Threshold Switching in Forming-Free Anodic Memristors Grown on Hf–Nb Combinatorial Thin-Film Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Memristors
2.2. Electrical Characterization of Memristive Devices
2.3. Microscopic and Spectroscopic Analysis
3. Results
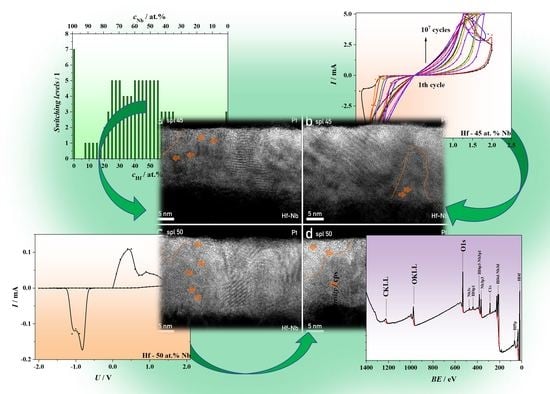
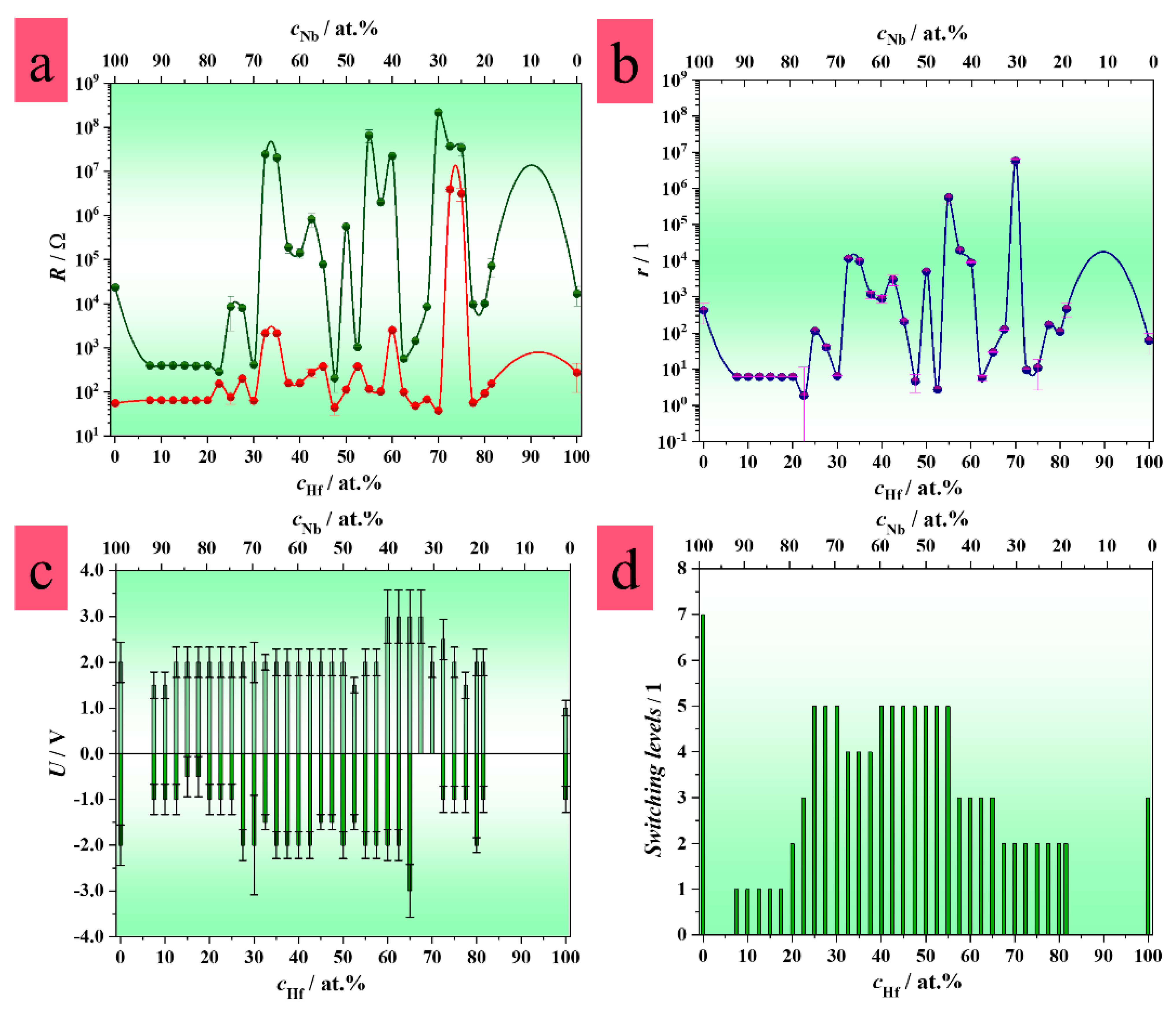
3.1. Combinatorial Screening of Memristive Behavior in Hf–Nb System
3.2. General Micro- and Nano-Scale Characterization
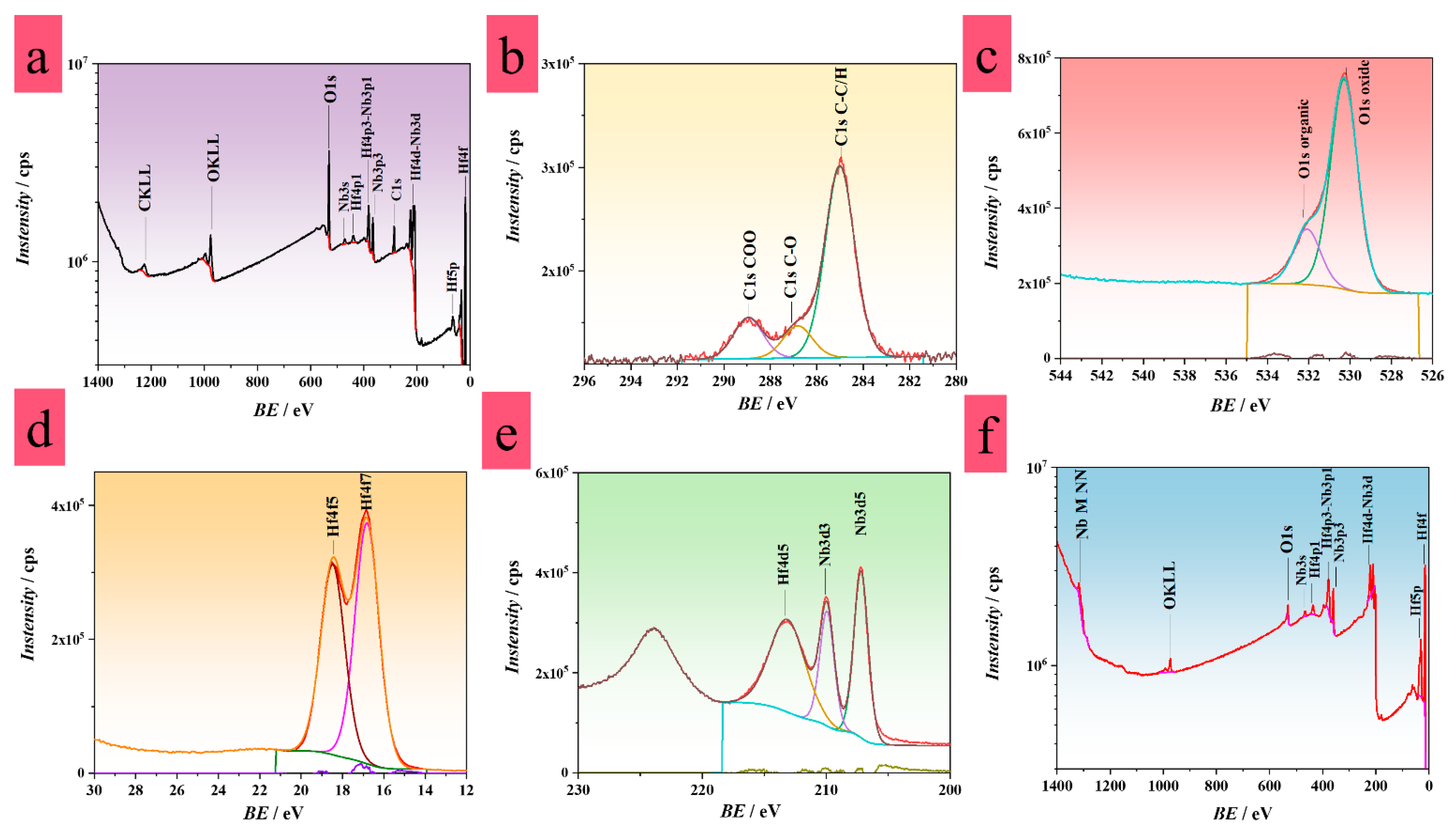
3.3. XPS Analysis
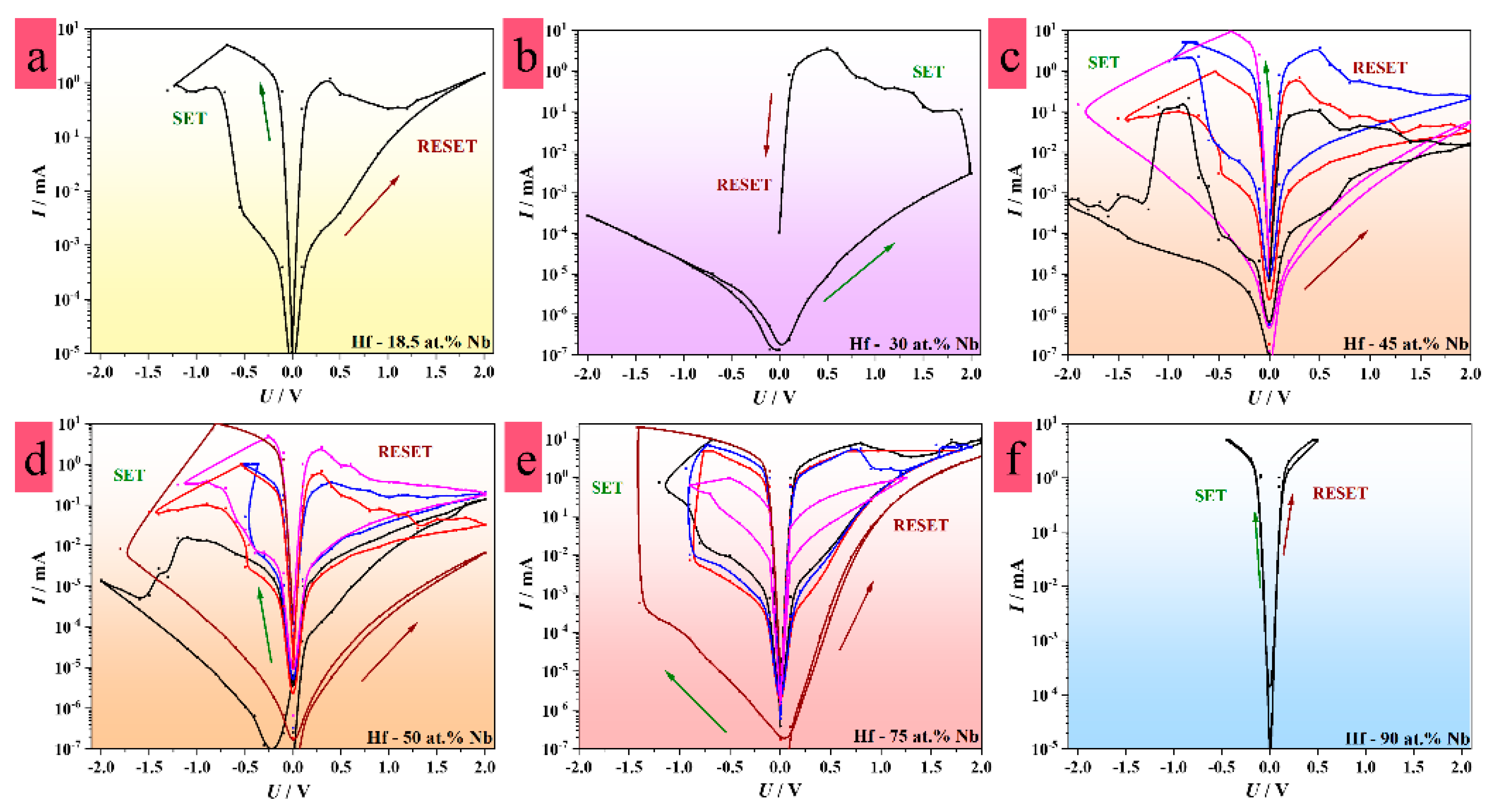
3.4. Memristive Switching of Anodized Hf–Nb Library
3.5. Electrical Characteristics of Hf–Nb Anodic Memristors
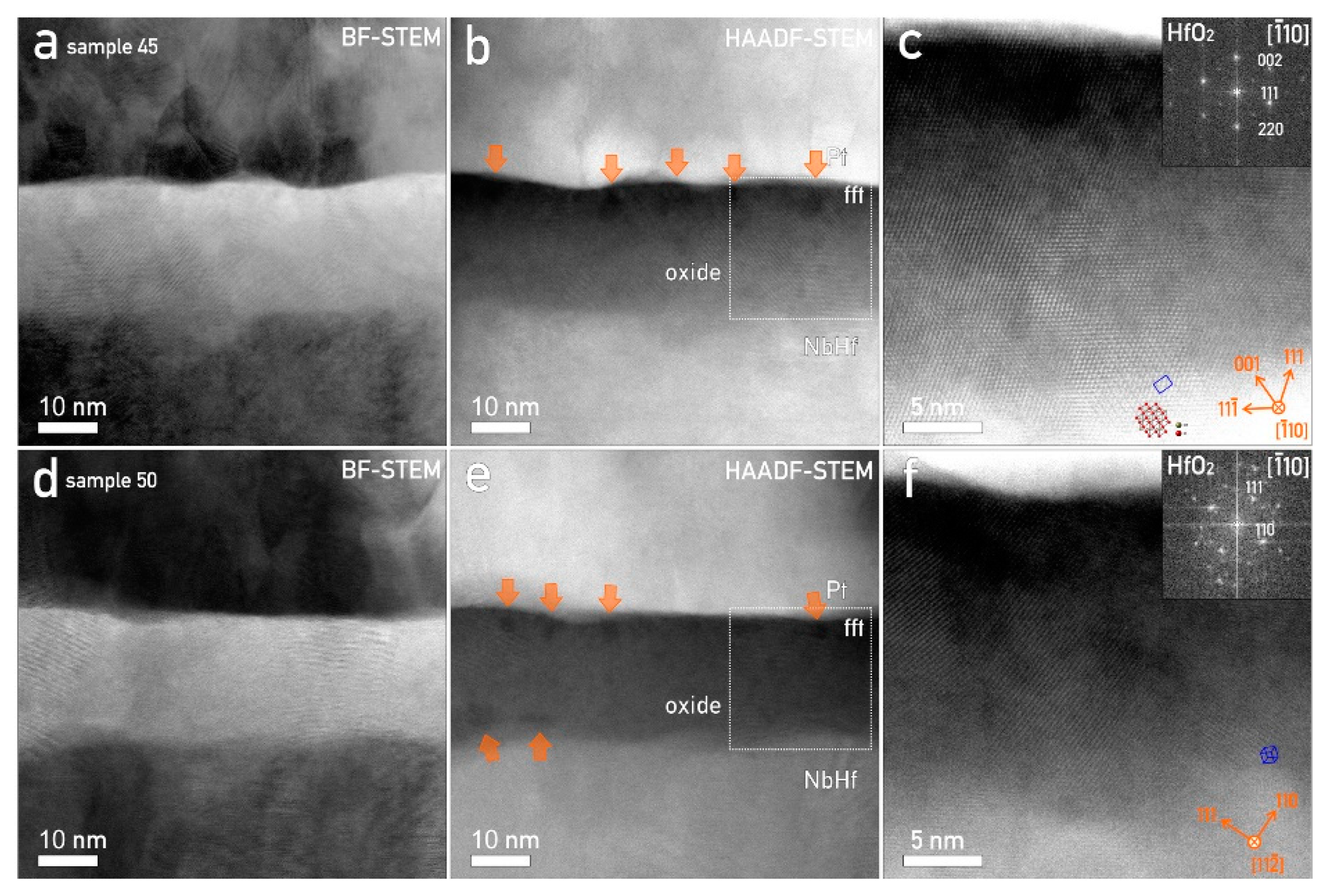
3.6. Nanostructural Characterization of Hf–Nb Anodic Memristors and CF Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Im, I.H.; Kim, S.J.; Jang, H.W. Memristive Devices for New Computing Paradigms. Adv. Intell. Syst. 2020, 2, 2000105. [Google Scholar] [CrossRef]
- Sun, K.; Chen, J.; Yan, X. The Future of Memristors: Materials Engineering and Neural Networks. Adv. Funct. Mater. 2021, 31, 2006773. [Google Scholar] [CrossRef]
- Sebastian, A.; Le Gallo, M.; Khaddam-Aljameh, R.; Eleftheriou, E. Memory devices and applications for in-memory computing. Nat. Nanotechnol. 2020, 15, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y. Advances in Non-Volatile Memory and Storage Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780857095008. [Google Scholar]
- Upadhyay, N.K.; Jiang, H.; Wang, Z.; Asapu, S.; Xia, Q.; Joshua Yang, J. Emerging Memory Devices for Neuromorphic Computing. Adv. Mater. Technol. 2019, 4, 201800589. [Google Scholar] [CrossRef] [Green Version]
- Ielmini, D. Resistive switching memories based on metal oxides: Mechanisms, reliability and scaling. Semicond. Sci. Technol. 2016, 31, 63002. [Google Scholar] [CrossRef]
- Yang, J.J.; Strukov, D.B.; Stewart, D.R. Memristive devices for computing. Nat. Nanotechnol. 2013, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Abbas, Y.; Hassan, G.; Sokolov, A.S.; Jeon, Y.R.; Ku, B.; Kang, C.J.; Choi, C. The coexistence of threshold and memory switching characteristics of ALD HfO2memristor synaptic arrays for energy-efficient neuromorphic computing. Nanoscale 2020, 12, 14120–14134. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Yin, S.; Qiao, L.; Wan, Q.; Wang, R.; Zeng, F.; Pan, F. Design of a Controllable Redox-Diffusive Threshold Switching Memristor. Adv. Electron. Mater. 2020, 6, 202000695. [Google Scholar] [CrossRef]
- Li, H.Y.; Huang, X.D.; Yuan, J.H.; Lu, Y.F.; Wan, T.Q.; Li, Y.; Xue, K.H.; He, Y.H.; Xu, M.; Tong, H.; et al. Controlled Memory and Threshold Switching Behaviors in a Heterogeneous Memristor for Neuromorphic Computing. Adv. Electron. Mater. 2020, 6, 202000309. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Gao, B.; Sun, W.; Hua, Q.; Zhang, W.; Li, X.; Zhang, W.; Qian, H.; Wu, H. High-Uniformity Threshold Switching HfO2-Based Selectors with Patterned Ag Nanodots. Adv. Sci. 2020, 7, 202002251. [Google Scholar] [CrossRef]
- Wang, Z.; Rao, M.; Midya, R.; Joshi, S.; Jiang, H.; Lin, P.; Song, W.; Asapu, S.; Zhuo, Y.; Li, C.; et al. Threshold Switching of Ag or Cu in Dielectrics: Materials, Mechanism, and Applications. Adv. Funct. Mater. 2018, 28, 201704862. [Google Scholar] [CrossRef]
- Xiao, M.; Shen, D.; Futscher, M.H.; Ehrler, B.; Musselman, K.P.; Duley, W.W.; Zhou, Y.N. Threshold Switching in Single Metal-Oxide Nanobelt Devices Emulating an Artificial Nociceptor. Adv. Electron. Mater. 2020, 6, 201900595. [Google Scholar] [CrossRef]
- Minnekhanov, A.A.; Emelyanov, A.V.; Lapkin, D.A.; Nikiruy, K.E.; Shvetsov, B.S.; Nesmelov, A.A.; Rylkov, V.V.; Demin, V.A.; Erokhin, V.V. Parylene Based Memristive Devices with Multilevel Resistive Switching for Neuromorphic Applications. Sci. Rep. 2019, 9, 10800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, G.W.; Shelby, R.M.; Sebastian, A.; Kim, S.; Kim, S.; Sidler, S.; Virwani, K.; Ishii, M.; Narayanan, P.; Fumarola, A.; et al. Neuromorphic computing using non-volatile memory. Adv. Phys. X 2017, 2, 89–124. [Google Scholar] [CrossRef]
- Sokolov, A.S.; Abbas, H.; Abbas, Y.; Choi, C. Towards engineering in memristors for emerging memory and neuromorphic computing: A review. J. Semicond. 2021, 42, 13101. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, A.; Hu, Q.; Xiao, Z.; Chu, P.K. Neuromorphic Computing with Memristor Crossbar. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1700875. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, S.; Savel’ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2017, 16, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yuan, P.; Fu, L.; Li, R.; Gao, X.; Tao, C. Coexistence of diode-like volatile and multilevel nonvolatile resistive switching in a ZrO2/TiO2 stack structure. Nanotechnology 2015, 26, 391001. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Md Sadaf, S.; Son, M.; Park, J.; Shin, J.; Lee, W.; Seo, K.; Lee, D.; Hwang, H. Co-occurrence of threshold switching and memory switching in Pt/NbO x/Pt cells for crosspoint memory applications. IEEE Electron Device Lett. 2012, 33, 236–238. [Google Scholar] [CrossRef]
- Abbas, H.; Ali, A.; Jung, J.; Hu, Q.; Park, M.R.; Lee, H.H.; Yoon, T.S.; Kang, C.J. Reversible transition of volatile to non-volatile resistive switching and compliance current-dependent multistate switching in IGZO/MnO RRAM devices. Appl. Phys. Lett. 2019, 114, 93503. [Google Scholar] [CrossRef]
- Mahne, H.; Wylezich, H.; Slesazeck, S.; Mikolajick, T.; Vesely, J.; Klemm, V.; Rafaja, D. Room temperature fabricated NbOx/Nb2O5 memory switching device with threshold switching effect. In Proceedings of the 2013 5th IEEE International Memory Workshop 2013, Monterey, CA, USA, 26–29 May 2013; pp. 174–177. [Google Scholar] [CrossRef]
- System, N.N.X.; Luo, Q.; Zhang, X.; Member, S.; Yu, J.; Wang, W. Memory Switching and Threshold Switching in a 3D nanoscaled NbO x system. IEEE Electron Device Lett. 2019, 40, 718–721. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Pisoni, R.; Cologni, A.; Brenna, A.; Corinto, F.; Pedeferri, M.P. Anodic oxidation as a means to produce memristive films. J. Appl. Biomater. Funct. Mater. 2016, 14, e290–e295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundozerova, T.V.; Grishin, A.M.; Stefanovich, G.B.; Velichko, A.A. Anodic Nb2O5 nonvolatile RRAM. IEEE Trans. Electron Devices 2012, 59, 1144–1148. [Google Scholar] [CrossRef]
- Aglieri, V.; Zaffora, A.; Lullo, G.; Santamaria, M.; Di Franco, F.; Lo Cicero, U.; Mosca, M.; Macaluso, R. Resistive switching in microscale anodic titanium dioxide-based memristors. Superlattices Microstruct. 2018, 113, 135–142. [Google Scholar] [CrossRef]
- Zrinski, I.; Mardare, C.C.; Jinga, L.I.; Kollender, J.P.; Socol, G.; Minenkov, A.; Hassel, A.W.; Mardare, A.I. Electrolyte-dependent modification of resistive switching in anodic hafnia. Nanomaterials 2021, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Zrinski, I.; Löfler, M.; Zavašnik, J.; Cancellieri, C.; Jeurgens, L.P.H.; Hassel, A.W.; Mardare, A.I. Impact of Electrolyte Incorporation in Anodized Niobium on Its Resistive Switching. Nanomaterials 2022, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Kim, H.; Rehman, S.; Hur, J.H.; Song, Y.H.; Khan, M.F.; Kim, D. kee Effect of oxygen stoichiometry on the threshold switching of RF-sputtered NbOx (x = 2.0–2.5) films. Mater. Res. Bull. 2021, 144, 111492. [Google Scholar] [CrossRef]
- Park, K.; Ryu, J.; Sahu, D.P.; Kim, H.M.; Yoon, T.S. Electroforming-free threshold switching of NbOx-based selector devices by controlling conducting phases in the NbOx layer for the application to crossbar array architectures. RSC Adv. 2022, 12, 18547–18558. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Zhu, M.; Jin, R. Atomically precise alloy nanoclusters: Syntheses, structures, and properties. Chem. Soc. Rev. 2020, 49, 6443–6514. [Google Scholar] [CrossRef]
- Sun, B.; Guo, T.; Zhou, G.; Ranjan, S.; Jiao, Y.; Wei, L.; Zhou, Y.N.; Wu, Y.A. Synaptic devices based neuromorphic computing applications in artificial intelligence. Mater. Today Phys. 2021, 18, 100393. [Google Scholar] [CrossRef]
- Ryu, J.H.; Mahata, C.; Kim, S. Long-term and short-term plasticity of Ta2O5/HfO2 memristor for hardware neuromorphic application. J. Alloys Compd. 2021, 850, 156675. [Google Scholar] [CrossRef]
- Gao, B.; Bi, Y.; Chen, H.Y.; Liu, R.; Huang, P.; Chen, B.; Liu, L.; Liu, X.; Yu, S.; Wong, H.S.P.; et al. Ultra-low-energy three-dimensional oxide-based electronic synapses for implementation of robust high-accuracy neuromorphic computation systems. ACS Nano 2014, 8, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Zrinski, I.; Minenkov, A.; Cancellieri, C.; Hauert, R.; Mardare, C.C.; Kollender, J.P.; Jeurgens, L.P.H.; Groiss, H.; Hassel, A.W.; Mardare, A.I. Mixed anodic oxides for forming-free memristors revealed by combinatorial screening of hafnium-tantalum system. Appl. Mater. Today 2022, 26, 101270. [Google Scholar] [CrossRef]
- Borghetti, J.; Snider, G.S.; Kuekes, P.J.; Yang, J.J.; Stewart, D.R.; Williams, R.S. Memristive switches enable stateful logic operations via material implication. Nature 2010, 464, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Cai, F.; Zidan, M.A.; Ma, W.; Lee, S.H.; Lu, W.D. Reservoir computing using dynamic memristors for temporal information processing. Nat. Commun. 2017, 8, 2204. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zhou, Z.; Chen, J.; Choy, T.H.; Wang, J.; Zhang, N.; Lin, Z.; Yu, S.; Kang, J.; Wong, H.S.P.; et al. Optoelectronic resistive random access memory for neuromorphic vision sensors. Nat. Nanotechnol. 2019, 14, 776–782. [Google Scholar] [CrossRef]
- Mardare, A.I.; Ludwig, A.; Savan, A.; Wieck, A.D.; Hassel, A.W. Combinatorial investigation of Hf-Ta thin films and their anodic oxides. Electrochim. Acta 2010, 55, 7884–7891. [Google Scholar] [CrossRef]
- Sodium Phosphate. Cold Spring Harb. Protoc. 2006, 2006, pdb.rec8303. [CrossRef]
- Zrinski, I.; Minenkov, A.; Mardare, C.C.; Kollender, J.P.; Lone, S.A.; Hassel, A.W.; Mardare, A.I. Influence of electrolyte selection on performance of tantalum anodic oxide memristors. Appl. Surf. Sci. 2021, 565, 150608. [Google Scholar] [CrossRef]
- Mardare, A.I.; Ludwig, A.; Savan, A.; Hassel, A.W. Scanning droplet cell microscopy on a wide range hafnium-niobium thin film combinatorial library. Electrochim. Acta 2013, 110, 539–549. [Google Scholar] [CrossRef]
- Mardare, A.I.; Ludwig, A.; Savan, A.; Hassel, A.W. Properties of anodic oxides grown on a hafnium-tantalum-titanium thin film library. Sci. Technol. Adv. Mater. 2014, 15, 15006. [Google Scholar] [CrossRef]
- Mardare, A.I.; Savan, A.; Ludwig, A.; Wieck, A.D.; Hassel, A.W. A combinatorial passivation study of Ta-Ti alloys. Corros. Sci. 2009, 51, 1519–1527. [Google Scholar] [CrossRef]
- Mardare, A.I.; Savan, A.; Ludwig, A.; Wieck, A.D.; Hassel, A.W. High-throughput synthesis and characterization of anodic oxides on Nb-Ti alloys. Electrochim. Acta 2009, 54, 5973–5980. [Google Scholar] [CrossRef]
- Mardare, A.I.; Yadav, A.P.; Wieck, A.D.; Stratmann, M.; Hassel, A.W. Combinatorial electrochemistry on Al-Fe alloys. Sci. Technol. Adv. Mater. 2008, 9, 35009. [Google Scholar] [CrossRef]
- Zrinski, I.; Minenkov, A.; Mardare, C.C.; Hassel, A.W.; Mardare, A.I. Composite Memristors by Nanoscale Modification of Hf/Ta Anodic Oxides. J. Phys. Chem. Lett. 2021, 12, 8917–8923. [Google Scholar] [CrossRef]
- Lanza, M.; Wong, H.S.P.; Pop, E.; Ielmini, D.; Strukov, D.; Regan, B.C.; Larcher, L.; Villena, M.A.; Yang, J.J.; Goux, L.; et al. Recommended Methods to Study Resistive Switching Devices. Adv. Electron. Mater. 2019, 5, 201800143. [Google Scholar] [CrossRef] [Green Version]
- Beamson, G.; Briggs, D. High resolution monochromated X-ray photoelectron spectroscopy of organic polymers: A comparison between solid state data for organic polymers and gas phase data for small molecules. Mol. Phys. Int. J. Interface Chem. Phys. 1992, 76, 919–936. [Google Scholar] [CrossRef]
- Briggs, D. X-ray photoelectron spectroscopy (XPS). In Handbook of Adhesion, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2005; pp. 621–622. [Google Scholar] [CrossRef]
- Barreca, D.; Milanov, A.; Fischer, R.A.; Devi, A.; Tondello, E. Hafnium oxide thin film grown by ALD: An XPS study. Surf. Sci. Spectra 2007, 14, 34–40. [Google Scholar] [CrossRef]
- Zagorenko, A.I.; Zaporozchenko, V.I.; Ivanova, O.P. Quantitative Auger analysis of metal oxides. Surf. Interface Anal. 1992, 18, 496–498. [Google Scholar] [CrossRef]
- Mcguire, G.E.; Schweitzer, G.K.; Carlson, T.A. Core electron binding energies in some Group IIIA, VB, and VIB compounds. Inorg. Chem. 1973, 12, 2450–2453. [Google Scholar] [CrossRef]
- Nyholm, R.; Berndtsson, A.; Martensson, N. Core level binding energies for the elements Hf to Bi (Z=72-83). J. Phys. C Solid State Phys. 1980, 13, L1091. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Nandi, S.K.; Elliman, R.G. Anatomy of filamentary threshold switching in amorphous niobium oxide. Nanotechnology 2018, 29, 375705. [Google Scholar] [CrossRef]
- Nath, S.K.; Nandi, S.K.; Li, S.; Elliman, R.G. Metal-oxide interface reactions and their effect on integrated resistive/threshold switching in NbOx. Nanotechnology 2020, 31, 235701. [Google Scholar] [CrossRef] [Green Version]
- Aziz, J.; Kim, H.; Rehman, S.; Kadam, K.D.; Patil, H.; Aftab, S.; Khan, M.F.; Kim, D. kee Discrete memristive levels and logic gate applications of Nb2O5 devices. J. Alloys Compd. 2021, 879, 160385. [Google Scholar] [CrossRef]
- Zrinski, I.; Mardare, C.C.; Jinga, L.-I.; Kollender, J.P.; Socol, G.; Hassel, A.W.; Mardare, A.I. Phosphate incorporation in anodic hafnium oxide memristors. Appl. Surf. Sci. 2021, 548, 149093. [Google Scholar] [CrossRef]
- Mikolajick, T.; Wylezich, H.; Maehne, H.; Slesazeck, S. Versatile resistive switching in niobium oxide. Proc.—IEEE Int. Symp. Circuits Syst. 2016, 2016, 381–384. [Google Scholar] [CrossRef]
- Chen, A.; Ma, G.; He, Y.; Chen, Q.; Liu, C.; Wang, H.; Chang, T.C. Research on Temperature Effect in Insulator-Metal Transition Selector Based on NbOx Thin Films. IEEE Trans. Electron Devices 2018, 65, 5448–5452. [Google Scholar] [CrossRef]
- Kim, S.; Liu, X.; Park, J.; Jung, S.; Lee, W.; Woo, J.; Shin, J.; Choi, G.; Cho, C.; Park, S.; et al. Ultrathin (<10 nm) Nb2O5/NbO2 hybrid memory with both memory and selector characteristics for high density 3D vertically stackable RRAM applications. In Proceedings of the 2012 Symposium on VLSI Technology (VLSIT), Honolulu, HI, USA, 12–14 June 2012; pp. 155–156. [Google Scholar] [CrossRef]
- Liu, X.; Nandi, S.K.; Venkatachalam, D.K.; Belay, K.; Song, S.; Elliman, R.G. Reduced threshold current in NbO2selector by engineering device structure. IEEE Electron Device Lett. 2014, 35, 1055–1057. [Google Scholar] [CrossRef]
- Kang, M.; Yu, S.; Son, J. Voltage-induced insulator-to-metal transition of hydrogen-treated NbO2 thin films. J. Phys. D Appl. Phys. 2015, 48, 95301. [Google Scholar] [CrossRef]
- Rathi, S.; Park, J.H.; Lee, I.Y.; Baik, J.M.; Yi, K.S.; Kim, G.H. Unravelling the switching mechanisms in electric field induced insulator-metal transitions in VO2 nanobeams. J. Phys. D Appl. Phys. 2014, 47, 295101. [Google Scholar] [CrossRef]
- Gentle, A.; Smith, G.B. Dual metal-insulator and insulator-insulator switching in nanoscale and Al doped VO2. J. Phys. D Appl. Phys. 2008, 41, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Sun, B.; Ranjan, S.; Zhu, X.; Zhou, G.; Zhao, H.; Mao, S.; Wang, H.; Zhao, Y.; Fu, G. Mechanism analysis of a flexible organic memristive memory with capacitance effect and negative differential resistance state. APL Mater. 2019, 7, 81117. [Google Scholar] [CrossRef]
- Goux, L.; Lisoni, J.G.; Jurczak, M.; Wouters, D.J.; Courtade, L.; Muller, C. Coexistence of the bipolar and unipolar resistive-switching modes in NiO cells made by thermal oxidation of Ni layers. J. Appl. Phys. 2010, 107, 202115. [Google Scholar] [CrossRef]
- Mohammad, B.; Jaoude, M.A.; Kumar, V.; Al Homouz, D.M.; Nahla, H.A.; Al-Qutayri, M.; Christoforou, N. State of the art of metal oxide memristor devices. Nanotechnol. Rev. 2016, 5, 311–329. [Google Scholar] [CrossRef]
- Du, H.; Chen, J.; Tu, M.; Luo, S.; Li, S.; Yuan, S.; Gong, T.; Huang, W.; Jie, W.; Hao, J. Transition from nonvolatile bipolar memory switching to bidirectional threshold switching in layered MoO3 nanobelts. J. Mater. Chem. C 2019, 7, 12160–12169. [Google Scholar] [CrossRef]
- Ma, Y.; Yeoh, P.P.; Shen, L.; Goodwill, J.M.; Bain, J.A.; Skowronski, M. Evolution of the conductive filament with cycling in TaOx-based resistive switching devices. J. Appl. Phys. 2020, 128, 194501. [Google Scholar] [CrossRef]
- Kim, G.S.; Park, T.H.; Kim, H.J.; Ha, T.J.; Park, W.Y.; Kim, S.G.; Hwang, C.S. Investigation of the retention performance of an ultra-thin HfO2 resistance switching layer in an integrated memory device. J. Appl. Phys. 2018, 124, 24102. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zrinski, I.; Zavašnik, J.; Duchoslav, J.; Hassel, A.W.; Mardare, A.I. Threshold Switching in Forming-Free Anodic Memristors Grown on Hf–Nb Combinatorial Thin-Film Alloys. Nanomaterials 2022, 12, 3944. https://doi.org/10.3390/nano12223944
Zrinski I, Zavašnik J, Duchoslav J, Hassel AW, Mardare AI. Threshold Switching in Forming-Free Anodic Memristors Grown on Hf–Nb Combinatorial Thin-Film Alloys. Nanomaterials. 2022; 12(22):3944. https://doi.org/10.3390/nano12223944
Chicago/Turabian StyleZrinski, Ivana, Janez Zavašnik, Jiri Duchoslav, Achim Walter Hassel, and Andrei Ionut Mardare. 2022. "Threshold Switching in Forming-Free Anodic Memristors Grown on Hf–Nb Combinatorial Thin-Film Alloys" Nanomaterials 12, no. 22: 3944. https://doi.org/10.3390/nano12223944
APA StyleZrinski, I., Zavašnik, J., Duchoslav, J., Hassel, A. W., & Mardare, A. I. (2022). Threshold Switching in Forming-Free Anodic Memristors Grown on Hf–Nb Combinatorial Thin-Film Alloys. Nanomaterials, 12(22), 3944. https://doi.org/10.3390/nano12223944