Surface Structure Engineering of PdAg Alloys with Boosted CO2 Electrochemical Reduction Performance
Abstract
:1. Introduction
2. Materials and Methods
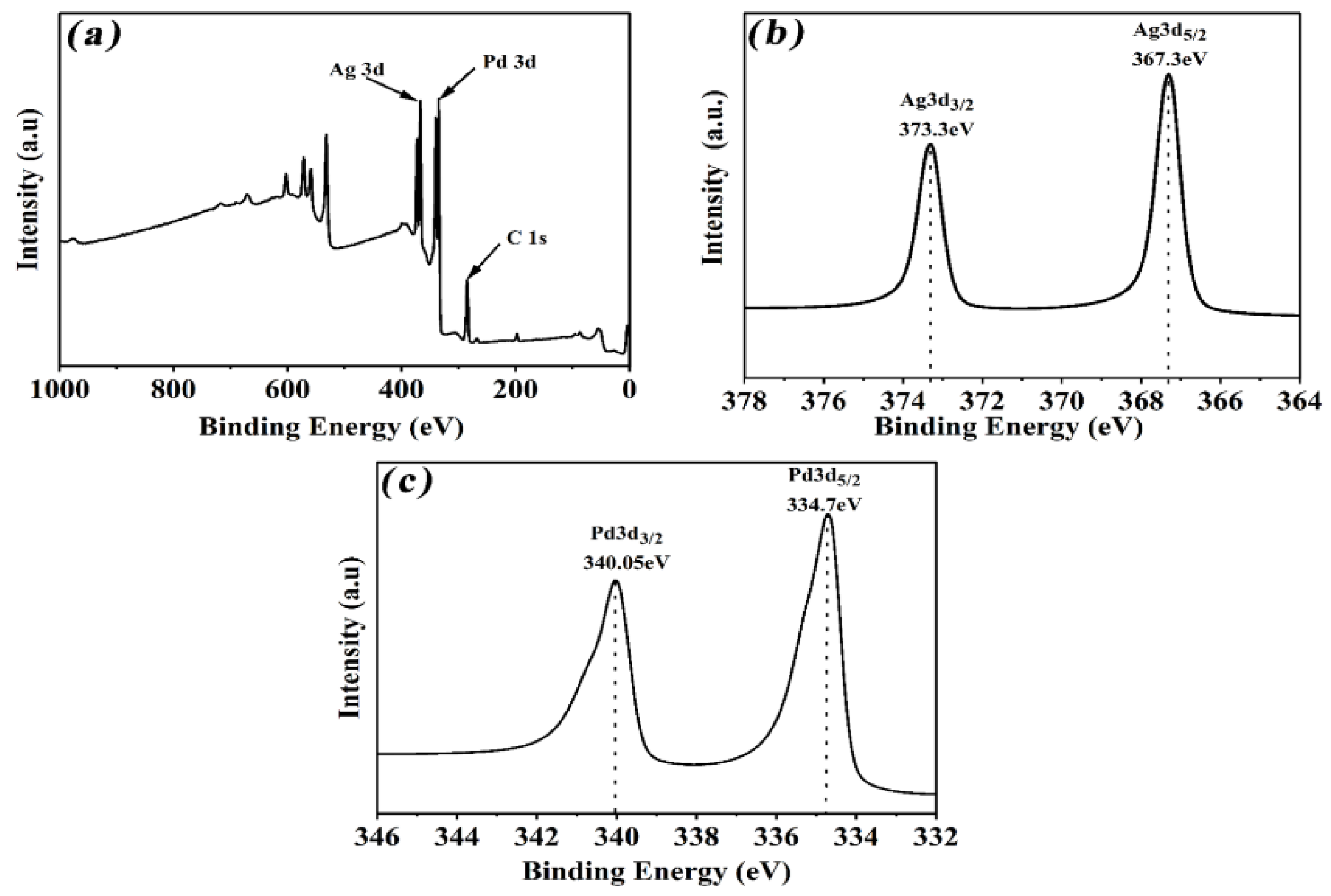
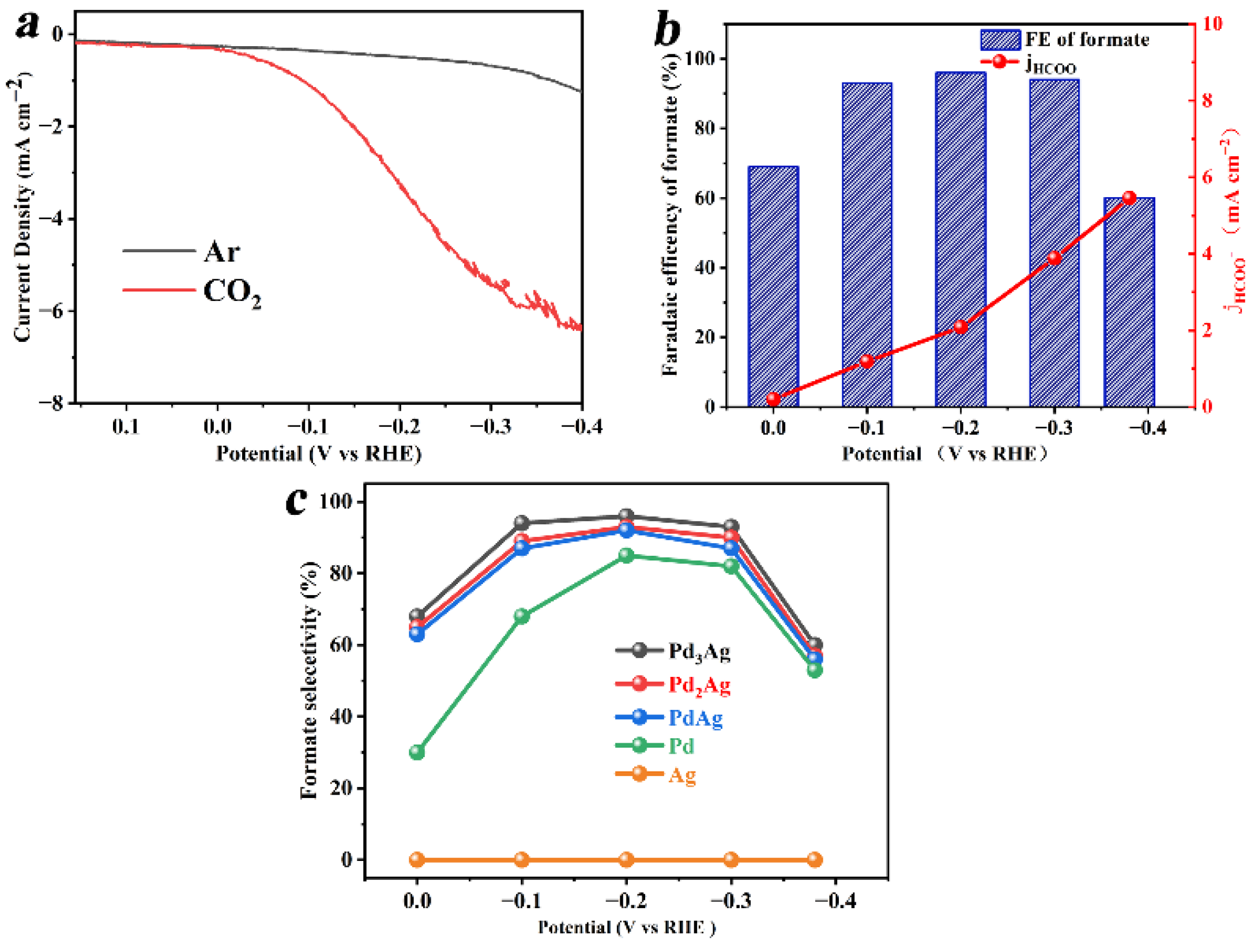
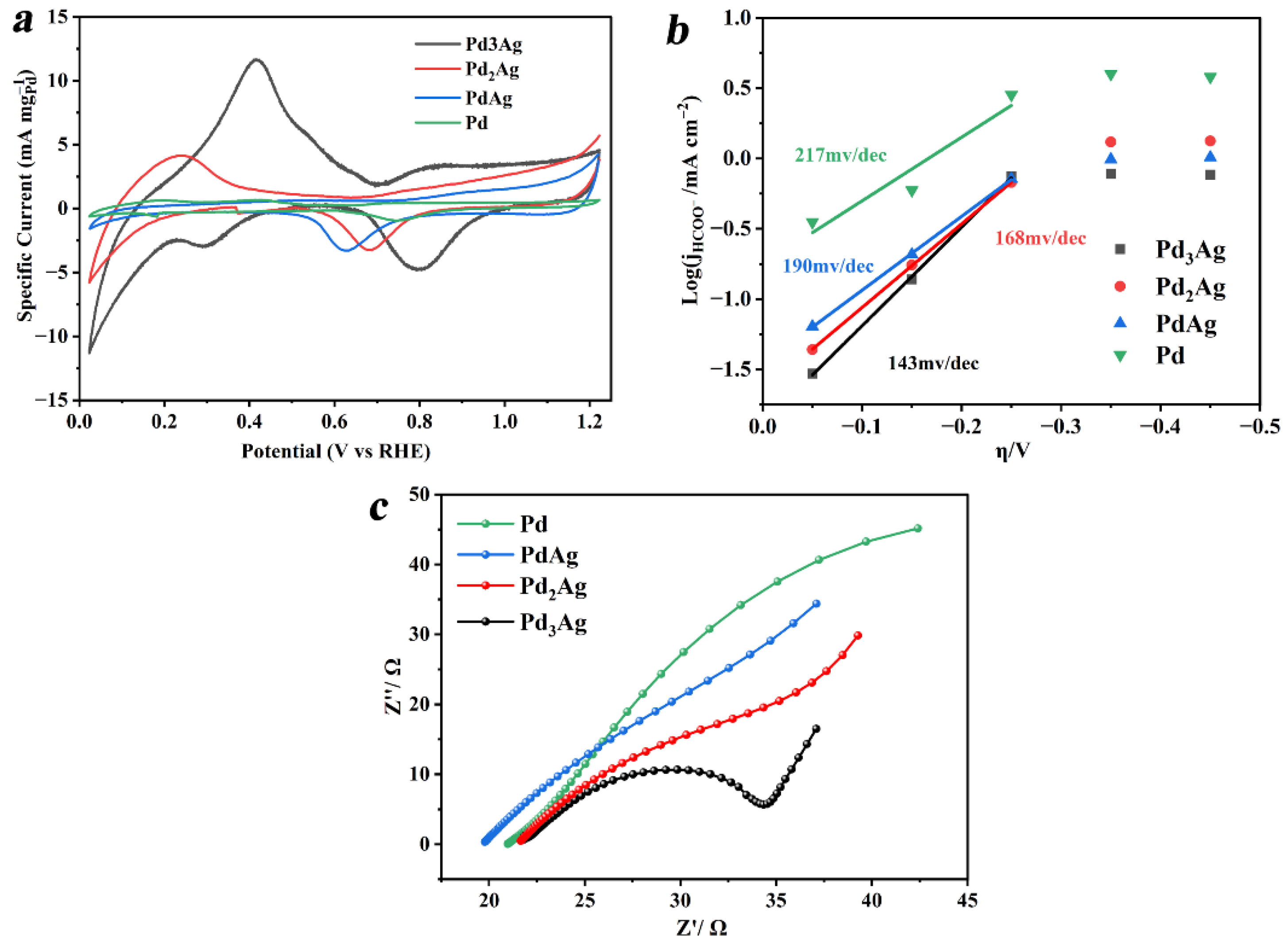
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.-Y.; Sun, S.-G. A breakthrough in electrocatalysis of CO2 conversion. Natl. Sci. Rev. 2017, 4, 155–156. [Google Scholar] [CrossRef] [Green Version]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.-F.; Guo, R.-T.; Gu, J.-W.; Liu, Y.-Z.; Wu, G.-L.; Duan, C.-P.; Zhang, X.-D.; Pan, W.-G. Fabrication of β-In2S3/NiAl-LDH heterojunction photocatalyst with enhanced separation of charge carriers for efficient CO2 photocatalytic reduction. Appl. Surf. Sci. 2020, 527, 146792. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Lv, X.; Niu, X.; Lin, X.; Zhong, S.; Wang, D.; Lin, H.; Chen, J.; Bai, S. Stacking Engineering of Semiconductor Heterojunctions on Hollow Carbon Spheres for Boosting Photocatalytic CO2 Reduction. ACS Catal. 2022, 12, 2569–2580. [Google Scholar] [CrossRef]
- He, C.; Duan, D.; Low, J.; Bai, Y.; Jiang, Y.; Wang, X.; Chen, S.; Long, R.; Song, L.; Xiong, Y. Cu2−xS derived copper nanoparticles: A platform for unraveling the role of surface reconstruction in efficient electrocatalytic CO2-to-C2H4 conversion. Nano Res. 2021. [Google Scholar] [CrossRef]
- Duo, J.; Jin, F.; Wang, Y.; Zhong, H.; Lyu, L.; Yao, G.; Huo, Z. NaHCO3-enhanced hydrogen production from water with Fe and in situ highly efficient and autocatalytic NaHCO3 reduction into formic acid. Chem. Commun. 2016, 52, 3316–3319. [Google Scholar] [CrossRef]
- Sekimoto, T.; Hashiba, H.; Shinagawa, S.; Uetake, Y.; Deguchi, M.; Yotsuhashi, S.; Ohkawa, K. Analysis of Products from Photoelectrochemical Reduction of 13CO2 by GaN-Si Based Tandem Photoelectrode. J. Phys. Chem. C 2016, 120, 13970–13975. [Google Scholar] [CrossRef]
- Lu, Y.; Zou, Y.; Zhao, W.; Wang, M.; Li, C.; Liu, S.; Wang, S. Nanostructured electrocatalysts for electrochemical carboxylation with CO2. Nano Select 2020, 1, 135–151. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, S. An Investigation of Active Sites for electrochemical CO2 Reduction Reactions: From In Situ Characterization to Rational Design. Adv. Sci. 2021, 8, 2003579. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.J.; Zhang, H.; Lv, H.; Li, Q.; Michalsky, R.; Peterson, A.A.; Sun, S. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135. [Google Scholar] [CrossRef]
- Lv, H.; Lv, F.; Qin, H.; Min, X.; Sun, L.; Han, N.; Xu, D.; Li, Y.; Liu, B. Single-Crystalline Mesoporous Palladium and Palladium-Copper Nanocubes for Highly Efficient Electrochemical CO2 Reduction. CCS Chem. 2021, 4, 1435–1444. [Google Scholar]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 2020, 3, 478–487. [Google Scholar] [CrossRef]
- Rong, L.; Xu, Z.; Sun, J.; Guo, G. New methyl formate synthesis method: Coal to methyl formate. J. Energy Chem. 2018, 27, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.J.; Dunn, E.; DuChene, J.S.; Atwater, H.A. Bicarbonate or Carbonate Processes for Coupling Carbon Dioxide Capture and Electrochemical Conversion. ACS Energy Lett. 2020, 5, 940–945. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698–9725. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Huang, X.; Wang, L. Metal (Sn, Bi, Pb, Cd) in-situ anchored on mesoporous hollow kapok-tubes for outstanding electrocatalytic CO2 reduction to formate. Electrochim. Acta 2019, 325, 134923. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S.; Ren, W.; Zhao, Y.; Chen, X.; Jia, C.; Liu, J.; Zhao, C. Nanostructured amalgams with tuneable silver–mercury bonding sites for selective electroreduction of carbon dioxide into formate and carbon monoxide. J. Mater. Chem. A 2019, 7, 15907–15912. [Google Scholar] [CrossRef]
- Yu, W.; Wen, L.; Gao, J.; Chen, S.; He, Z.; Wang, D.; Shen, Y.; Song, S. Facile treatment tuning the morphology of Pb with state-of-the-art selectivity in CO2 electroreduction to formate. Chem. Commun. 2021, 57, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liang, Y.; Li, H.; Zhang, B.; Liu, Z.; Chang, Q.; Zhang, H.; Zhu, C.F.; Geng, Z.; Zhu, W.; et al. In-Situ Surface Reconstruction of InN Nanosheets for Efficient CO2 Electroreduction into Formate. Nano Lett. 2020, 20, 8229–8235. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Han, N.; Deng, J.; Wu, J.; Wang, Y.; Hu, Y.; Ding, P.; Li, Y.; Li, Y.; Lu, J. Selective CO2 Reduction on 2D Mesoporous Bi Nanosheets. Adv. Energy Mater. 2018, 8, 1801536. [Google Scholar] [CrossRef]
- Min, X.; Kanan, M.W. Pd-catalyzed electrohydrogenation of carbon dioxide to formate: High mass activity at low overpotential and identification of the deactivation pathway. J. Am. Chem. Soc. 2015, 137, 4701–4708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, N.; Li, Y. Recent Progress on Pd-based Nanomaterials for Electrochemical CO2 Reduction. Acta Phys.-Chim. Sin. 2020, 36, 2001041. [Google Scholar] [CrossRef]
- Kortlever, R.; Peters, I.; Koper, S.; Koper, M.T.M. Electrochemical CO2 Reduction to Formic Acid at Low Overpotential and with High Faradaic Efficiency on Carbon-Supported Bimetallic Pd–Pt Nanoparticles. ACS Catal. 2015, 5, 3916–3923. [Google Scholar] [CrossRef]
- He, L.-q.; Yang, H.; Huang, J.-j.; Lu, X.-h.; Li, G.-R.; Liu, X.-q.; Fang, P.-p.; Tong, Y.-x. Enhanced catalytic activity of Au core Pd shell Pt cluster trimetallic nanorods for CO2 reduction. RSC Adv. 2019, 9, 10168–10173. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Sun, M.; Xu, J.; Zhao, X.; Zhou, R.; Pan, B.; Wang, L.; Han, N.; Huang, B.; Li, Y. Phase-Dependent Electrocatalytic CO2 Reduction on Pd3Bi Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 21741–21745. [Google Scholar] [CrossRef]
- Wang, W.J.; Hwang, S.; Kim, T.; Ha, S.; Scudiero, L. Study of carbon supported CuPd alloy nanoparticles with Pd-rich surface for the electrochemical formate oxidation and CO2 reduction. Electrochim. Acta 2021, 387, 138531. [Google Scholar] [CrossRef]
- Zhou, R.; Fan, X.; Ke, X.; Xu, J.; Zhao, X.; Jia, L.; Pan, B.; Han, N.; Li, L.; Liu, X.; et al. Two-Dimensional Palladium-Copper Alloy Nanodendrites for Highly Stable and Selective Electrochemical Formate Production. Nano Lett. 2021, 21, 4092–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xie, R.-K.; Mao, M.-J.; Chai, G.-L.; Yi, J.-D.; Zhao, S.-S.; Huang, Y.-B.; Cao, R. Integration of Strong Electron Transporter Tetrathiafulvalene into Metalloporphyrin-Based Covalent Organic Framework for Highly Efficient Electroreduction of CO2. ACS Energy Lett. 2020, 5, 1005–1012. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, R.; Zhu, X.; Han, N.; Song, B.; Liu, T.; Hu, G.; Li, Y.; Lu, J.; Li, Y. Mesoporous PdAg Nanospheres for Stable Electrochemical CO2 Reduction to Formate. Adv. Mater. 2020, 32, 2000992. [Google Scholar] [CrossRef]
- Han, N.; Sun, M.; Zhou, Y.; Xu, J.; Cheng, C.; Zhou, R.; Zhang, L.; Luo, J.; Huang, B.; Li, Y. Alloyed Palladium-Silver Nanowires Enabling Ultrastable Carbon Dioxide Reduction to Formate. Adv. Mater. 2021, 33, 2005821. [Google Scholar] [CrossRef]
- Huang, W.; Kang, X.; Xu, C.; Zhou, J.; Deng, J.; Li, Y.; Cheng, S. 2D PdAg Alloy Nanodendrites for Enhanced Ethanol Electroxidation. Adv. Mater. 2018, 30, 1706962. [Google Scholar] [CrossRef]
- Lin, R.; Ma, X.; Cheong, W.-C.; Zhang, C.; Zhu, W.; Pei, J.; Zhang, K.; Wang, B.; Liang, S.; Liu, Y.; et al. PdAg bimetallic electrocatalyst for highly selective reduction of CO2 with low COOH* formation energy and facile CO desorption. Nano Res. 2019, 12, 2866–2871. [Google Scholar] [CrossRef]
- Ma, L.; Wang, C.; Xia, B.Y.; Mao, K.; He, J.; Wu, X.; Xiong, Y.; Lou, X.W. Platinum multicubes prepared by Ni2+-mediated shape evolution exhibit high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 5666–5671. [Google Scholar] [CrossRef]
- Chen, Z.P.; Yao, S.Y.; Liu, L.C. 3D hierarchical porous structured carbon nanotube aerogel-supported Sn spheroidal particles: An efficient and selective catalyst for electrochemical reduction of CO2 to formate. J. Mater. Chem. A. 2017, 5, 24651–24656. [Google Scholar] [CrossRef]
- Kortlever, R.; Balemans, C.; Kwon, Y.; Koper, M.T.M. Electrochemical CO2 reduction to formic acid on a Pd-based formic acid oxidation catalyst. Catal. Today 2015, 244, 58–62. [Google Scholar] [CrossRef]
- Zhou, F.L.; Li, H.T.; Fournier, M.; MacFarlane, D.R. Electrocatalytic CO2 Reduction to Formate at Low Overpotentials on Electrodeposited Pd Films: Stabilized Performance by Suppression of CO Formation. ChemSusChem 2017, 10, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, W.; Zhang, J.; Wang, Z.; Jin, X. Nanoporous Ag-Sn derived from codeposited AgCl-SnO2 for the electrocatalytic reduction of CO2 with high formate selectivity. Electrochem. Commun. 2019, 102, 52–56. [Google Scholar] [CrossRef]
- Luc, W.; Collins, C.; Wang, S.W.; Xin, H.L.; He, K.; Kang, Y.J.; Jiao, F. Ag-Sn Bimetallic Catalyst with a Core-Shell Structure for CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, X.G.; Jiang, K.; Wu, D.Y.; Cai, W.B. Boosting Formate Production in Electrocatalytic CO2 Reduction over Wide Potential Window on Pd Surfaces. J. Am. Chem. Soc. 2018, 140, 2880–2889. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, L.; Sheveleva, A.; Han, X.; Li, J.; Liu, L.; Tuna, F.; McInnes, E.J.L.; Han, B.; Yang, S.; et al. Electro-reduction of carbon dioxide at low over-potential at a metal-organic framework decorated cathode. Nat. Commun. 2020, 11, 5464. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wu, S.; Zhang, Q.; Qiu, S.; Wang, Y.; Tan, J.; Ma, L.; Wang, T.; Xia, Y. Surface Structure Engineering of PdAg Alloys with Boosted CO2 Electrochemical Reduction Performance. Nanomaterials 2022, 12, 3860. https://doi.org/10.3390/nano12213860
Yang X, Wu S, Zhang Q, Qiu S, Wang Y, Tan J, Ma L, Wang T, Xia Y. Surface Structure Engineering of PdAg Alloys with Boosted CO2 Electrochemical Reduction Performance. Nanomaterials. 2022; 12(21):3860. https://doi.org/10.3390/nano12213860
Chicago/Turabian StyleYang, Xianghua, Shiqing Wu, Qian Zhang, Songbai Qiu, Yuan Wang, Junjun Tan, Liang Ma, Tiejun Wang, and Yongde Xia. 2022. "Surface Structure Engineering of PdAg Alloys with Boosted CO2 Electrochemical Reduction Performance" Nanomaterials 12, no. 21: 3860. https://doi.org/10.3390/nano12213860





