Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution
Abstract
1. Introduction
2. Experimentation
2.1. Chemicals and Instruments
2.2. Chitosan Synthesis
2.3. Nanosilica Synthesis
2.4. Silica/Chitosan Synthesis
2.5. Uranium Adsorption
2.6. Uranium Desorption
3. Results and Discussion
3.1. Characteristics
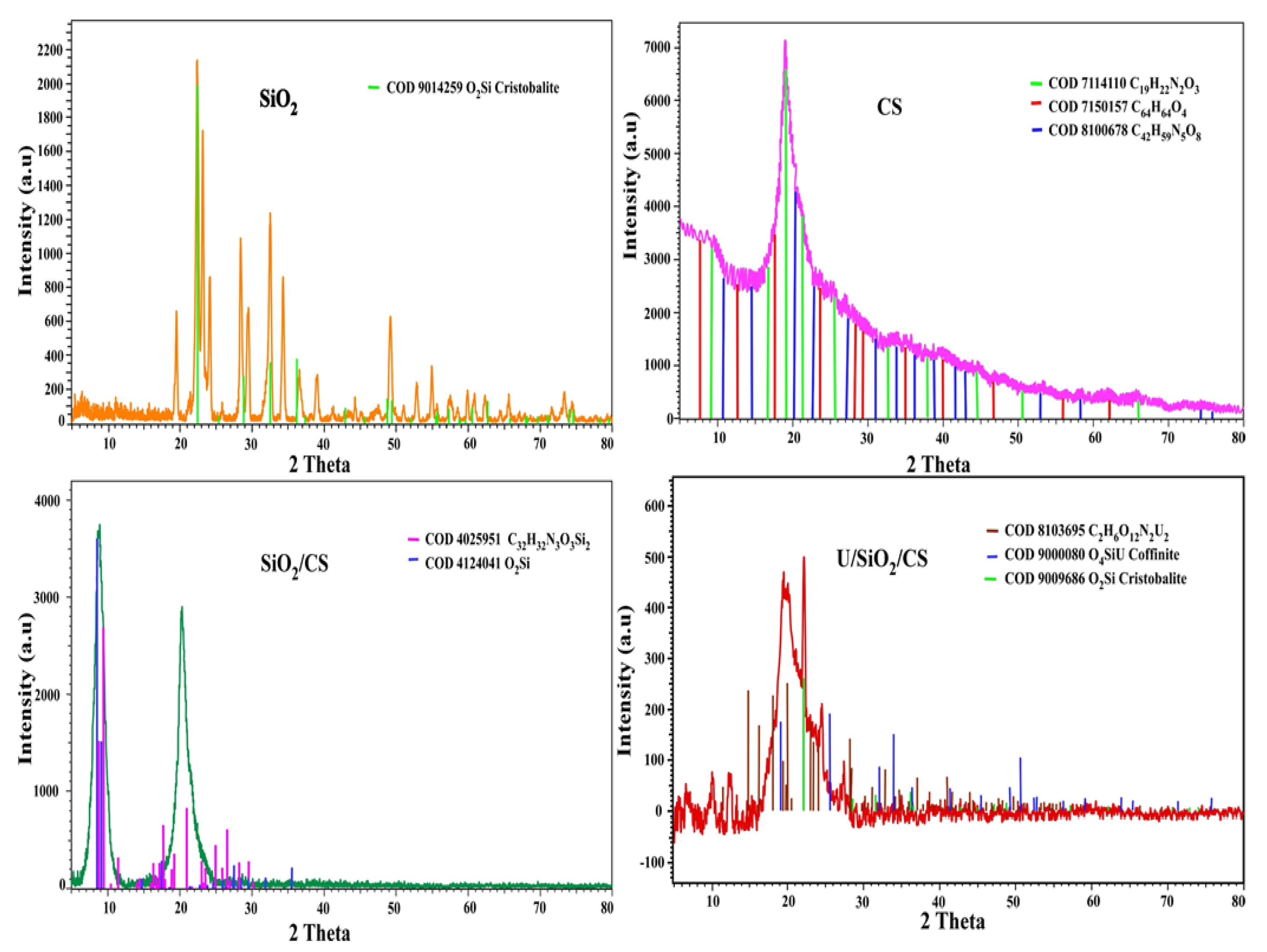
3.1.1. X-ray Diffraction Analysis
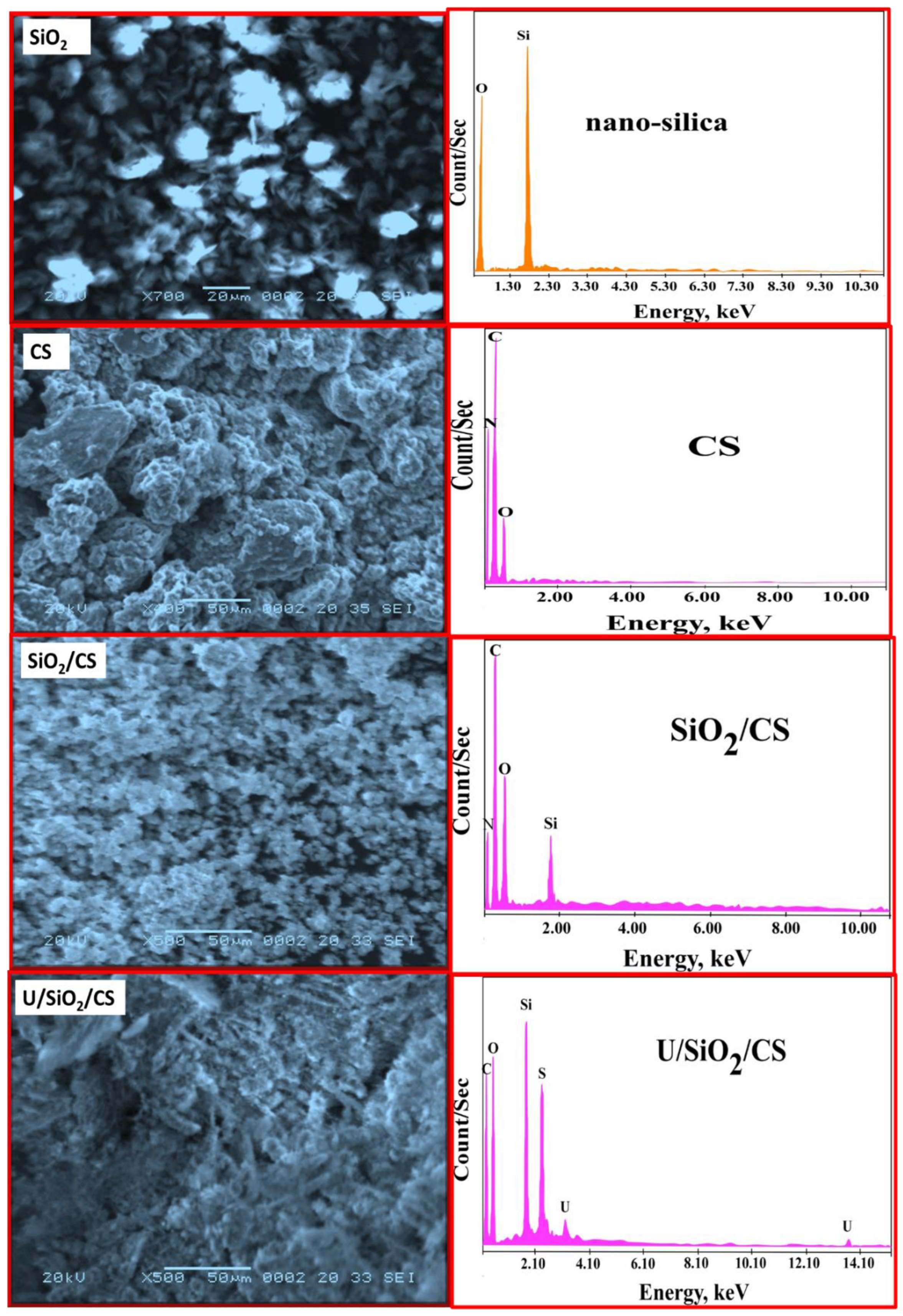
3.1.2. EDX and SEM Images
3.1.3. BET Surface Examination
3.1.4. FTIR Investigation
3.2. U(VI) Sorption
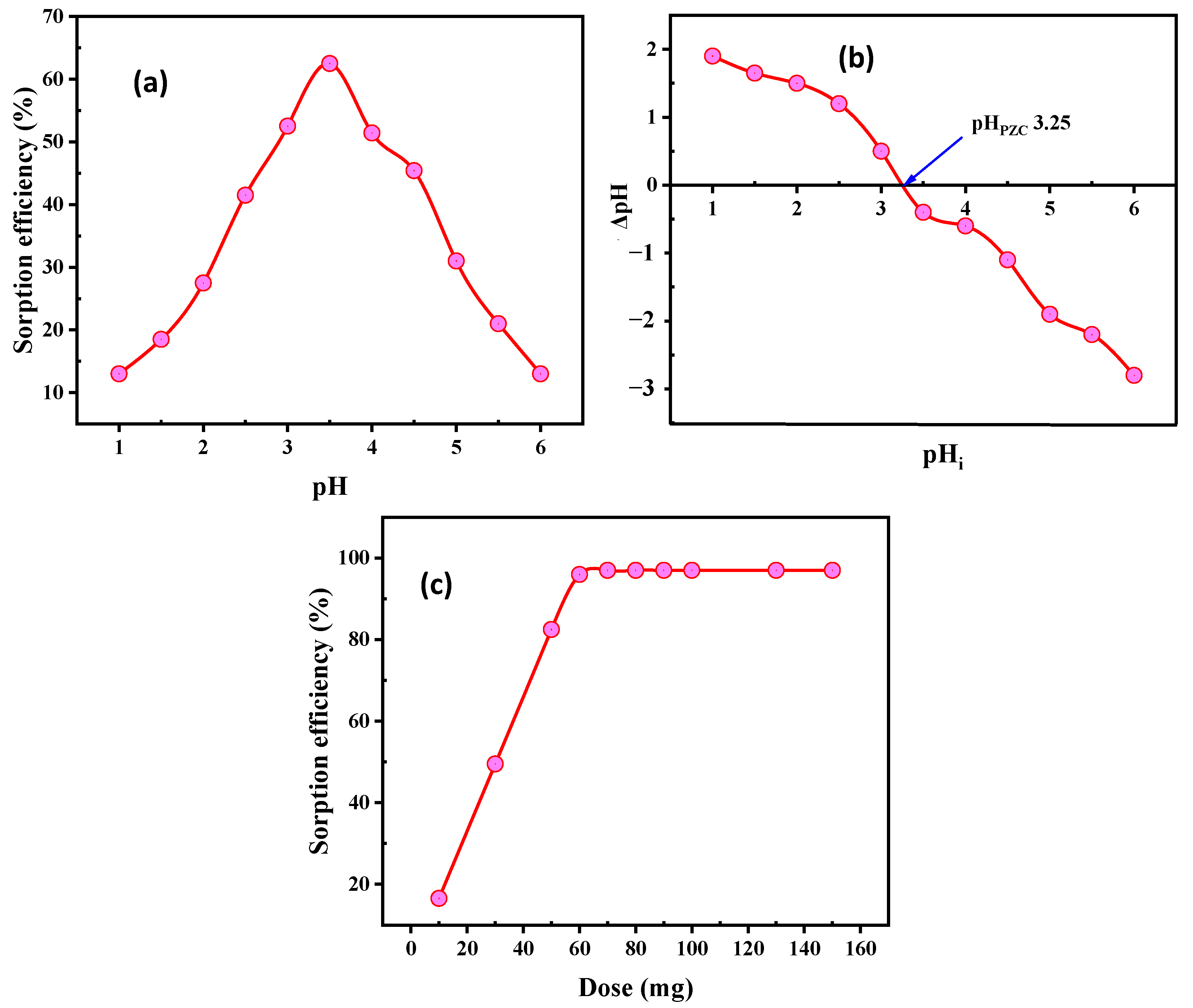
3.2.1. Influence of pH
3.2.2. Influence of SiO2/CS Dose
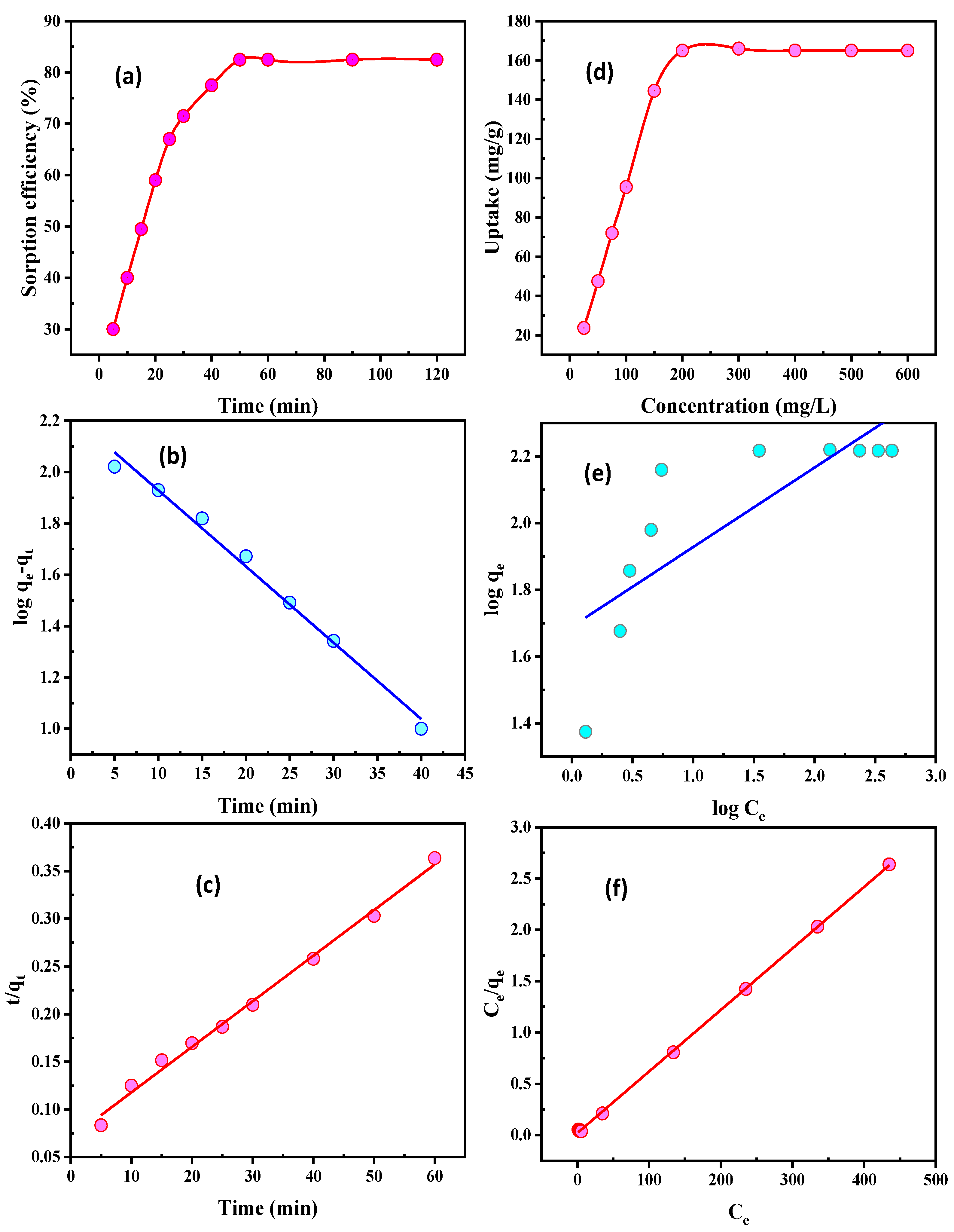
3.2.3. Contact Time
3.2.4. U(VI) Concentration Influence
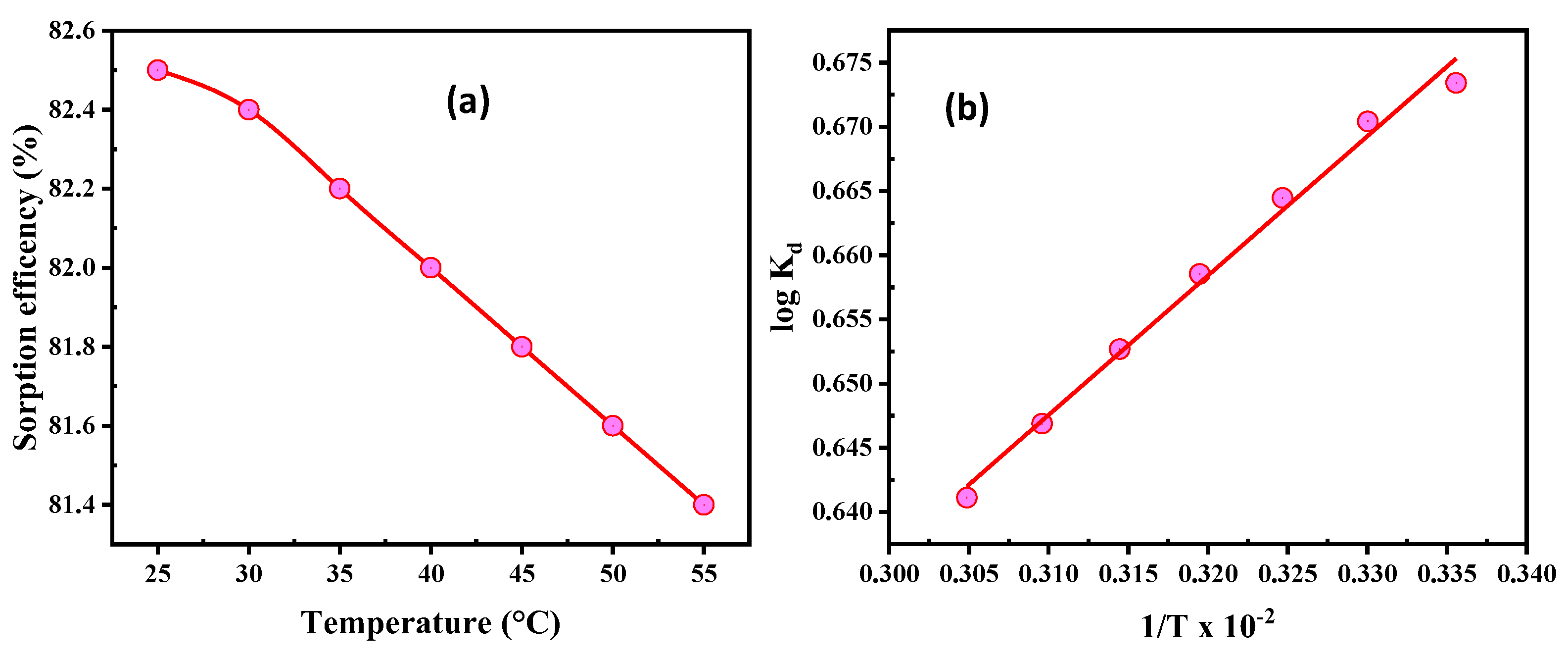
3.2.5. Temperature and Thermodynamics
3.2.6. Diverse Ions Effect
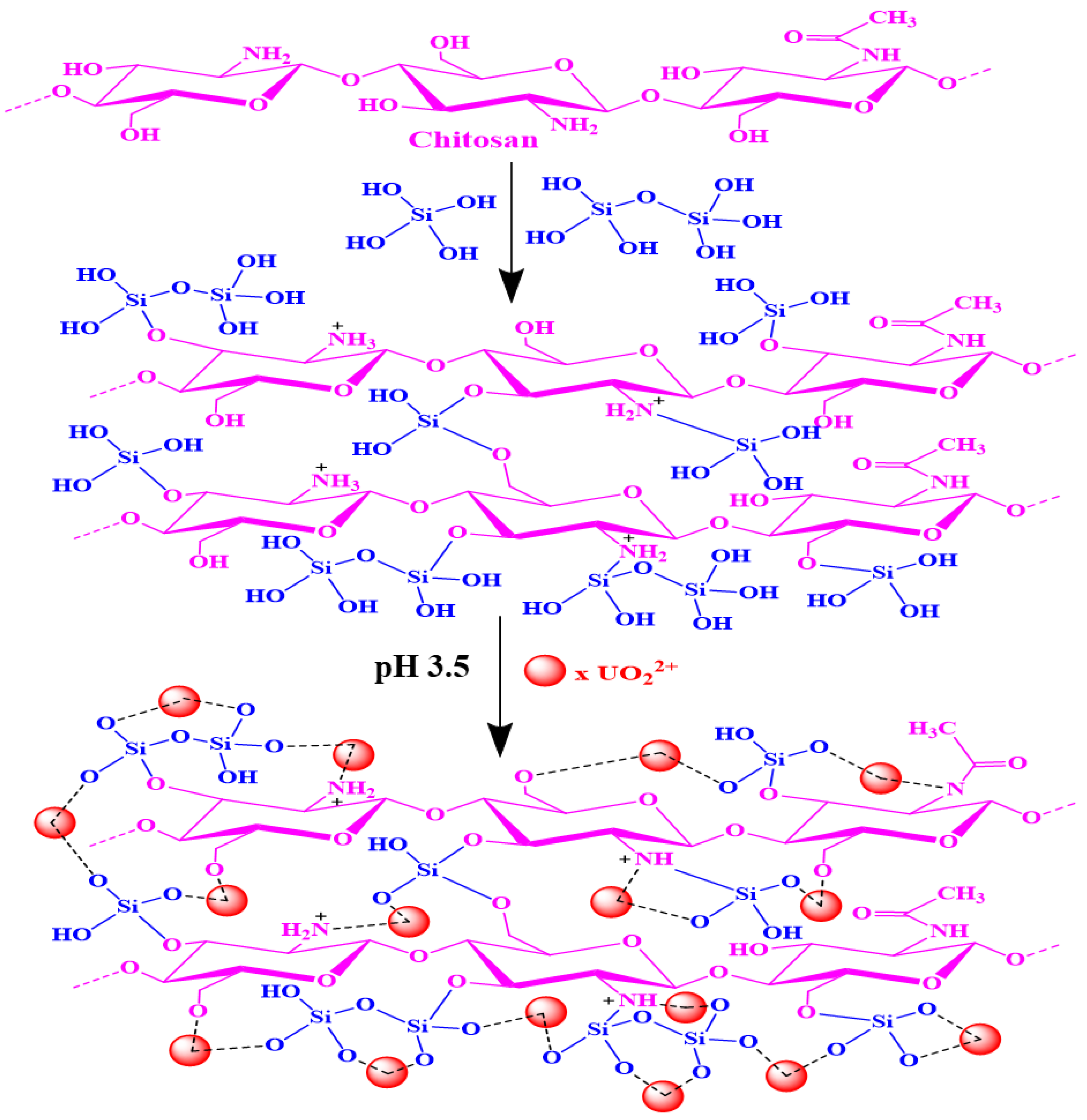
3.3. Adsorption Mechanism
3.4. U(VI) Desorption
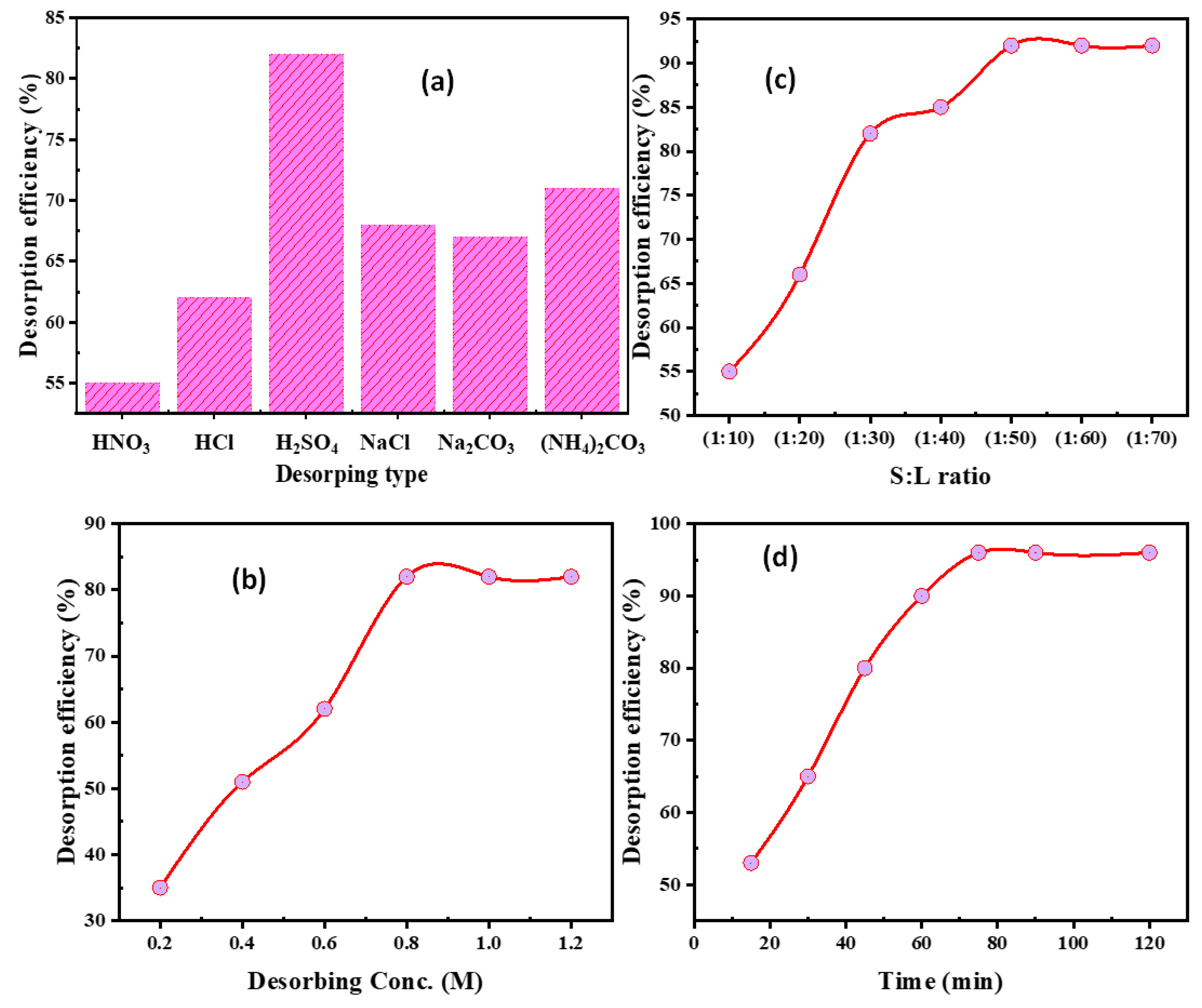
3.4.1. Eluting Type
3.4.2. H2SO4 Concentration
3.4.3. S:L Phase Ratio
3.4.4. Desorbing Time
3.5. Regeneration of the SiO2/CS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020, 266, 121867. [Google Scholar] [CrossRef]
- Das, T.K.; Poater, A. Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J. Environ. Radioact. 2016, 162, 134–145. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, J.; Chen, F.; Dionysiou, D.D.; Feng, Y.; Liang, B.; Cheng, H.-Y.; Qin, Z.; Tang, X.; Li, H.; et al. Removal and recovery of aqueous U(VI) by heterogeneous photocatalysis: Progress and challenges. Chem. Eng. J. 2022, 450, 138317. [Google Scholar] [CrossRef]
- Du, J.; Waite, T.D.; Biesheuvel, P.M.; Tang, W. Recent advances and prospects in electrochemical coupling technologies for metal recovery from water. J. Hazard. Mater. 2023, 442, 130023. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Sakr, A.K.; Al-Hamarneh, I.F.; Gomaa, H.; Abdel Aal, M.M.; Hanfi, M.Y.; Sayyed, M.I.; Khandaler, M.U.; Cheira, M.F. Removal of uranium from nuclear effluent using regenerated bleaching earth steeped in β-naphthol. Radiat. Phys. Chem. 2022, 200, 110204. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Yang, T.; Chen, X.; Liu, X.; Ding, X. Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydr. Polym. 2015, 121, 79–85. [Google Scholar] [CrossRef]
- Pastoor, K.J.; Miskowiec, A.J.; Niedziela, J.L.; Christian, J.H.; Foley, B.J.; Isbill, S.B.; Shields, A.E.; Manjón-Sanz, A.M.; Nykwest, E.C.; Spano, T.L.; et al. Structural Characterization of Uranium Tetrafluoride Hydrate (UF4·2.5H2O). J. Phys. Chem. C 2022, 126, 13256–13267. [Google Scholar] [CrossRef]
- Sang, K.; Mei, D.; Wang, Y.; Liu, L.; Li, H.; Yang, G.; Ma, F.; Zhang, C.; Dong, H. Amidoxime-functionalized zeolitic imidazolate frameworks with antimicrobial property for the removal of U (VI) from wastewater. J. Environ. Chem. Eng. 2022, 10, 108344. [Google Scholar] [CrossRef]
- Xiong, T.; Jia, L.; Li, Q.; Zhang, Y.; Zhu, W. Efficient removal of uranium by hydroxyapatite modified kaolin aerogel. Sep. Purif. Technol. 2022, 299, 121776. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Wang, X.; Xie, Z.; Deng, N. Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef]
- Ao, X.; Zhou, L.; Yu, H.; Ouyang, J.; Liu, Z.; Liu, Y.; Adesina, A.A. Polyethyleneimine incorporated chitosan/α-MnO2 nanorod honeycomb-like composite foams with remarkable elasticity and ultralight property for the effective removal of U(VI) from aqueous solution. Int. J. Biol. Macromol. 2022, 218, 190–201. [Google Scholar] [CrossRef]
- Anpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, Z. Characterisation and reactivity of oxygen species at the surface of metal oxides. J. Catal. 2021, 393, 259–280. [Google Scholar] [CrossRef]
- Vassileva, E.; Proinova, I.; Hadjiivanov, K. Solid-phase extraction of heavy metal ions on a high surface area titanium dioxide (anatase). Analyst 1996, 121, 607–612. [Google Scholar] [CrossRef]
- Fan, F.-L.; Qin, Z.; Bai, J.; Rong, W.-D.; Fan, F.-Y.; Tian, W.; Wu, X.-L.; Wang, Y.; Zhao, L. Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J. Environ. Radioact. 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Moradi Abdoluosofi, L.; Pakniat, M.; Montazerozohori, M. Sodium dodecyl sulfate coated alumina modified with a new Schiff’s base as a uranyl ion selective adsorbent. J. Hazard. Mater. 2011, 187, 75–81. [Google Scholar] [CrossRef]
- Sylwester, E.R.; Hudson, E.A.; Allen, P.G. The structure of uranium (VI) sorption complexes on silica, alumina, and montmorillonite. Geochim. Cosmochim. Acta 2000, 64, 2431–2438. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Extraction and adsorption of U(VI) from aqueous solution using affinity ligand-based technologies: An overview. Rev. Environ. Sci. Bio/Technol. 2019, 18, 437–452. [Google Scholar] [CrossRef]
- Hassanin, M.A.; El-Gendy, H.S.; Cheira, M.F.; Atia, B.M. Uranium ions extraction from the waste solution by thiosemicarbazide anchored cellulose acetate. Int. J. Environ. Anal. Chem. 2021, 101, 351–369. [Google Scholar] [CrossRef]
- Gunathilake, C.; Górka, J.; Dai, S.; Jaroniec, M. Amidoxime-modified mesoporous silica for uranium adsorption under seawater conditions. J. Mater. Chem. A 2015, 3, 11650–11659. [Google Scholar] [CrossRef]
- Amesh, P.; Venkatesan, K.A.; Suneesh, A.S.; Gupta, D.K.; Ravindran, T.R. Adsorption of uranium by diethylenetriamine functionalized magnetic mesoporous silica. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100583. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Antoniou, Ε.; Terpiłowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyiannis, I.A.; et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef]
- Hamza, M.F.; Gamal, A.; Hussein, G.; Nagar, M.S.; Abdel-Rahman, A.A.H.; Wei, Y.; Guibal, E. Uranium(VI) and zirconium(IV) sorption on magnetic chitosan derivatives—Effect of different functional groups on separation properties. J. Chem. Technol. Biotechnol. 2019, 94, 3866–3882. [Google Scholar] [CrossRef]
- Cheira, M.F. Performance of poly sulfonamide/nano-silica composite for adsorption of thorium ions from sulfate solution. SN Appl. Sci. 2020, 2, 398. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Tan, Y.; Chen, B.; Yang, P. Removal of U(VI) from wastewater by sulfhydryl-functionalized biomass carbon supported nano-zero-valent iron through synergistic effect of adsorption and reduction. Mater. Sci. Eng. B 2022, 284, 115891. [Google Scholar] [CrossRef]
- Huynh, J.; Palacio, R.; Safizadeh, F.; Lefèvre, G.; Descostes, M.; Eloy, L.; Guignard, N.; Rousseau, J.; Royer, S.; Tertre, E.; et al. Adsorption of Uranium over NH2-Functionalized Ordered Silica in Aqueous Solutions. ACS Appl. Mater. Interfaces 2017, 9, 15672–15684. [Google Scholar] [CrossRef] [PubMed]
- Jamali, M.R.; Assadi, Y.; Shemirani, F.; Hosseini, M.R.M.; Kozani, R.R.; Masteri-Farahani, M.; Salavati-Niasari, M. Synthesis of salicylaldehyde-modified mesoporous silica and its application as a new sorbent for separation, preconcentration and determination of uranium by inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2006, 579, 68–73. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz Antonino, R.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Sharma, M.; Laddha, H.; Yadav, P.; Jain, Y.; Sachdev, K.; Janu, V.C.; Gupta, R. Selective removal of uranium from an aqueous solution of mixed radionuclides of uranium, cesium, and strontium via a viable recyclable GO@chitosan based magnetic nanocomposite. Mater. Today Commun. 2022, 32, 104020. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, M.A.; Negm, S.H.; Youssef, M.A.; Sakr, A.K.; Mira, H.I.; Mohammaden, T.F.; Al-Otaibi, J.S.; Hanfi, M.Y.; Sayyed, M.I.; Cheira, M.F. Sustainable Remedy Waste to Generate SiO2 Functionalized on Graphene Oxide for Removal of U(VI) Ions. Sustainability 2022, 14, 2699. [Google Scholar] [CrossRef]
- Bao, M.; Zhu, G.; Wang, L.; Wang, M.; Gao, C. Preparation of monodispersed spherical mesoporous nanosilica–polyamide thin film composite reverse osmosis membranes via interfacial polymerization. Desalination 2013, 309, 261–266. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Pylypchuk, I.V.; Tertykh, V.A.; Yanovska, E.S.; Kolodynska, D. Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res. Lett. 2015, 10, 87. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Ismael, M.K.; Gönen, M. Synthesis of Chitosan-Silica Nanocomposite for Removal of Methyl Orange from Water: Composite Characterization and Adsorption Performance. IOP Conf. Ser. Mater. Sci. Eng. 2020, 745, 012084. [Google Scholar] [CrossRef]
- Abou Rida, M.; Harb, F. Synthesis and characterization of amorphous silica nanoparitcles from aqueous silicates uisng cationic surfactants. J. Met. Mater. Miner. 2014, 24, 37–42. [Google Scholar]
- Guo, Q.; Yang, G.; Huang, D.; Cao, W.; Ge, L.; Li, L. Synthesis and characterization of spherical silica nanoparticles by modified Stöber process assisted by slow-hydrolysis catalyst. Colloid Polym. Sci. 2018, 296, 379–384. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Highly pure amorphous silica nano-disks from rice straw. Powder Technol. 2012, 225, 149–155. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Gu, D.; Zhao, X.; Pan, Q. In situ Preparation of Chitosan/ZIF-8 Composite Beads for Highly Efficient Removal of U(VI). Front. Chem. 2019, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Monier, M.; Elsayed, N.H. Selective extraction of uranyl ions using ion-imprinted chelating microspheres. J. Colloid Interface Sci. 2014, 423, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, D.; Chen, B.; Kong, L.; Su, M. Enhanced uranium(VI) adsorption by chitosan modified phosphate rock. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 141–147. [Google Scholar] [CrossRef]
- Sureshkumar, M.K.; Das, D.; Mallia, M.B.; Gupta, P.C. Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J. Hazard. Mater. 2010, 184, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zidan, I.H.; Cheira, M.F.; Bakry, A.R.; Atia, B.M. Potentiality of uranium recovery from G.Gattar leach liquor using Duolite ES-467 chelating resin: Kinetic, thermodynamic and isotherm features. Int. J. Environ. Anal. Chem. 2022, 102, 2102–2124. [Google Scholar] [CrossRef]
- Kausar, A.; Naeem, K.; Hussain, T.; Nazli, Z.-i.-H.; Bhatti, H.N.; Jubeen, F.; Nazir, A.; Iqbal, M. Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: Comparison of linear and non-linear regression methods. J. Mater. Res. Technol. 2019, 8, 1161–1174. [Google Scholar] [CrossRef]
- Sakr, A.K.; Cheira, M.F.; Hassanin, M.A.; Mira, H.I.; Mohamed, S.A.; Khandaker, M.U.; Osman, H.; Eed, E.M.; Sayyed, M.I.; Hanfi, M.Y. Adsorption of Yttrium Ions on 3-Amino-5-Hydroxypyrazole Impregnated Bleaching Clay, a Novel Sorbent Material. Appl. Sci. 2021, 11, 10320. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Imam, E.A.; El-Tantawy El-Sayed, I.; Mahfouz, M.G.; Tolba, A.A.; Akashi, T.; Galhoum, A.A.; Guibal, E. Synthesis of α-aminophosphonate functionalized chitosan sorbents: Effect of methyl vs phenyl group on uranium sorption. Chem. Eng. J. 2018, 352, 1022–1034. [Google Scholar] [CrossRef]
- Cheira, M.F.; Mira, H.I.; Sakr, A.K.; Mohamed, S.A. Adsorption of U(VI) from acid solution on a low-cost sorbent: Equilibrium, kinetic, and thermodynamic assessments. Nucl. Sci. Tech. 2019, 30, 156. [Google Scholar] [CrossRef]
- Ilaiyaraja, P.; Singha Deb, A.K.; Sivasubramanian, K.; Ponraju, D.; Venkatraman, B. Adsorption of uranium from aqueous solution by PAMAM dendron functionalized styrene divinylbenzene. J. Hazard. Mater. 2013, 250, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Weshahy, A.R.; Sakr, A.K.; Gouda, A.A.; Atia, B.M.; Somaily, H.H.; Hanfi, M.Y.; Sayyed, M.I.; El Sheikh, R.; El-Sheikh, E.M.; Radwan, H.A.; et al. Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives. J. Mol. Sci. 2022, 23, 8677. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, S.; Park, S.-J.; Kim, J.M. Application of polymer-modified nanoporous silica to adsorbents of uranyl ions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 162–166. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Desouky, O.A.; Elshehy, E.A. Selective separation of U(VI) from its solutions using amine modified silica gel produced from leached zircon. Int. J. Miner. Process. 2011, 101, 81–88. [Google Scholar] [CrossRef]
- Mishima, K.; Du, X.; Miyamoto, N.; Kano, N.; Imaizumi, H. Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan. J. Funct. Biomater. 2018, 9, 49. [Google Scholar] [CrossRef]
- Negm, S.H.; Abd El-Magied, M.O.; El Maadawy, W.M.; Abdel Aal, M.M.; Abd El Dayem, S.M.; Taher, M.A.; Abd El-Rahem, K.A.; Rashed, M.N.; Cheira, M.F. Appreciatively Efficient Sorption Achievement to U(VI) from the El Sela Area by ZrO2/Chitosan. Separations 2022, 9, 311. [Google Scholar] [CrossRef]
- Szlachta, M.; Neitola, R.; Peräniemi, S.; Vepsäläinen, J. Effective separation of uranium from mine process effluents using chitosan as a recyclable natural adsorbent. Sep. Purif. Technol. 2020, 253, 117493. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Zhao, J.; Wei, P.; Wang, C.; Liu, P.; Zhao, X.; Zeng, K.; Wu, F.; Liu, Z. Unexpected ultrafast and highly efficient removal of uranium from aqueous solutions by a phosphonic acid and amine functionalized polymer adsorbent. New J. Chem. 2021, 45, 10777–10787. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wang, Z.; Zhang, Z.; Wang, X.; Yang, Z. Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J. Alloys Compd. 2021, 852, 156993. [Google Scholar] [CrossRef]
- Abdel Geleel, M.; Atwa, S.T.; Sakr, A.K. Removal of Cr (III) from aqueous waste using Spent Activated Clay. J. Am. Sci. 2013, 9, 256–262. [Google Scholar]
- Mahmoud, N.S.; Atwa, S.T.; Sakr, A.K.; Abdel Geleel, M. Kinetic and thermodynamic study of the adsorption of Ni (II) using Spent Activated clay Mineral. N. Y. Sci. J. 2012, 5, 62–68. [Google Scholar] [CrossRef]
- Atia, B.M.; Sakr, A.K.; Gado, M.A.; El-Gendy, H.S.; Abdelazeem, N.M.; El-Sheikh, E.M.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Synthesis of a New Chelating Iminophosphorane Derivative (Phosphazene) for U(VI) Recovery. Polymers 2022, 14, 1687. [Google Scholar] [CrossRef] [PubMed]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Cetylpyridinium Bromide/Polyvinyl Chloride for Substantially Efficient Capture of Rare Earth Elements from Chloride Solution. Polymers 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Cheira, M.F.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I. Rare Earth Group Separation after Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride from Lamprophyre Dykes Leachate. Materials 2022, 15, 1211. [Google Scholar] [CrossRef]
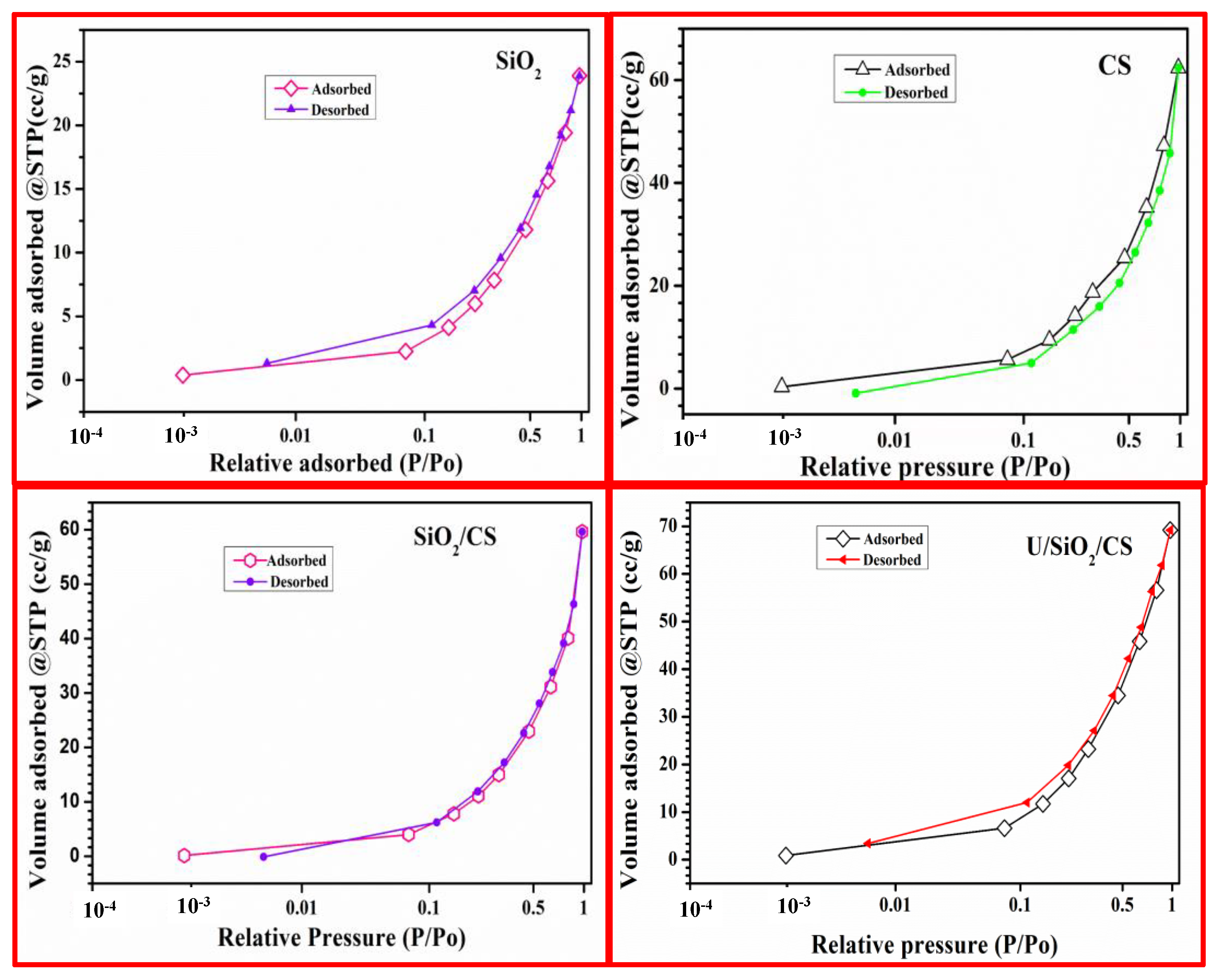

| Materials | SBET, m2/g | Pore Size, nm | Pore Volume, cc/g |
|---|---|---|---|
| SiO2 | 25.85 ± 0.21 | 2.87 ± 0.08 | 0.037 ± 0.006 |
| CS | 19.77 ± 0.32 | 2.65 ± 0.09 | 0.033 ± 0.005 |
| SiO2/CS | 24.55 ± 0.43 | 2.75 ± 0.07 | 0.035 ± 0.007 |
| U/SiO2/CS | 22.71 ± 0.26 | 2.59 ± 0.08 | 0.032 ± 0.008 |
| Pseudo-1st-Order | Pseudo-2nd-Order | ||||
|---|---|---|---|---|---|
| qe(cal) (mg/g) | k1 (1/min) | R2 | qe(cal) (mg/g) | k2 (g/mg.min) | R2 |
| 106.19 | 0.068 | 0.942 | 181.82 | 5.69 × 10−4 | 0.995 |
| Freundlich | Langmuir | ||||
|---|---|---|---|---|---|
| Kf (mg/g) | n (mg.min/g) | R2 | qmax | b (L/mg) | R2 |
| 48.99 ± 2.13 | 4.196 ± 0.93 | 0.641 | 166.66 ± 3.77 | 0.290 ± 0.04 | 0.9998 |
| Sorbent | Uptake (mg/g) | References |
|---|---|---|
| Free silica gel | 21.40 | [19] |
| Nanoporous silica | 29.40 | [54] |
| SiO2/graphene oxide | 145.00 | [34] |
| Triamine modified silica (TAMS) | 90.30 | [55] |
| Pentamine modified silica (PAMS) | 112.00 | [55] |
| Chitosan | 68.0 | [56] |
| ZrO2/Chitosan | 175.00 | [57] |
| Deacetylated chitosan | 17.44 | [58] |
| Amidoxime/chitosan/bentonite | 49.09 | [59] |
| Chitosan/attapulgite | 53.5 | [60] |
| SiO2/CS | 165.00 | This work |
| T, K | 298 | 303 | 308 | 313 | 318 | 323 | 328 |
| ∆G°, kJ/mol | −0.392 ± 0.05 | −0.369 ± 0.08 | −0.347 ± 0.09 | −0.325 ±0.06 | −0.302 ± 0.05 | −0.279 ± 0.07 | −0.257± 0.06 |
| ∆H°, kJ/mol | −1.73 ± 0.09 | ||||||
| ∆S°, kJ/(mol. K) | −0.45 × 10−2 ± 0.009 | ||||||
| R2 | 0.9915 | ||||||
| Diverse Ions | Tolerance Limit, W/W * | Adsorption Efficiency, % | Separation Factor, β |
|---|---|---|---|
| K+ | 2000 ± 20.17 | 99 ±1.89 | 1 × 105 ± 42 |
| Na+ | 2000 ± 19.34 | 99 ± 1.93 | 1 × 105 ± 71 |
| Mg2+ | 2000 ± 21.45 | 99 ± 2.01 | 1 × 105 ± 64 |
| Ca2+ | 2000 ± 22.44 | 99 ± 1.99 | 1 × 105 ± 55 |
| Al3+ | 2000 ± 18.58 | 99 ± 1.79 | 1 × 105 ± 65 |
| Si4+ | 2000 ± 19.77 | 99 ± 1.94 | 1 × 105 ± 64 |
| P5+ | 2000 ± 20.78 | 99 ± 1.97 | 1 × 105 ± 63 |
| Ba2+ | 2000 ± 21.23 | 99 ± 1.89 | 1 × 105 ± 63 |
| V5+ | 400 ± 12.22 | 99 ± 1.99 | 1 × 105 ± 58 |
| Zn2+ | 400 ± 12.22 | 99 ± 1.92 | 1.6 × 105 ± 59 |
| Ni2+ | 400 ± 11.45 | 98 ± 1.88 | 1.6 × 104 ± 47 |
| Mn2+ | 500 ± 10.76 | 99 ± 1.91 | 2.5 × 104 ± 45 |
| Cr3+ | 400 ± 9.79 | 98± 1.89 | 1.6 × 104 ± 51 |
| Fe3+ | 500 ± 11.23 | 98 ± 1.98 | 5 × 103 ± 52 |
| Pb2+ | 300 ± 8.85 | 98 ± 1.96 | 1.4 × 104 ± 61 |
| Zr4+ | 300 ± 8.81 | 98 ± 1.95 | 8.9 × 103 ± 71 |
| Ti4+ | 500 ± 10.21 | 99 ± 1.92 | 2.5 × 104 ± 59 |
| Cu2+ | 350 ± 8.89 | 99 ± 1.97 | 1.6 × 104 ± 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, A.K.; Abdel Aal, M.M.; Abd El-Rahem, K.A.; Allam, E.M.; Abdel Dayem, S.M.; Elshehy, E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Cheira, M.F. Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials 2022, 12, 3866. https://doi.org/10.3390/nano12213866
Sakr AK, Abdel Aal MM, Abd El-Rahem KA, Allam EM, Abdel Dayem SM, Elshehy EA, Hanfi MY, Alqahtani MS, Cheira MF. Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials. 2022; 12(21):3866. https://doi.org/10.3390/nano12213866
Chicago/Turabian StyleSakr, Ahmed K., Mostafa M. Abdel Aal, Khaled A. Abd El-Rahem, Eman M. Allam, Samia M. Abdel Dayem, Emad A. Elshehy, Mohamed Y. Hanfi, Mohammed S. Alqahtani, and Mohamed F. Cheira. 2022. "Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution" Nanomaterials 12, no. 21: 3866. https://doi.org/10.3390/nano12213866
APA StyleSakr, A. K., Abdel Aal, M. M., Abd El-Rahem, K. A., Allam, E. M., Abdel Dayem, S. M., Elshehy, E. A., Hanfi, M. Y., Alqahtani, M. S., & Cheira, M. F. (2022). Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials, 12(21), 3866. https://doi.org/10.3390/nano12213866







