Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Characterization
3. Results and Discussion
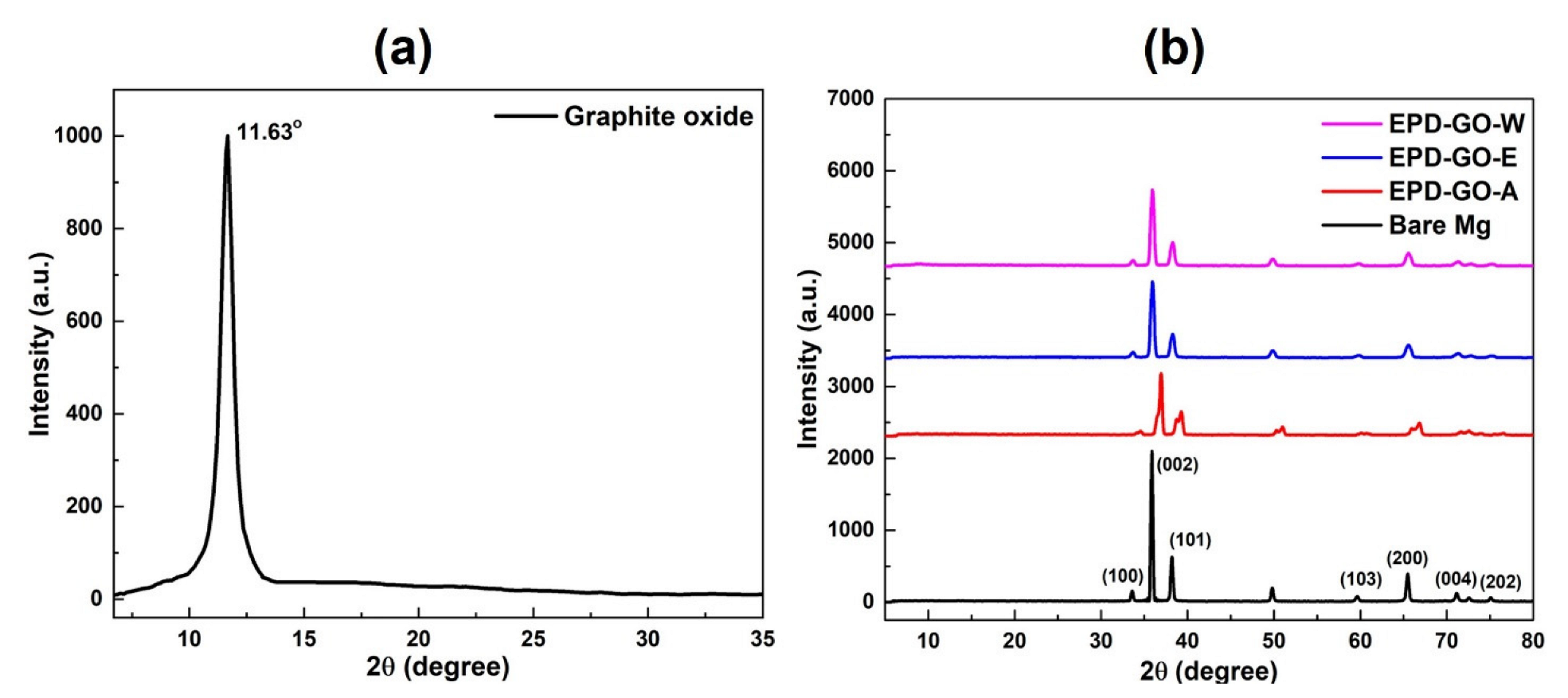
3.1. XRD
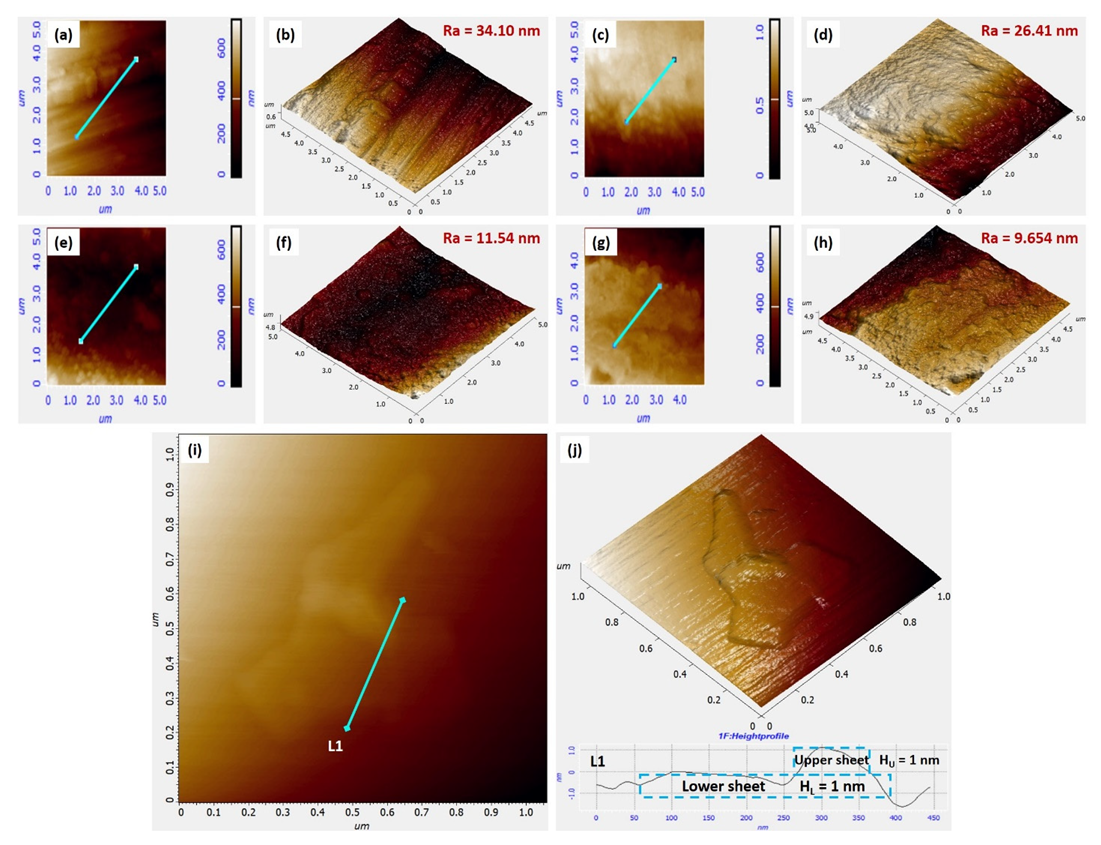
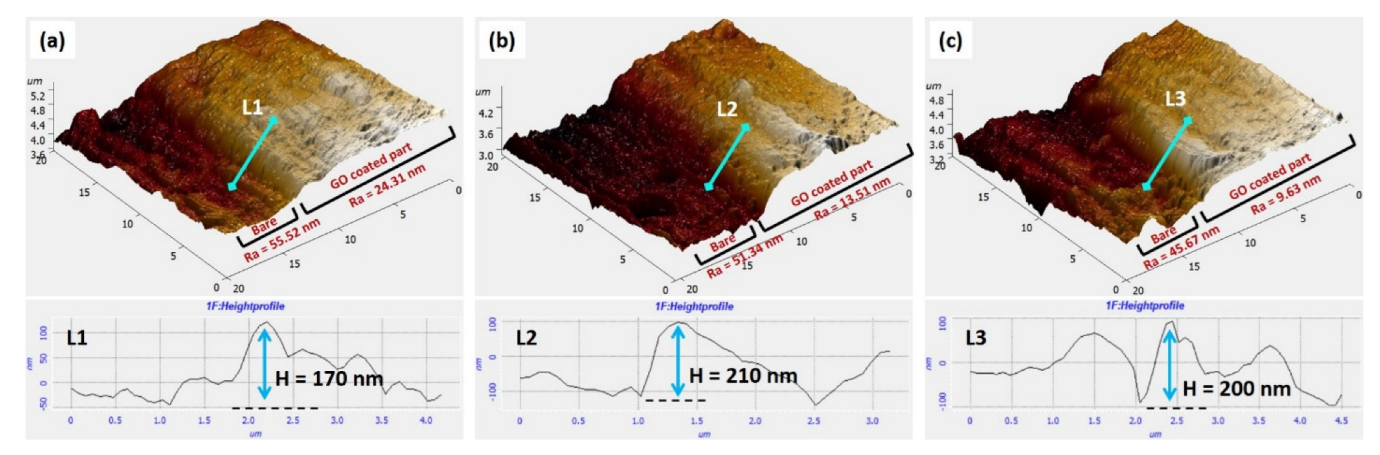
3.2. AFM
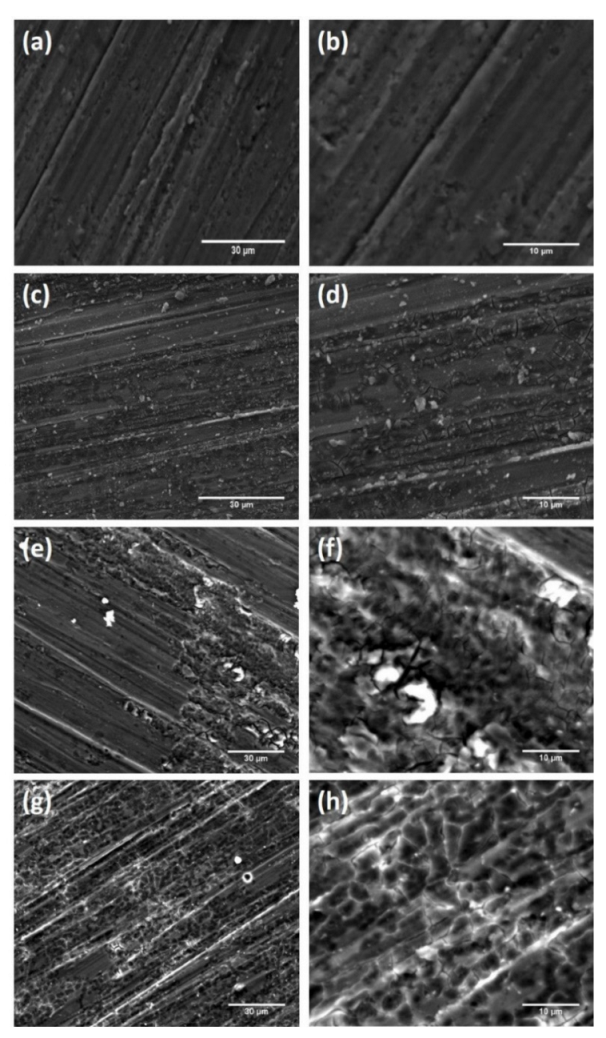
3.3. SEM
3.4. Electrochemical Testing
3.4.1. Tafel
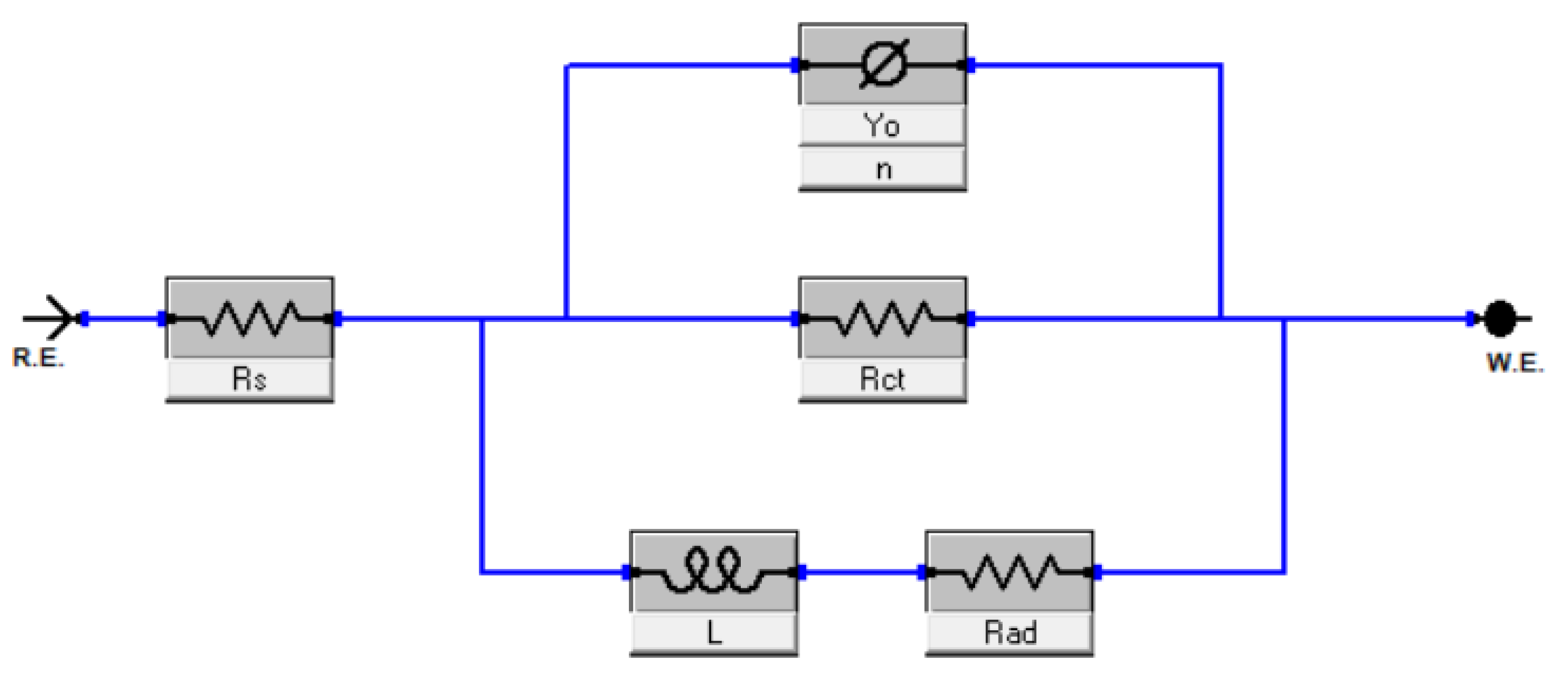
3.4.2. Electrochemical Impedance Spectroscopy (EIS)
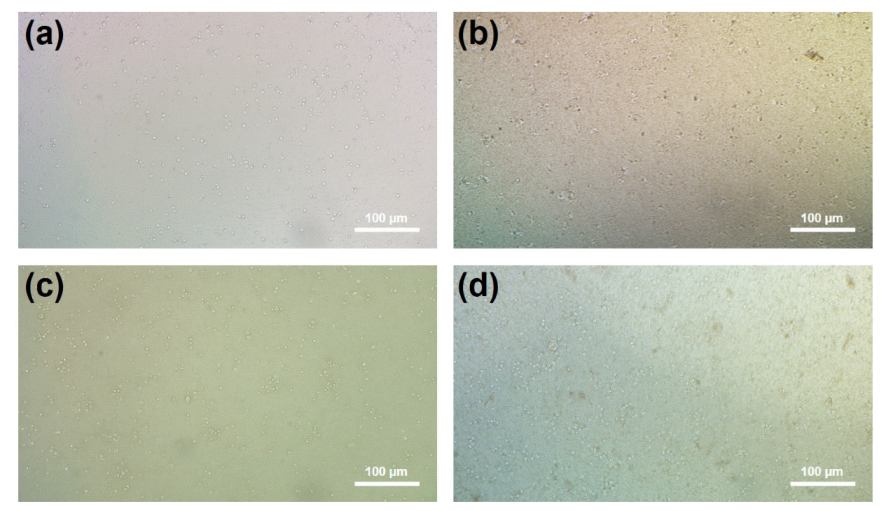
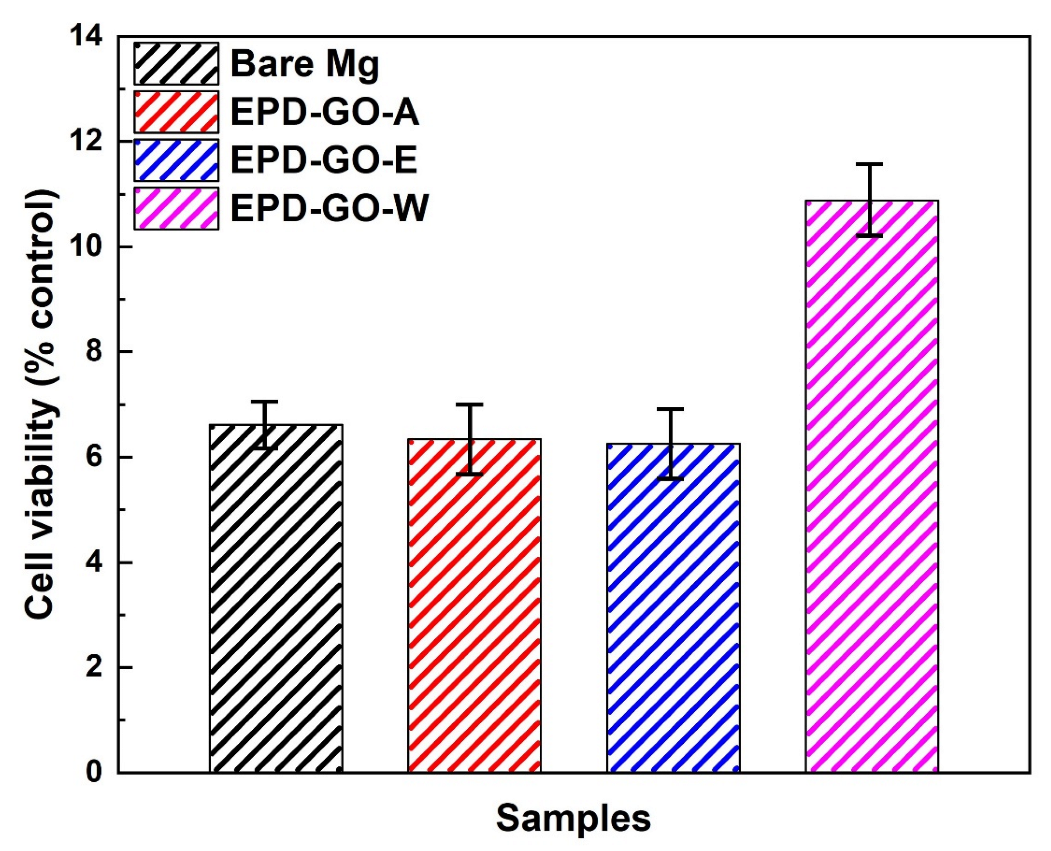
3.5. Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, D.; Liu, X.; Zhang, Y. Recent progress in superhydrophobic coating on Mg alloys: A general review. J. Magnes. Alloy. 2021, 9, 1471–1486. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloy. 2021, 10, 627–669. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of magnesium and its alloys: A review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.; Coy, A.E.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion behaviour of magnesium/aluminium alloys in 3.5 wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng. C 2015, 46, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y.; Qin, L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, J.; Martinelli, E.; Weinberg, A.M.; Becker, M.; Mingler, B.; Uggowitzer, P.J.; Löffler, J.F. Assessing the degradation performance of ultrahigh-purity magnesium in vitro and in vivo. Corros. Sci. 2015, 91, 29–36. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R. Enhancing corrosion and wear performance of PEO coatings on Mg alloys using graphene and graphene oxide additions: A review. FlatChem 2021, 27, 100241. [Google Scholar] [CrossRef]
- Saadati, A.; Khiarak, B.N.; Zahraei, A.A.; Nourbakhsh, A.; Mohammadzadeh, H. Electrochemical characterization of electrophoretically deposited hydroxyapatite/chitosan/graphene oxide composite coating on Mg substrate. Surf. Interfaces 2021, 25, 101290. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Etim, I.P.; Tan, L.; Yang, K.; Misra, R.; Wang, J.; Su, X. Recent Advances on Development of Hydroxyapatite Coating on Biodegradable Magnesium Alloys: A Review. Materials 2021, 14, 5550. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Superior corrosion protection and adhesion strength of epoxy coating applied on AZ31 magnesium alloy pre-treated by PEO/Silane with inorganic and organic corrosion inhibitors. Corros. Sci. 2021, 178, 109065. [Google Scholar] [CrossRef]
- Johari, N.; Alias, J.; Zanurin, A.; Mohamed, N.; Alang, N.; Zain, M. Recent progress of self-healing coatings for magnesium alloys protection. J. Coat. Technol. Res. 2022, 19, 757–774. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, T.; Liu, Z.; Pu, J.; Liu, W.; Zhao, H.; Li, X.; Wang, L. Superior corrosion resistance and self-healable epoxy coating pigmented with silanzied trianiline-intercalated graphene. Carbon 2019, 142, 164–176. [Google Scholar] [CrossRef]
- Ghauri, F.A.; Raza, M.A.; Baig, M.S.; Ibrahim, S. Corrosion study of the graphene oxide and reduced graphene oxide-based epoxy coatings. Mater. Res. Express 2017, 4, 125601. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Wang, Q.-q.; Wen, M.-l.; Tang, T.-t.; Liu, Y.; Yuan, R.; Li, Y.-f.; An, M.-w. Research progress on the biomedical uses of graphene and its derivatives. N. Carbon Mater. 2021, 36, 779–793. [Google Scholar] [CrossRef]
- Raza, M.A.; Maqsood, M.F.; Rehman, Z.U.; Westwood, A.; Inam, A.; Sattar, M.M.S.; Ghauri, F.A.; Ilyas, M.T. Thermally Reduced Graphene Oxide-Reinforced Acrylonitrile Butadiene Styrene Composites Developed by Combined Solution and Melt Mixing Method. Arab. J. Sci. Eng. 2020, 45, 9559–9568. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of ultra-narrow band graphene refractive index sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Kotov, N.A. Materials science: Carbon sheet solutions. Nature 2006, 442, 254–255. [Google Scholar] [CrossRef] [Green Version]
- Hoche, H.; Groß, S.; Oechsner, M. Development of new PVD coatings for magnesium alloys with improved corrosion properties. Surf. Coat. Technol. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Kang, Z.; Lai, X.; Sang, J.; Li, Y. Fabrication of hydrophobic/super-hydrophobic nanofilms on magnesium alloys by polymer plating. Thin Solid Films 2011, 520, 800–806. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—a critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, H.; Yao, B.; Qin, Z.; Zhang, Q. Corrosion resistance property of the ceramic coating obtained through microarc oxidation on the AZ31 magnesium alloy surfaces. Surf. Coat. Technol. 2007, 201, 4905–4908. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A. Biomedical coatings on magnesium alloys–a review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.F.; Raza, M.A.; Ghauri, F.A.; Rehman, Z.U.; Ilyas, M.T. Corrosion study of graphene oxide coatings on AZ31B magnesium alloy. J. Coat. Technol. Res. 2020, 17, 1321–1329. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.; Li, H.; Zhao, R.; Li, G.; Zhang, S.; Ding, L.; Cui, X.; Zhao, Y.; Zhang, R. Performance and failure process of green recycling solutions for preparing high degradation resistance coating on biomedical magnesium alloys. Green Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Raza, M.A.; Rehman, Z.U.; Ghauri, F.A. Corrosion study of silane-functionalized graphene oxide coatings on copper. Thin Solid Films 2018, 663, 93–99. [Google Scholar] [CrossRef]
- An, S.J.; Zhu, Y.; Lee, S.H.; Stoller, M.D.; Emilsson, T.; Park, S.; Velamakanni, A.; An, J.; Ruoff, R.S. Thin film fabrication and simultaneous anodic reduction of deposited graphene oxide platelets by electrophoretic deposition. J. Phys. Chem. Lett. 2010, 1, 1259–1263. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Paredes, J.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar]
- Raza, M.A.; Rehman, Z.U.; Ghauri, F.A.; Ahmad, A.; Ahmad, R.; Raffi, M. Corrosion study of electrophoretically deposited graphene oxide coatings on copper metal. Thin Solid Films 2016, 620, 150–159. [Google Scholar] [CrossRef]
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R. No cytotoxicity or genotoxicity of graphene and graphene oxide in murine lung epithelial FE1 cells in vitro. Environ. Mol. Mutagenesis 2016, 57, 469–482. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Carpio, I.E.M.; Mangadlao, J.D.; Nguyen, H.N.; Advincula, R.C.; Rodrigues, D.F. Graphene oxide functionalized with ethylenediamine triacetic acid for heavy metal adsorption and anti-microbial applications. Carbon 2014, 77, 289–301. [Google Scholar] [CrossRef]
- Ordikhani, F.; Farani, M.R.; Dehghani, M.; Tamjid, E.; Simchi, A. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity. Carbon 2015, 84, 91–102. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.F.; Zubair, M.A.A.; Raza, M.A.; Mehdi, S.M.Z.; Lee, N.; Rehman, Z.U.; Park, K.; Bhatti, M.U.; Latif, U.; Tawakkal, A. Fabrication and characterization of graphene oxide and glass fiber-based hybrid epoxy composites. Polym. Compos. 2022. [Google Scholar] [CrossRef]
- Masood Chaudry, U.; Farooq, A.; Malik, A.; Nabeel, M.; Sufyan, M.; Tayyeb, A.; Asif, S.; Inam, A.; Elbalaawy, A.; Hafez, E. Biodegradable properties of AZ31-0.5 Ca magnesium alloy. Mater. Technol. 2022, 37, 2230–2241. [Google Scholar] [CrossRef]
- Kartick, B.; Srivastava, S. Green synthesis of graphene. J. Nanosci. Nanotechnol. 2013, 13, 4320–4324. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Du, C. 1Influence of graphene oxide additive on the tribological and electrochemical corrosion properties of a PEO coating prepared on AZ31 magnesium alloy. Tribol. Int. 2020, 146, 106135. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Titovich, K.A.; Ivanovich, K.V.; Vyacheslavovich, S.A.; Dash, S. Lubrication properties of chemically aged reduced graphene-oxide additives. Surf. Interfaces 2017, 7, 6–13. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, R.; Wu, J.; Zhang, Y.; Qu, Y.; Wu, X. Graphene oxide as a corrosion-inhibitive coating on magnesium alloys. RSC Adv. 2015, 5, 44149–44159. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Zhang, C. Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci. 2017, 114, 146–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Liu, Z.; Wang, X.; Du, C. Influence of graphene oxide on the antiwear and antifriction performance of MAO coating fabricated on MgLi alloy. Surf. Coat. Technol. 2019, 364, 144–156. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Takadoum, J.; Bennani, H.H. Influence of substrate roughness and coating thickness on adhesion, friction and wear of TiN films. Surf. Coat. Technol. 1997, 96, 272–282. [Google Scholar] [CrossRef]
- Raza, M.A.; Ali, A.; Ghauri, F.A.; Aslam, A.; Yaqoob, K.; Wasay, A.; Raffi, M. Electrochemical behavior of graphene coatings deposited on copper metal by electrophoretic deposition and chemical vapor deposition. Surf. Coat. Technol. 2017, 332, 112–119. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, P.; Wang, X.; Wang, Z.; Liu, D.; Yang, B.; Cao, W. CVD growth of large area and uniform graphene on tilted copper foil for high performance flexible transparent conductive film. J. Mater. Chem. 2012, 22, 18283–18290. [Google Scholar] [CrossRef]
- Diba, M.; García-Gallastegui, A.; Taylor, R.N.K.; Pishbin, F.; Ryan, M.P.; Shaffer, M.S.; Boccaccini, A.R. Quantitative evaluation of electrophoretic deposition kinetics of graphene oxide. Carbon 2014, 67, 656–661. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. Electrochem. Methods 2001, 2, 580–632. [Google Scholar]
- Wei, Y.; Wang, J.; Jia, X. Electrochemical Studies of Corrosion Inhibiting Effect of Polyaniline Coatings; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Rafique, M.; Afzal, N.; Mukhtar, R.; Younas, I.; Bashir, S.; Imran, M.; Mahmood, K.; Farooq, A. Surface and Structural Modifications of Tungsten by Laser Irradiation for Enhanced Electrochemical Corrosion Resistance. J. Mater. Eng. Perform. 2021, 31, 1904–1913. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Rahimi, S.; Yarmand, B.; Mozafari, M. Electrophoretic deposition of graphene oxide on plasma electrolytic oxidized-magnesium implants for bone tissue engineering applications. Mater. Today Proc. 2018, 5, 15603–15612. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Wang, D.; Liu, L.; Zhang, Y.; Liu, X. Enhanced corrosion resistance and biocompatibility of magnesium alloy by hydroxyapatite/graphene oxide bilayer coating. Mater. Lett. 2020, 264, 127322. [Google Scholar] [CrossRef]
- Soliman, H.; Qian, J.; Tang, S.; Chen, Y.; Makhlouf, A.-S.; Wan, G. Hydroxyquinoline/nano-graphene oxide composite coating of self-healing functionality on treated Mg alloys AZ31. Surf. Coat. Technol. 2020, 385, 125395. [Google Scholar] [CrossRef]
- Deng, Y.; Xia, L.; Song, G.-L.; Zhao, Y.; Zhang, Y.; Xu, Y.; Zheng, D. Development of a curcumin-based antifouling and anticorrosion sustainable polybenzoxazine resin composite coating. Compos. Part B Eng. 2021, 225, 109263. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance spectroscopy: Emphasizing solid materials and systems. Appl. Opt. 1989, 28, 1083. [Google Scholar]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.; Chu, P.K. Electrochemical corrosion behavior of biodegradable Mg–Y–RE and Mg–Zn–Zr alloys in Ringer’s solution and simulated body fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Li, J.; Shi, H.; Liu, F.; Han, E.-H. Self-healing epoxy coating based on tung oil-containing microcapsules for corrosion protection. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Chen, Y.; Zhong, Z.; Ci, W.; Wu, J.; Xie, Z.; Yuan, Y.; Pan, F. A self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating. Corros. Sci. 2022, 194, 109941. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, T.; Xu, Y.; Guo, Y.; Li, G.; Lian, J. A polydopamine-based calcium phosphate/graphene oxide composite coating on magnesium alloy to improve corrosion resistance and biocompatibility for biomedical applications. Materialia 2022, 21, 101315. [Google Scholar] [CrossRef]
- Malik, M.U.; Tabish, M.; Yasin, G.; Anjum, M.J.; Jameel, S.; Tang, Y.; Zhang, X.; Manzoor, S.; Ibraheem, S.; Khan, W.Q. Electroless codeposition of GO incorporated silane nanocomposite coating onto AZ91 Mg alloy: Effect of GO content on its morphology, mechanical and corrosion protection properties. J. Alloys Compd. 2021, 883, 160790. [Google Scholar] [CrossRef]
- Tabish, M.; Malik, M.U.; Khan, M.A.; Anjum, M.J.; Muhammad, N.; Ahmad, A.; Ibraheem, S.; Kumar, A.; Nguyen, T.A.; Yasin, G. Boosting the hydrophobicity and mechanical properties of fluoroalkylsilane hydrolyzed 3-glycidyloxypropyl/graphene oxide-based nanocomposite coating for enhanced corrosion resistance. Thin Solid Films 2022, 756, 139373. [Google Scholar] [CrossRef]
- Gao, F.; Xu, C.; Hu, H.; Wang, Q.; Gao, Y.; Chen, H.; Guo, Q.; Chen, D.; Eder, D. Biomimetic synthesis and characterization of hydroxyapatite/graphene oxide hybrid coating on Mg alloy with enhanced corrosion resistance. Mater. Lett. 2015, 138, 25–28. [Google Scholar] [CrossRef]
- Askarnia, R.; Fardi, S.R.; Sobhani, M.; Staji, H.; Aghamohammadi, H. Effect of graphene oxide on properties of AZ91 magnesium alloys coating developed by micro-arc oxidation process. J. Alloys Compd. 2022, 892, 162106. [Google Scholar] [CrossRef]
- Askarnia, R.; Ghasemi, B.; Fardi, S.R.; Adabifiroozjaei, E. Improvement of tribological, mechanical and chemical properties of Mg alloy (AZ91D) by electrophoretic deposition of alumina/GO coating. Surf. Coat. Technol. 2020, 403, 126410. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, H.; Zhao, R.; Deng, X.; Chen, G.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhang, K. Construction of a magnesium hydroxide/graphene oxide/hydroxyapatite composite coating on Mg–Ca–Zn–Ag alloy to inhibit bacterial infection and promote bone regeneration. Bioact. Mater. 2022, 18, 354–367. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y.; Gao, H. Improving the corrosion resistance and osteogenic differentiation of ZK60 magnesium alloys by hydroxyapatite/graphene/graphene oxide composite coating. Ceram. Int. 2022, 48, 16131–16141. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, J.; Xu, C.; Wang, X.; Song, S.; Jiang, Z.; Kamado, S.; Cheng, L.; Zhang, H. Enhanced corrosion and wear resistances by graphene oxide coating on the surface of Mg-Zn-Ca alloy. Carbon 2016, 109, 340–351. [Google Scholar] [CrossRef]
- Chu, J.; Tong, L.; Wen, M.; Jiang, Z.; Zou, D.; Liu, S.; Zhang, H. Inhibited corrosion activity of biomimetic graphene-based coating on Mg alloy through a cerium intermediate layer. Carbon 2020, 161, 577–589. [Google Scholar] [CrossRef]
- Xue, Z.; Li, X.; Chu, J.; Li, M.; Zou, D.; Tong, L. Greatly enhanced corrosion/wear resistances of epoxy coating for Mg alloy through a synergistic effect between functionalized graphene and insulated blocking layer. J. Magnes. Alloy. 2022, in press. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rew. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
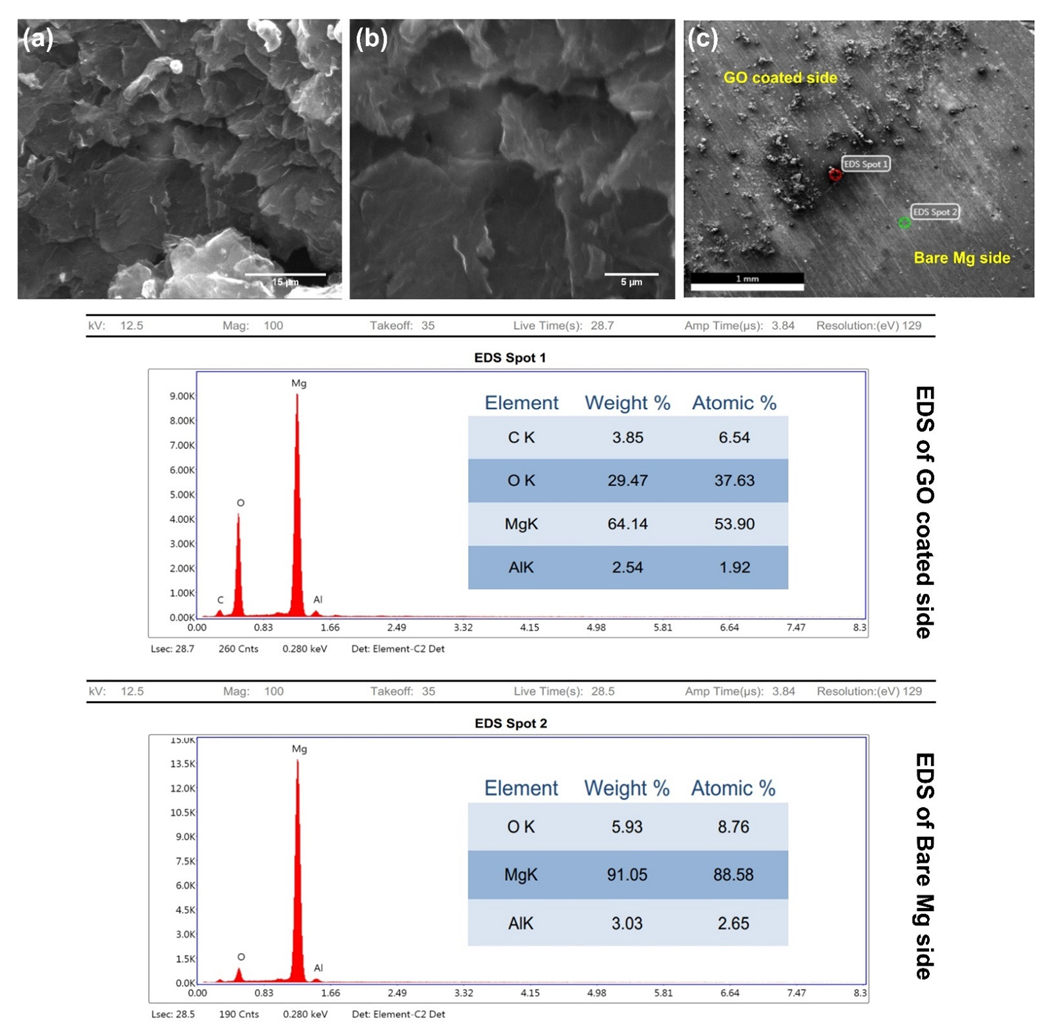
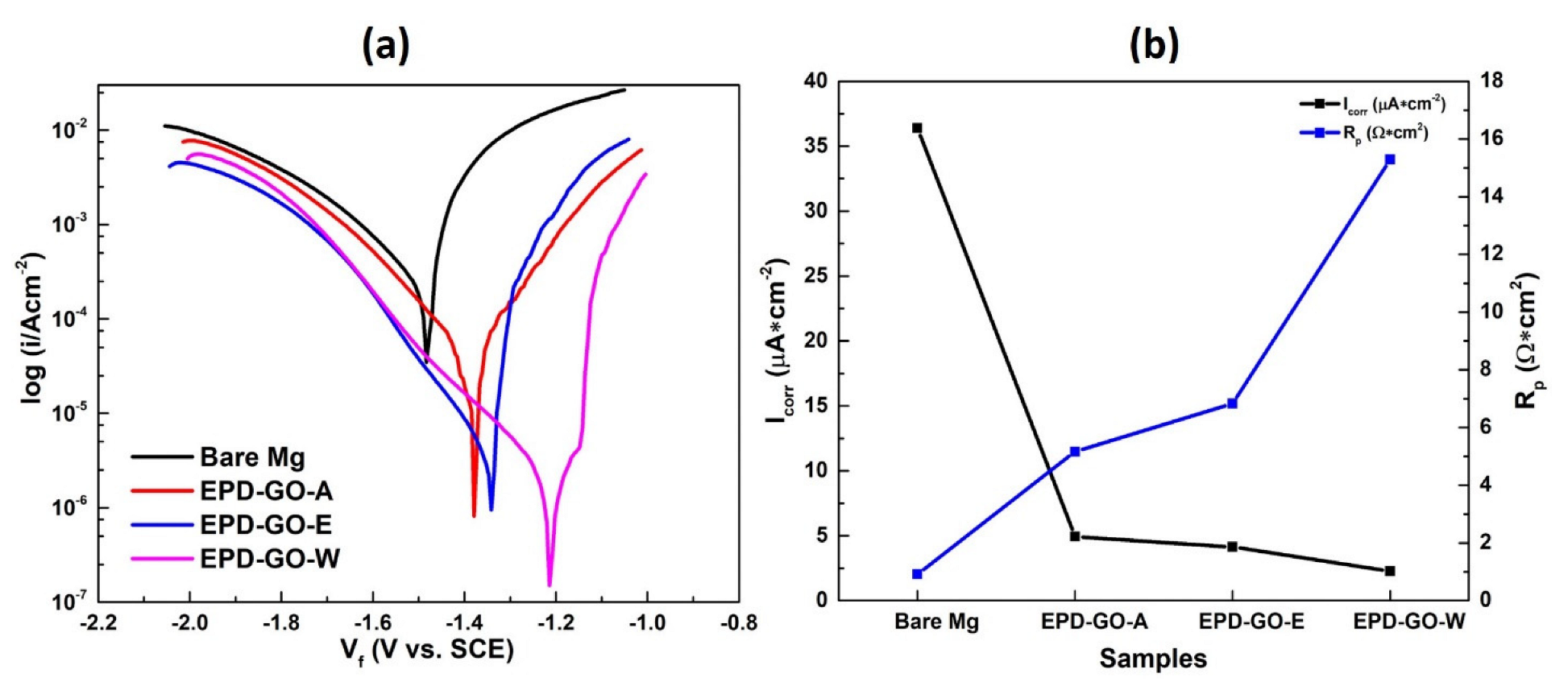


| Sample | βa (mV/decade) | βc (mV/decade) | Icorr (μA/cm2) | Εcorr (V) | Rp (Ω × cm2) | Avg. Corrosion Rate (mpy) | η (%) |
|---|---|---|---|---|---|---|---|
| Bare Mg | 100.9 | 314.7 | 36.38 ± 0.62 | −1.48 | 0.916 | 32.39 | --- |
| EPD-GO-A | 91.50 | 163.5 | 4.930 ± 0.079 | −1.40 | 5.167 | 4.573 | 86.46 |
| EPD-GO-E | 109.5 | 160.8 | 4.140 ± 0.043 | −1.34 | 6.832 | 3.678 | 88.63 |
| EPD-GO-W | 128.4 | 209.4 | 2.260 ± 0.021 | −1.21 | 15.29 | 2.004 | 93.79 |
| Sample | Rs (Ω × cm2) | Rct (Ω × cm2) | Rad (Ω × cm2) | L (H × cm2) | Yo (µS × sa/cm2) | n |
|---|---|---|---|---|---|---|
| Bare Mg | 17.29 | 150.8 | 93.92 | 63.88 | 32.89 | 0.91 ± 0.06 |
| EPD-GO-A | 35.10 | 242.9 | 135.7 | 159.5 | 31.19 | 0.87 ± 0.03 |
| EPD-GO-E | 56.32 | 601.2 | 190.4 | 428.9 | 18.45 | 0.85 ± 0.07 |
| EPD-GO-W | 28.96 | 873.5 | 299.2 | 708.3 | 10.41 | 0.93 ± 0.05 |
| Mg Alloy | Composites | Coating Technique | Electrolyte (wt.%) | Electrochemical Result | Ref. |
|---|---|---|---|---|---|
| AZ31B | GO-A, GO-E, GO-W | Electrophoretic deposition | Ringer’s lactate | ~6× increase in Rct for EPD-GO-W | This work |
| AZ31 | MAO-LDHs/8-HQ@GO | Ring-opening reaction, micro-arc oxidation and hydrothermal chemical transformation | 3.5% NaCl | ~1.5× increase in Rct even after 14 days | [73] |
| AZ31B | GO | Electrophoretic deposition | Ringer’s lactate | Decreased corrosion rate ~16× | [32] |
| AZ60 | PDA/CaP/GO | Biomimetic deposition and spin-coating | SBF | ~27× increase in Rct | [74] |
| AZ91 | GPTMS/GO | Electroless co-deposition | 3.5% NaCl | ~5× increase in Rct | [75] |
| AZ91 | GPTMS/GO/FAS | Electroless co-deposition | 3.5% NaCl | ~100% corrosion protection efficiency | [76] |
| AZ91 | HA/GO | Biomimetic method | SBF | Improves corrosion resistance due to positive shift of polarization curves | [77] |
| AZ91 | GO | Micro-arc oxidation process | SBF | ~2.5× increase in Rct | [78] |
| AZ91D | Alumina/GO | Electrophoretic deposition | 3.5% NaCl | ~17.5× increase in Rct | [79] |
| ZQ71 | Mg(OH)2/GO/HA | Electrophoretic and electrochemical deposition | PBS | ~98% corrosion protection efficiency | [80] |
| ZK60 | HA/G/GO | Hydrothermal method | PBS | Decreased corrosion rate ~28.5× | [81] |
| Mg-5.7Zn-0.8Ca alloy | APTES/GO | Hydrolysis process and silane agent | 3.5% NaCl | ~3× increase in Rct | [82] |
| Mg-4Zn- 4Sn-0.6Ca-0.5Mn | HA/chitosan/GO | Electrophoretic deposition | SBF | ~2× increase in Rct | [16] |
| Mg-6.0Zn-0.5Ca alloy | Ce(Ⅳ)/GO/PVA | Spin-casting method | 3.5% NaCl | ~2× increase in Rct | [83] |
| Mg-3.0Zn-0.5Ca alloy | Ce/WEP/GO | Spin-coating | 3.5% NaCl | Decreased corrosion rate ~550× | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Tayyeb, A.; Makhdoom, M.A.; Ghafoor, F.; Latif, U.; Khan, M.F. Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials 2022, 12, 3745. https://doi.org/10.3390/nano12213745
Maqsood MF, Raza MA, Rehman ZU, Tayyeb A, Makhdoom MA, Ghafoor F, Latif U, Khan MF. Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials. 2022; 12(21):3745. https://doi.org/10.3390/nano12213745
Chicago/Turabian StyleMaqsood, Muhammad Faheem, Mohsin Ali Raza, Zaeem Ur Rehman, Asima Tayyeb, Muhammad Atif Makhdoom, Faisal Ghafoor, Umar Latif, and Muhammad Farooq Khan. 2022. "Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis" Nanomaterials 12, no. 21: 3745. https://doi.org/10.3390/nano12213745
APA StyleMaqsood, M. F., Raza, M. A., Rehman, Z. U., Tayyeb, A., Makhdoom, M. A., Ghafoor, F., Latif, U., & Khan, M. F. (2022). Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials, 12(21), 3745. https://doi.org/10.3390/nano12213745








