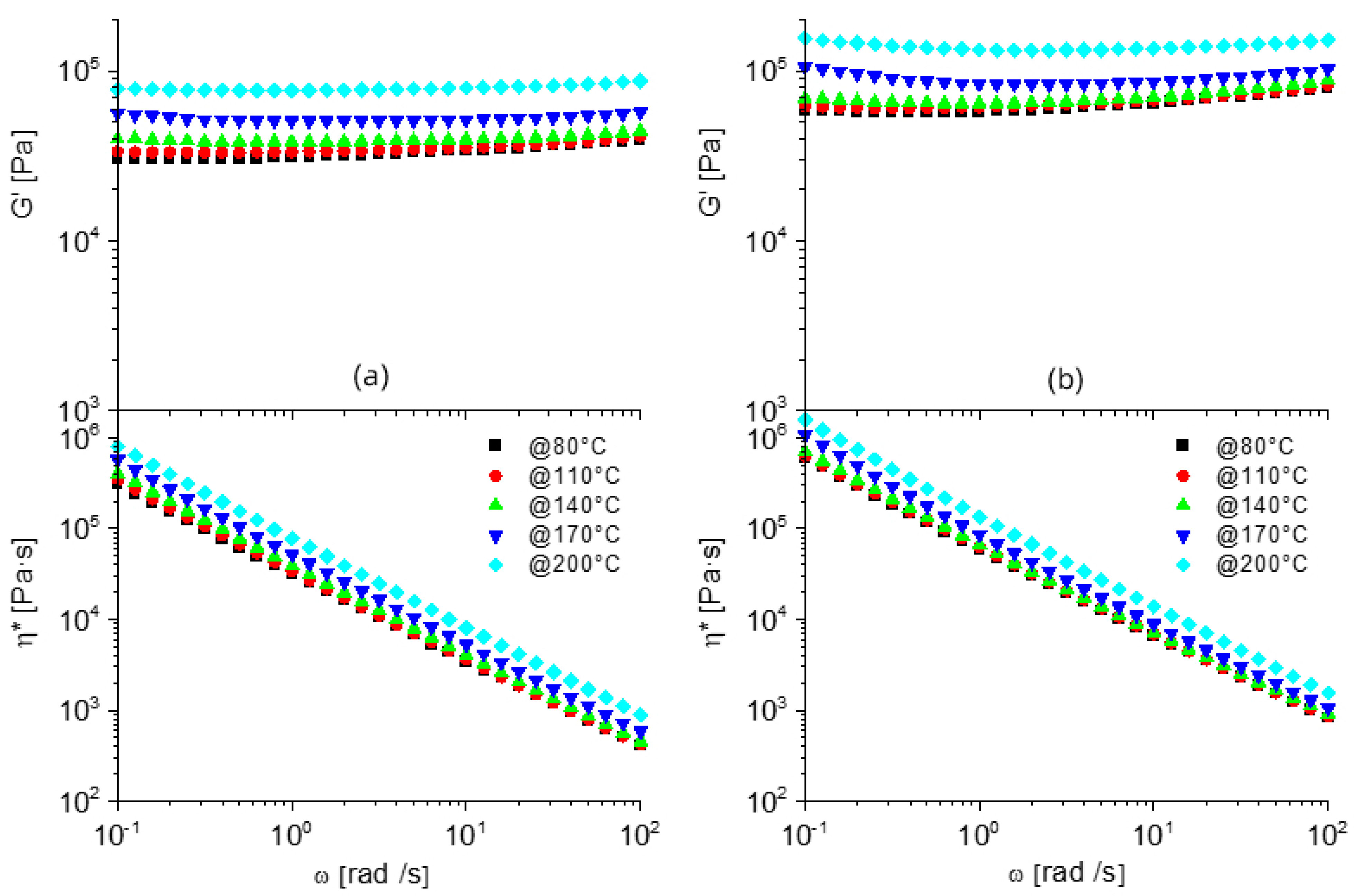
3.1. FT-IR, Swelling, Thermal and Rheological Analysis of Neat Polymers and Dynamic Networks
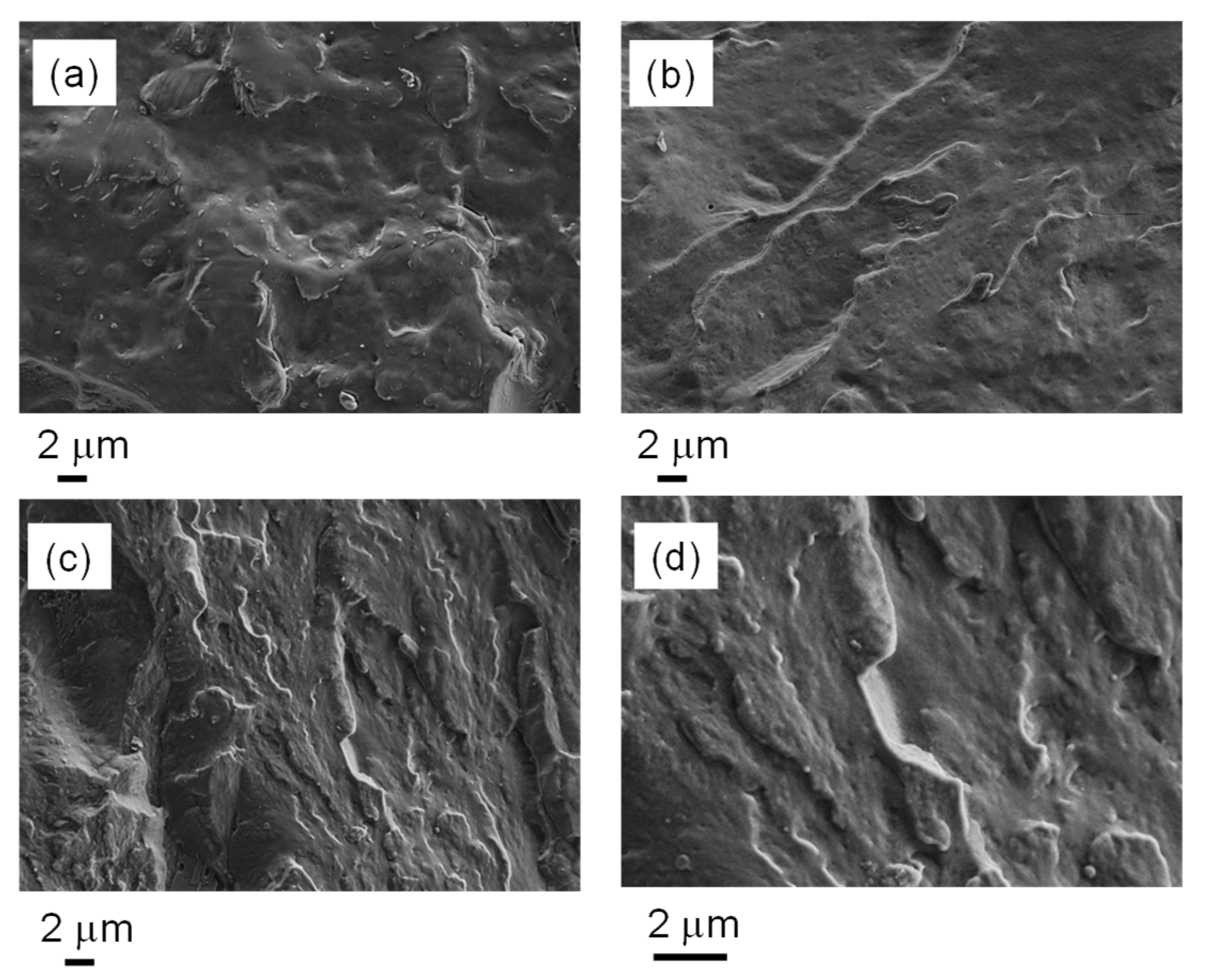
3.2. Morphological, Rheological and Thermal Analysis of Nanostructured Dynamic Networks
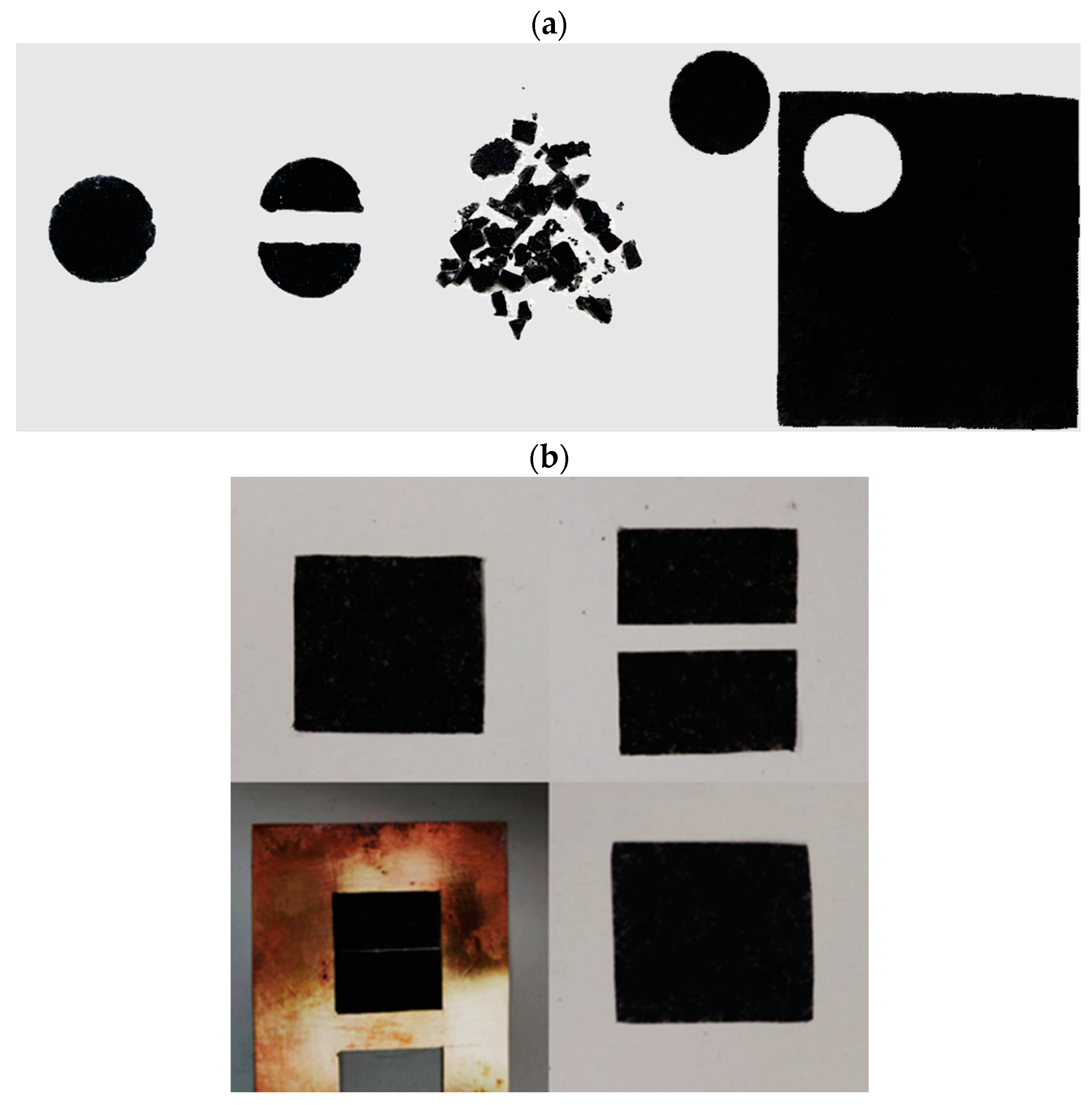
3.3. Recycling and Self-Healing Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Labeta, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Dai, X.-H.; Zhang, H.-D.; Dong, C.-M. Fabrication, biomolecular binding, in vitro drug release behavior of sugar-installed nanoparticles from star poly(3-caprolactone)/glycopolymer biohybrid with a dendrimer core. Polymer 2009, 50, 4626–4634. [Google Scholar] [CrossRef]
- Damonte, G.; Vallin, A.; Battegazzore, D.; Fina, A.; Monticelli, O. Synthesis and characterization of a novel star polycaprolactone to be applied in the development of graphite nanoplates-based nanopapers. React. Funct. Polym. 2021, 167, 105019. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Rafique, I.; Lu, Z. Star-shaped polycaprolactone bearing mussel-inspired catechol end-groups as a promising bio-adhesive. Eur. Polym. J. 2020, 139, 110025. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase structure, compatibility, and toughness of PLA/PCL blends: A review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Holešová, S.; Barabaszová, K.C.; Hundáková, M.; Šcuková, M.; Hrabovská, K.; Joszko, K.; Antonowicz, M.; Gzik-Zroska, B. Development of novel thin polycaprolactone (PCL)/clay nanocomposite films with antimicrobial activity promoted by the study of mechanical, thermal, and surface properties. Polymers 2021, 13, 3193. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Chen, S.; Jiang, G. In situ polymerization approach to poly(ε-caprolactone)-graphene oxide composites. Des. Monomers Polym. 2012, 15, 303–310. [Google Scholar] [CrossRef][Green Version]
- Wang, G.S.; Wei, Z.Y.; Sang, L.; Chen, G.Y.; Zhang, W.X.; Dong, X.F.; Qi, M. Morphology, crystallization and mechanical properties of poly(ε-caprolactone)/graphene oxide nanocomposites. Chin. J. Polym. Sci. 2013, 31, 1148–1160. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Poly(ε-caprolactone)/graphene oxide biocomposites: Mechanical properties and bioactivity. Biomed. Mater. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, Z. Morphology, crystallization behavior, and dynamic mechanical properties of biodegradable poly(ε-caprolactone)/thermally reduced graphene nanocomposites. Ind. Eng. Chem. Res. 2011, 50, 13885–13891. [Google Scholar] [CrossRef]
- Damonte, G.; Vallin, A.; Fina, A.; Monticelli, O. On the development of an effective method to produce conductive PCL film. Nanomaterials 2021, 11, 1385. [Google Scholar] [CrossRef]
- Bagheri, M.; Mahmoodzadeh, A. Polycaprolactone/graphene nanocomposites: Synthesis, characterization and mechanical properties of electrospun nanofibers. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1566–1577. [Google Scholar] [CrossRef]
- Cantamessa, F.; Damonte, G.; Monticelli, O.; Arrigo, R.; Fina, A. Thermoreversible cross-linked rubber prepared via melt blending and its nanocomposites. ACS Appl. Polym. Mater. 2022, 4, 4796–4807. [Google Scholar] [CrossRef] [PubMed]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef]
- Houbben, M.; Thomassin, J.-M.; Jèrôme, C. Supercritical CO2 blown poly(ε-caprolactone) covalent adaptable networks towards unprecedented low density shape memory foams. Mater. Adv. 2022, 3, 2918–2926. [Google Scholar] [CrossRef]
- Caprasse, J.; Riva, R.; Thomassin, J.-M.; Jèrôme, C. Hybrid covalent adaptable networks from cross-reactive poly(e-caprolactone) and poly(ethylene oxide) stars towards advanced shape-memory materials. Mater. Adv. 2021, 2, 7077–7087. [Google Scholar] [CrossRef]
- Cao, Y.; Rong, M.Z.; Zhang, M.Q. Covalent adaptable networks impart smart processability to multifunctional highly filled polymer composites. Compos. Part A Appl. Sci. Manuf. 2021, 151, 106647. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic control of the vitrimer glass transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Albisio, W.; Schlögl, S. The impact of vitrimers on the industry of the future: Chemistry, properties and sustainable forward-looking applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef]
- Joe, J.; Shin, J.; Choi, Y.-S.; Hwang, J.H.; Kim, S.H.; Han, J.; Park, B.; Lee, W.; Park, S.; Kim, Y.S.; et al. A 4D printable shape memory vitrimer with repairability and recyclability through network architecture tailoring from commercial poly(𝝐-caprolactone). Adv. Sci. 2021, 8, 2103682. [Google Scholar] [CrossRef]
- Maddalena, L.; Benselfelt, T.; Gomez, J.; Hamedi, M.M.; Fina, A.; Wagberg, L.; Carosio, F. Polyelectrolyte-Aasisted dispersions of reduced graphite oxide nanoplates in water and their gas-barrier application. ACS Appl. Mater. Interfaces 2021, 13, 43301–43313. [Google Scholar] [CrossRef] [PubMed]
- Colonna, S.; Battegazzore, D.; Eleuteri, M.; Arrigo, R.; Fina, A. Properties of graphene-related materials controlling the thermal conductivity of their polymer nanocomposites. Nanomaterials 2020, 10, 2167. [Google Scholar] [CrossRef]
- Damonte, G.; Maddalena, L.; Fina, A.; Cavallo, D.; Muller, A.J.; Caputo, M.R.; Mariani, A.; Monticelli, O. On novel hydrogels based on poly(2-hydroxyethyl acrylate) and polycaprolactone with improved mechanical properties prepared by frontal polymerization. Eur. Polym. J. 2022, 171, 11122. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 10, 1217–1256. [Google Scholar] [CrossRef]
- Biswas, M.; Libera, J.A.; Darling, S.B.; Elam, J.W. Polycaprolactone: A promising addition to the sequential infiltration synthesis polymer family identified through in situ infrared spectroscopy. ACS Appl. Polym. Mater. 2020, 2, 5501–5510. [Google Scholar] [CrossRef]
- Mandru, M.; Vald, S.; Ciobanu, C.; Lebrun, L.; Popa, M. Polyurethane-hydroxypropyl cellulose membranes for sustained release of nystatin. Cellulose Chem. Technol. 2013, 47, 5–12. [Google Scholar]
- Núñez, E.; Ferrando, C.; Malmström, E.; Claesson, H.; Gedde, U.W. Crystallization behavior and morphology of star polyesters with poly(ε-caprolactone) arms. J. Polym. Sci. B Polym. Phys. 2004, 43, 1143–1160. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Forouharshad, M.; Gardella, L.; Furfaro, D.; Galimberti, M.; Monticelli, O. A low-environmental-impact approach for novel bio-composites based on PLLA/PCL blends and high surface area graphite. Eur. Polym. J. 2015, 70, 28–36. [Google Scholar] [CrossRef]
- Li, K.; Battegazzore, D.; Pérez-Camargo, R.A.; Liu, G.; Monticelli, O.; Müller, A.J.; Fina, A. Polycaprolactone adsorption and nucleation onto graphite nanoplates for highly flexible, thermally conductive, and thermomechanically stiff nanopapers. ACS Appl. Mater. Interfaces 2021, 13, 59206–5922015. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, J.; Hu, G.; Zhang, B. Recycling strategies for vitrimers. Int. J. Smart Nano Mater. 2022, 13, 367–390. [Google Scholar] [CrossRef]
- Vashchuk, A.; Kobzar, Y. Chemical welding of polymer networks. Mater. Today Chem. 2022, 24, 100803. [Google Scholar] [CrossRef]



| Sample Code | Molar Ratio PCL-OH:MDI | Molar Ratio OH:NCO | Temp. [°C] | rGO [wt.-%] |
|---|---|---|---|---|
| PCL-OH_MDI_1:1_120 | 1:1 | 2:1 | 120 | - |
| PCL-OH_MDI_1:1.33_120 | 1:1.33 | 1.5:1 | 120 | - |
| PCL-OH_MDI_1:1_200 | 1:1 | 2:1 | 200 | - |
| PCL-OH_MDI_1:1.33_200 | 1:1.33 | 1.5:1 | 200 | - |
| PCL-OH_MDI_1:1_120_G | 1:1 | 2:1 | 120 | 1 |
| PCL-OH_MDI_1:1.33_120_G | 1:1.33 | 1.5:1 | 120 | 1 |
| PCL-OH_MDI_1:1_200_G | 1:1 | 2:1 | 200 | 1 |
| PCL-OH_MDI_1:1.33_200_G | 1:1.33 | 1.5:1 | 200 | 1 |
| Sample Code | GF [%] | SR [%] |
|---|---|---|
| PCL-OH_MDI_1:1_120 | 24 | 2600 |
| PCL-OH_MDI_1:1.33_120 | 43 | 3300 |
| PCL-OH_MDI_1:1_200 | 36 | 2800 |
| PCL-OH_MDI_1:1.33_200 | 73 | 3200 |
| Sample Code | ΔHc [J/g] | Tc [°C] | ΔHm [J/g] | Tm [°C] | Xc [%] |
|---|---|---|---|---|---|
| PCL | −65 | 30 | 69 | 57 | 50 |
| PCL-OH | −71 | 28 | 80 | 49 | 58 |
| PCL-OH_MDI_1:1_120 | −72 | 19 | 76 | 52 | 55 |
| PCL-OH_MDI_1:1.33_120 | −64 | 17 | 68 | 52 | 49 |
| PCL-OH_MDI_1:1_200 | −69 | 23 | 76 | 49 | 55 |
| PCL-OH_MDI_1:1.33_200 | −60 | 17 | 64 | 49 | 46 |
| Sample Code | ΔHc [J/g] | Tc [°C] | ΔHm [J/g] | Tm [°C] | χc [%] |
|---|---|---|---|---|---|
| PCL | −65 | 30 | 69 | 57 | 50 |
| PCL-OH | −71 | 28 | 80 | 49 | 58 |
| PCL-G | −67 | 34 | 75 | 56 | 54 |
| PCL-OH_MDI_1:1_200_G | −72 | 26 | 77 | 53 | 55 |
| PCL-OH_MDI_1:1.33_200_G | −74 | 28 | 76 | 50 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallin, A.; Battegazzore, D.; Damonte, G.; Fina, A.; Monticelli, O. On the Development of Nanocomposite Covalent Associative Networks Based on Polycaprolactone and Reduced Graphite Oxide. Nanomaterials 2022, 12, 3744. https://doi.org/10.3390/nano12213744
Vallin A, Battegazzore D, Damonte G, Fina A, Monticelli O. On the Development of Nanocomposite Covalent Associative Networks Based on Polycaprolactone and Reduced Graphite Oxide. Nanomaterials. 2022; 12(21):3744. https://doi.org/10.3390/nano12213744
Chicago/Turabian StyleVallin, Alberto, Daniele Battegazzore, Giacomo Damonte, Alberto Fina, and Orietta Monticelli. 2022. "On the Development of Nanocomposite Covalent Associative Networks Based on Polycaprolactone and Reduced Graphite Oxide" Nanomaterials 12, no. 21: 3744. https://doi.org/10.3390/nano12213744
APA StyleVallin, A., Battegazzore, D., Damonte, G., Fina, A., & Monticelli, O. (2022). On the Development of Nanocomposite Covalent Associative Networks Based on Polycaprolactone and Reduced Graphite Oxide. Nanomaterials, 12(21), 3744. https://doi.org/10.3390/nano12213744








