Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of COS
2.2. Preparation of BHA
2.3. Synthesis of Experimental COS-BHA Pulp-Capping Material
2.4. Chemical Characterisation
Fourier Transform Infrared Spectroscopy (FTIR)
Inductively Coupled Mass Spectrometry (ICP-MS) Analysis
Scanning Electron Microscopy (SEM)/Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
2.5. Physical Properties
Determination of pH and Solubility
Determination of Setting Time
Determination of Radiopacity
3. Results
3.1. Selection of the Appropriate Experimental Pulp-Capping Material
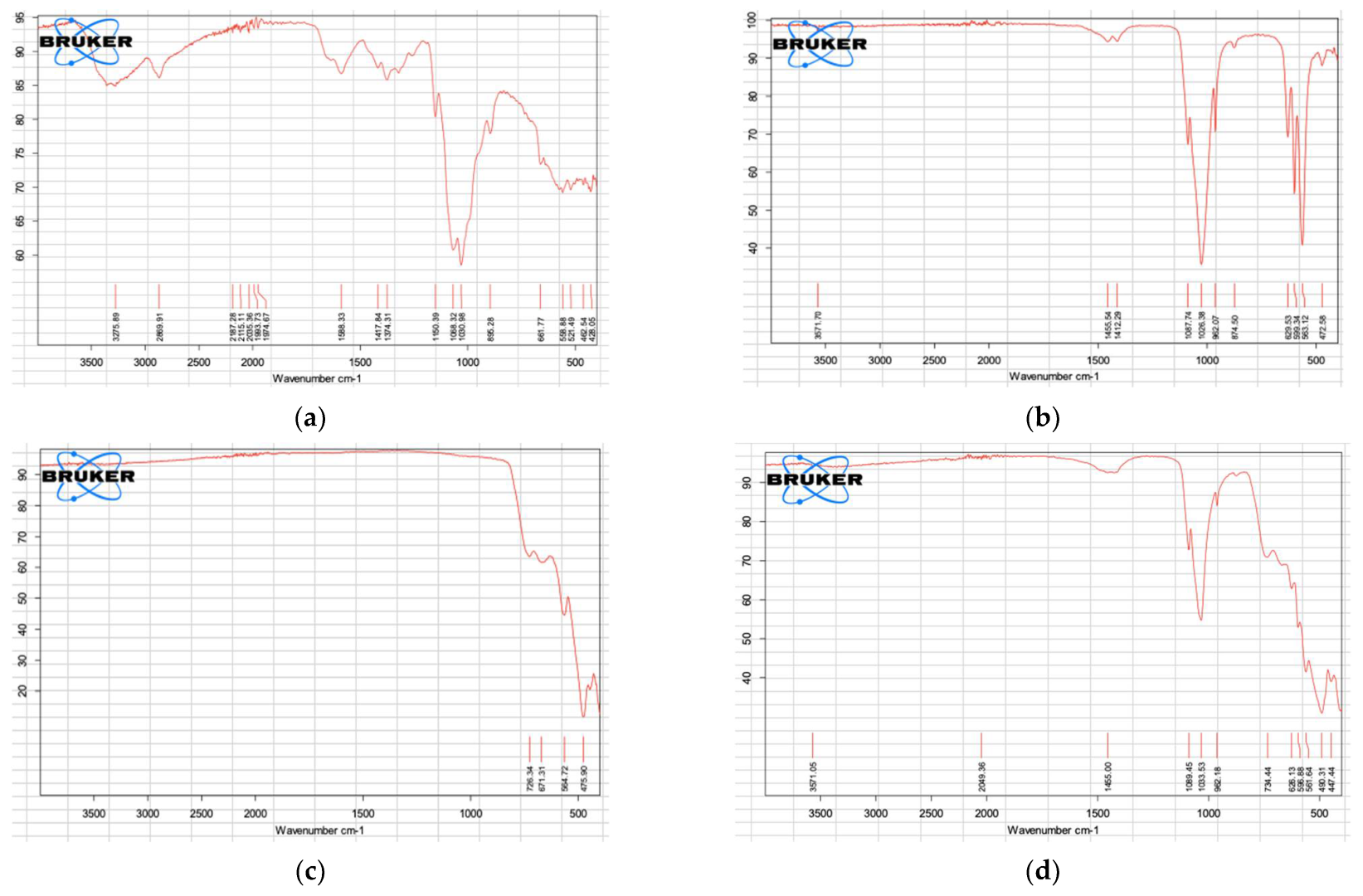
3.2. FTIR Analysis of COS, BHA, ZrO2, and the Biocomposite (Sample 4)
3.3. ICP-MS Biocomposite Analysis
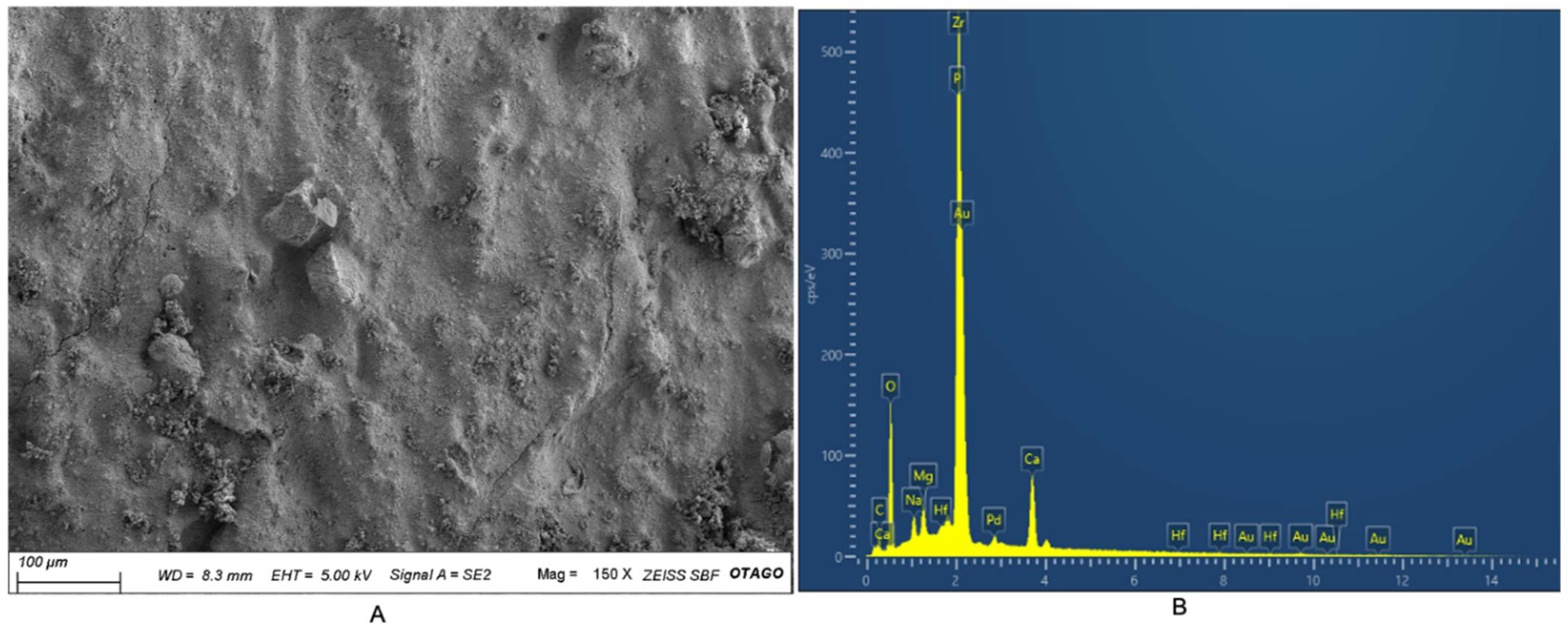
3.4. SEM-EDX Analysis
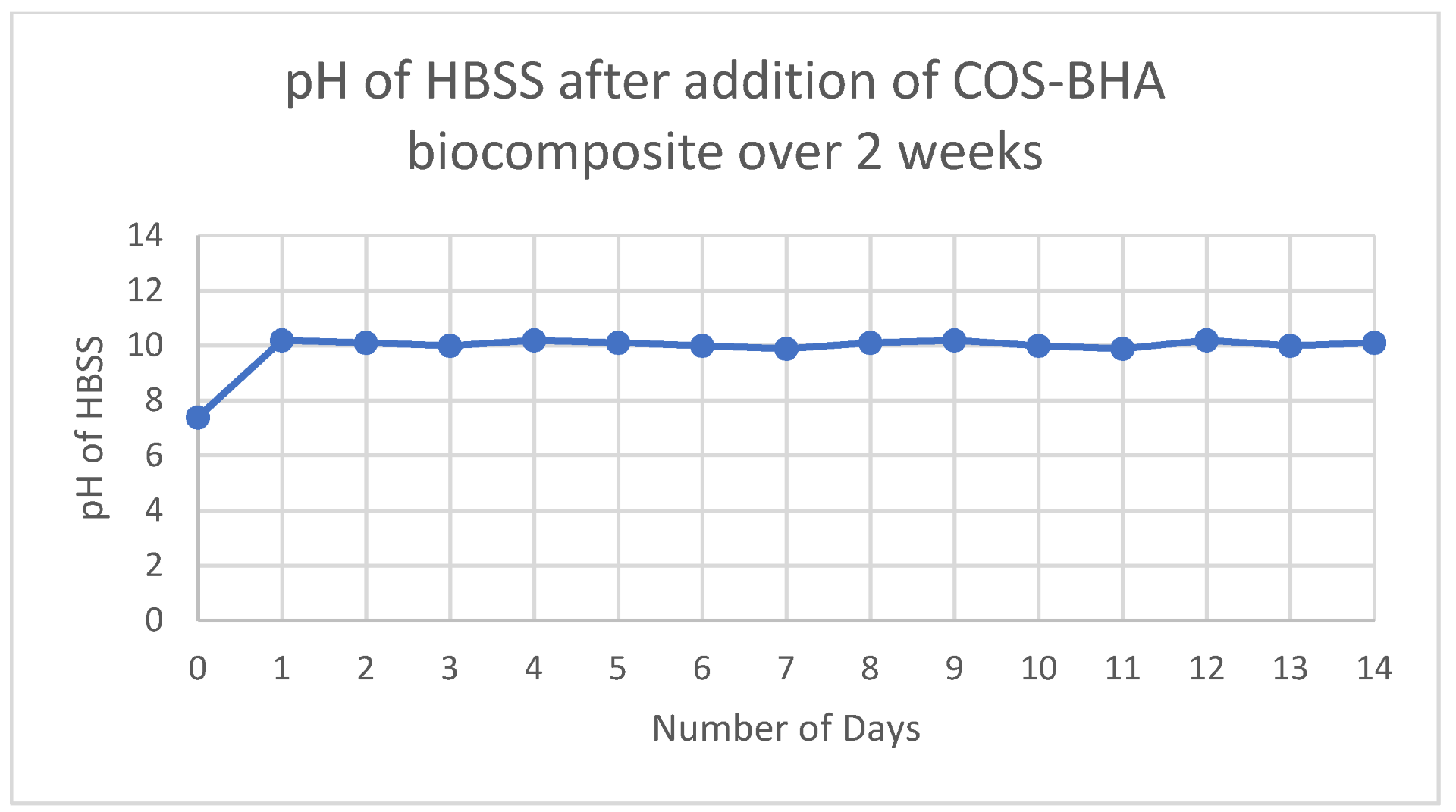
3.5. pH and Solubility of Sample 4
3.6. Biocomposite Setting Time
3.7. Biocomposite Radiopacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilton, T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, D.; Cathro, P. Conservative pulp therapy in the management of reversible and irreversible pulpitis. Aust. Dent. J. 2021, 66, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current status of direct pulp-capping materials for permanent teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Odeh, A.; Osifo, P.; Noemagus, H. Chitosan: A low cost material for the production of membrane for use in PEMFC—A review. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 152–163. [Google Scholar] [CrossRef]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental applications of systems based on hydroxyapatite nanoparticles—An evidence-based update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.; Lee, T. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Ratnayake, J.T.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar] [CrossRef]
- Huang, J.; Ratnayake, J.; Ramesh, N.; Dias, G.J. Development and characterization of a biocomposite material from chitosan and New Zealand-sourced bovine-derived hydroxyapatite for bone regeneration. ACS Omega 2020, 5, 16537–16546. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Choi, J.J.E.; Cathro, P.; Cooper, P.R.; Dias, G.; Huang, J.; Ratnayake, J. Development and Analysis of a Hydroxyapatite Supplemented Calcium Silicate Cement for Endodontic Treatment. Materials 2022, 15, 1176. [Google Scholar] [CrossRef] [PubMed]
- Radha, E.; Sudha, P. Synthesis and characterization of glutaraldehyde crosslinked chitosan oligosaccharide-graft-glycidylmethacrylate/poly propylene glycol blend. World J. Pharm. Res. 2017, 7, 1310–1324. [Google Scholar]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Das, R.S.; Warkhade, S.K.; Kumar, A.; Wankhade, A.V. Graphene oxide-based zirconium oxide nanocomposite for enhanced visible light-driven photocatalytic activity. Res. Chem. Intermed. 2019, 45, 1689–1705. [Google Scholar] [CrossRef]
- Coleman, N.J.; Li, Q. The impact of zirconium oxide radiopacifier on the early hydration behaviour of white Portland cement. Mater. Sci. Eng. C 2013, 33, 427–433. [Google Scholar] [CrossRef]
- Primus, C.M. Comments on testing for the presence of arsenic in MTA and portland cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 4, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhou, X.-N.; Chen, W.-M. Self-etching adhesives: Possible new pulp capping agents to vital pulp therapy. Front. Med. 2011, 5, 77–79. [Google Scholar] [CrossRef] [PubMed]


| Sample | COS Sol (% by wt) | COS Sol (g) | BHA (% by wt) | BHA (g) | ZrO2 (% by wt) | ZrO2 (g) |
|---|---|---|---|---|---|---|
| 1 | 55 | 5 | 10 | 0.91 | 35 | 3.18 |
| 2 | 45 | 5 | 20 | 2.22 | 35 | 3.89 |
| 3 | 35 | 5 | 30 | 4.29 | 35 | 5.00 |
| 4 | 25 | 5 | 40 | 8.00 | 35 | 7.00 |
| 5 | 15 | 5 | 50 | 16.67 | 35 | 11.67 |
| Element (mg/kg) | Experimental Pulp-Capping Material |
|---|---|
| Zr | 68,100 |
| Ca | 55,300 |
| P | 14,600 |
| Na | 1320 |
| Mg | 914 |
| K | <900 |
| Al | <200 |
| B | <200 |
| Zn | <90 |
| Fe | <50 |
| Sr | 43 |
| Ba | 19 |
| Element | Weight (%) |
|---|---|
| C | 13.47 |
| O | 31.02 |
| Na | 1.17 |
| Mg | 1.28 |
| P | 2.66 |
| Ca | 6.01 |
| Zr | 43.72 |
| Hf | 0.66 |
| Total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Ratnayake, J.; Cathro, P.; Gould, M.; Ali, A. Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials 2022, 12, 3925. https://doi.org/10.3390/nano12213925
Cai M, Ratnayake J, Cathro P, Gould M, Ali A. Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials. 2022; 12(21):3925. https://doi.org/10.3390/nano12213925
Chicago/Turabian StyleCai, Mingkai, Jithendra Ratnayake, Peter Cathro, Maree Gould, and Azam Ali. 2022. "Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy" Nanomaterials 12, no. 21: 3925. https://doi.org/10.3390/nano12213925
APA StyleCai, M., Ratnayake, J., Cathro, P., Gould, M., & Ali, A. (2022). Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials, 12(21), 3925. https://doi.org/10.3390/nano12213925








