Efficient Mesoporous MgO/g-C3N4 for Heavy Metal Uptake: Modeling Process and Adsorption Mechanism
Abstract
1. Introduction
2. Experimental
2.1. Fabrication of MGCN
2.2. Characterization
2.3. Adsorption Measurements
3. Results and Discussion
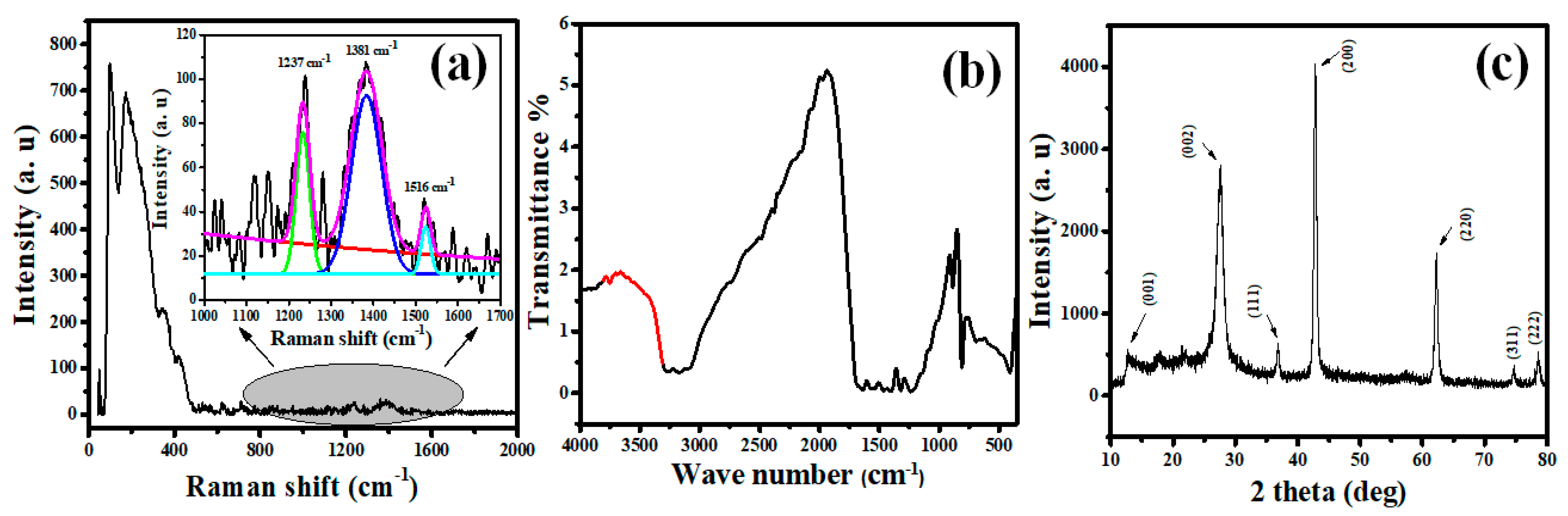
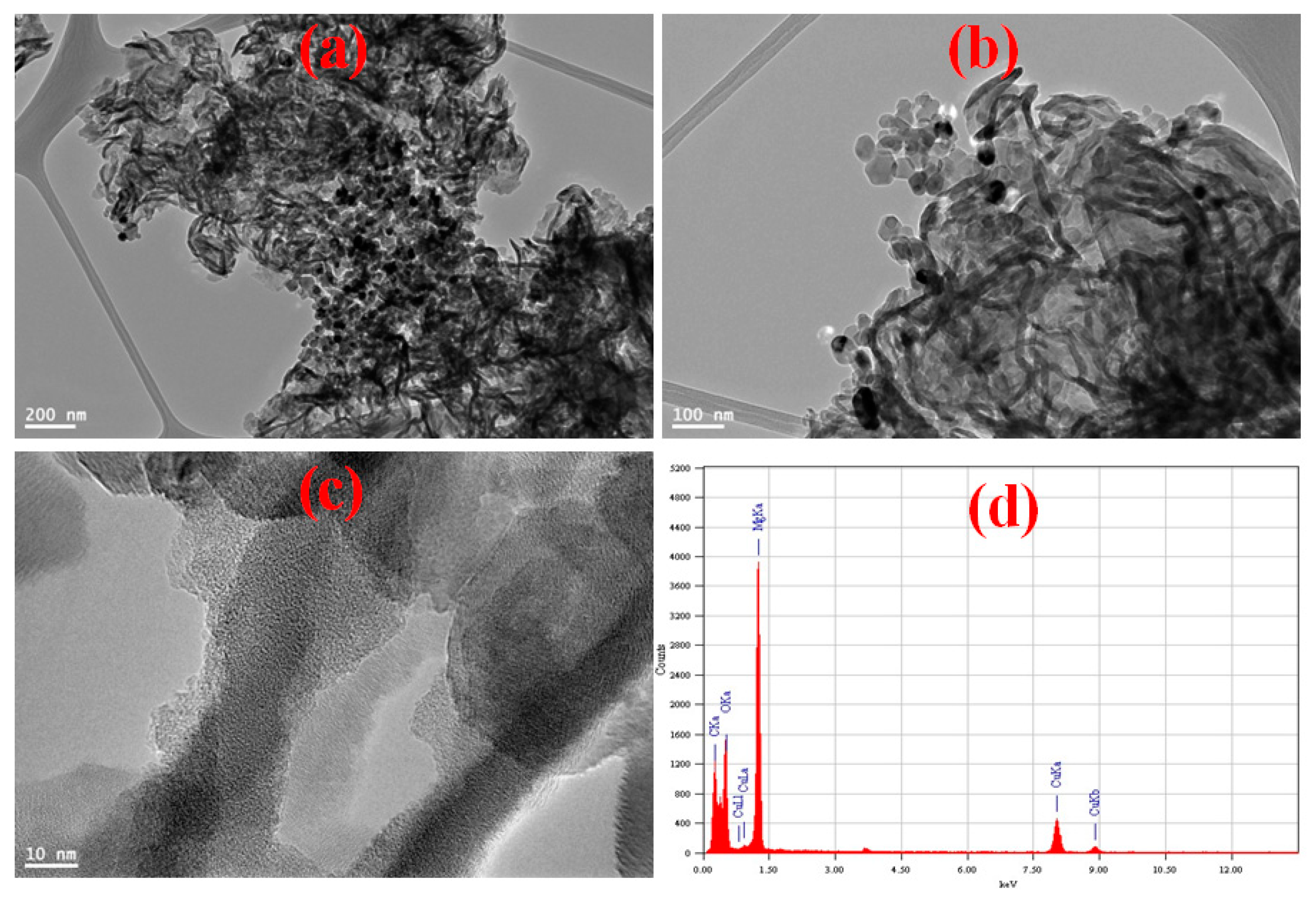
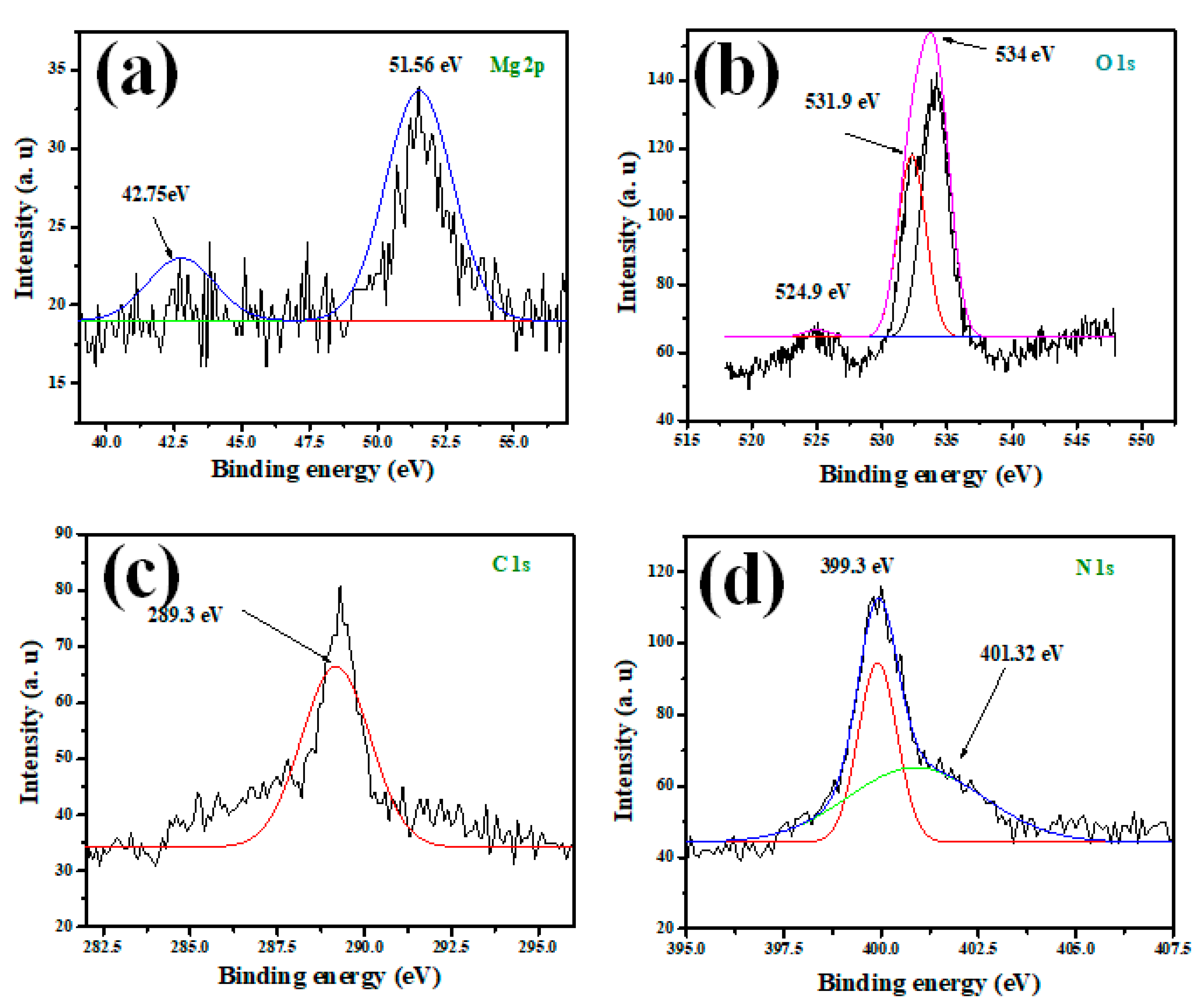
3.1. MGCN Nanomaterial’s Surface Properties
3.2. MGCN Adsorption Study
3.2.1. Comparative Analysis of the Adsorption Capabilities of MgO, g-C3N4, and MGCN
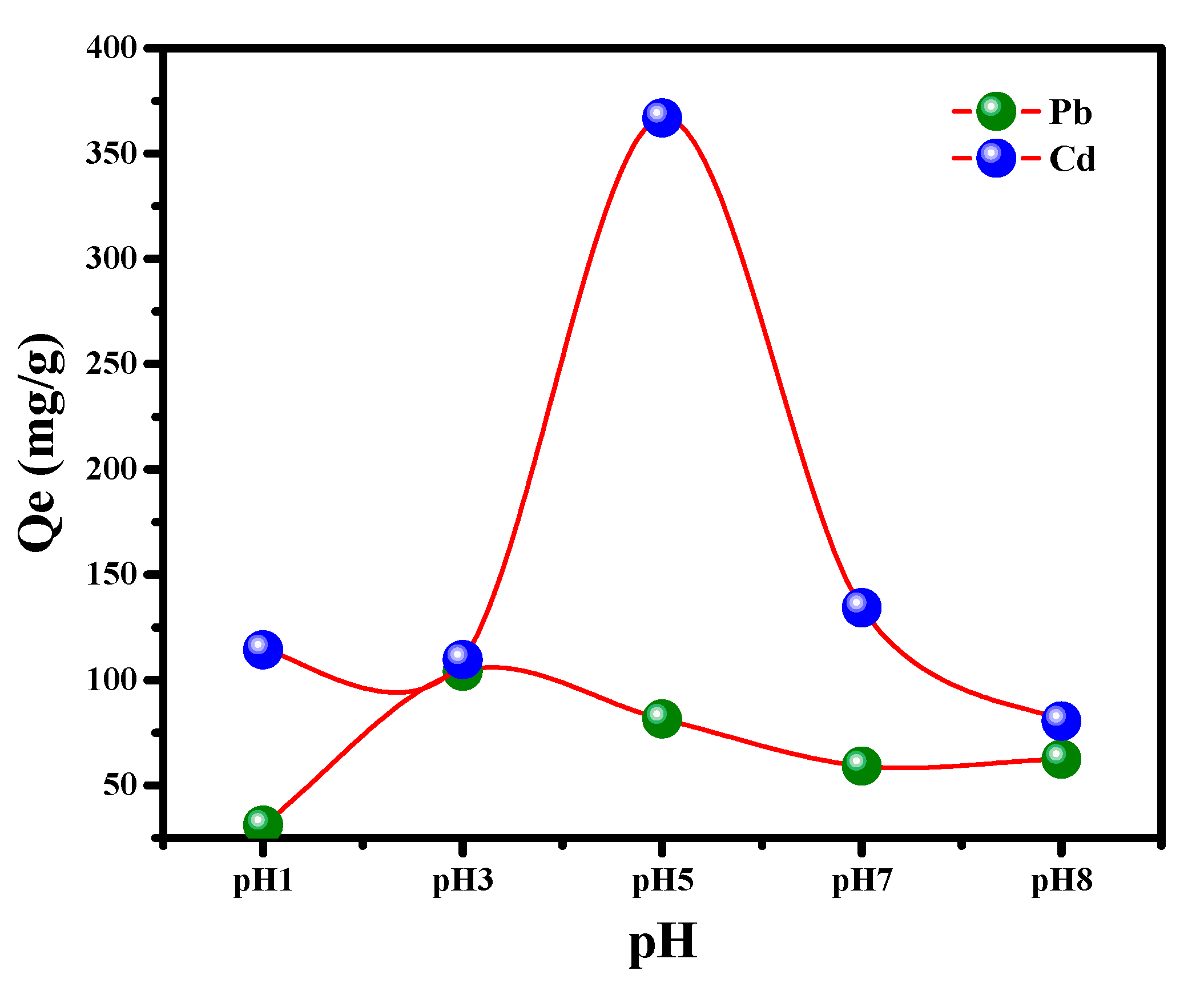
3.2.2. Impact of pH on the Uptake Process
3.2.3. Adsorption Kinetics
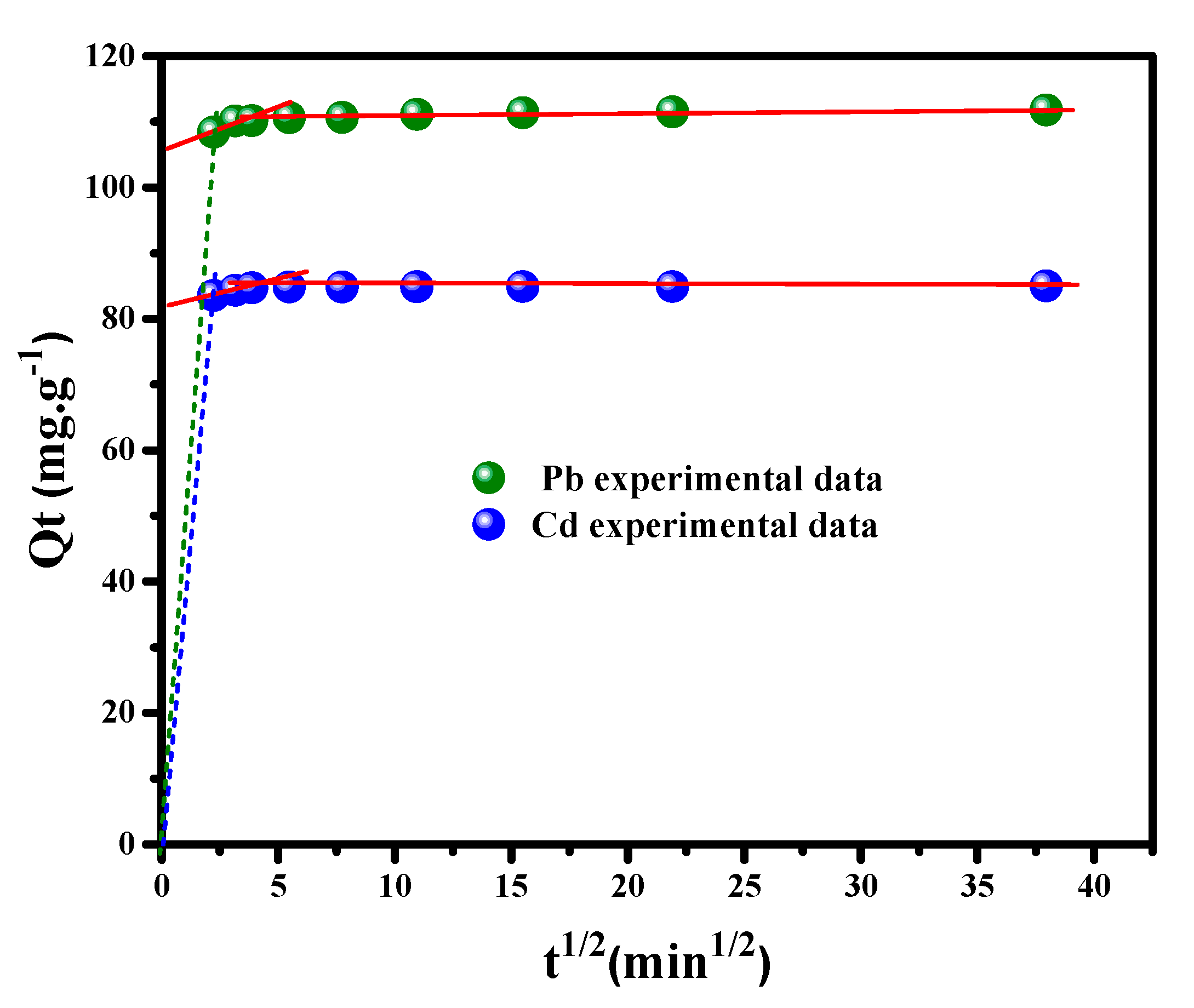
3.2.4. Intra-Particle Diffusion Study
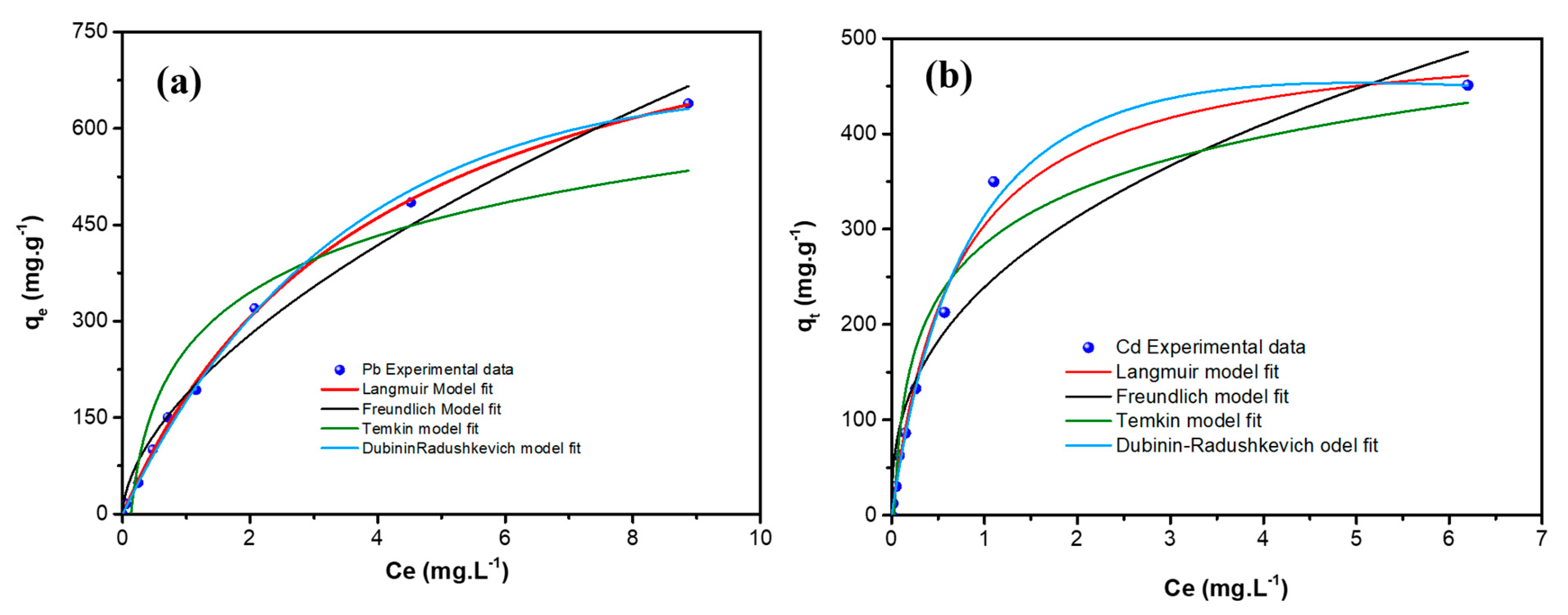
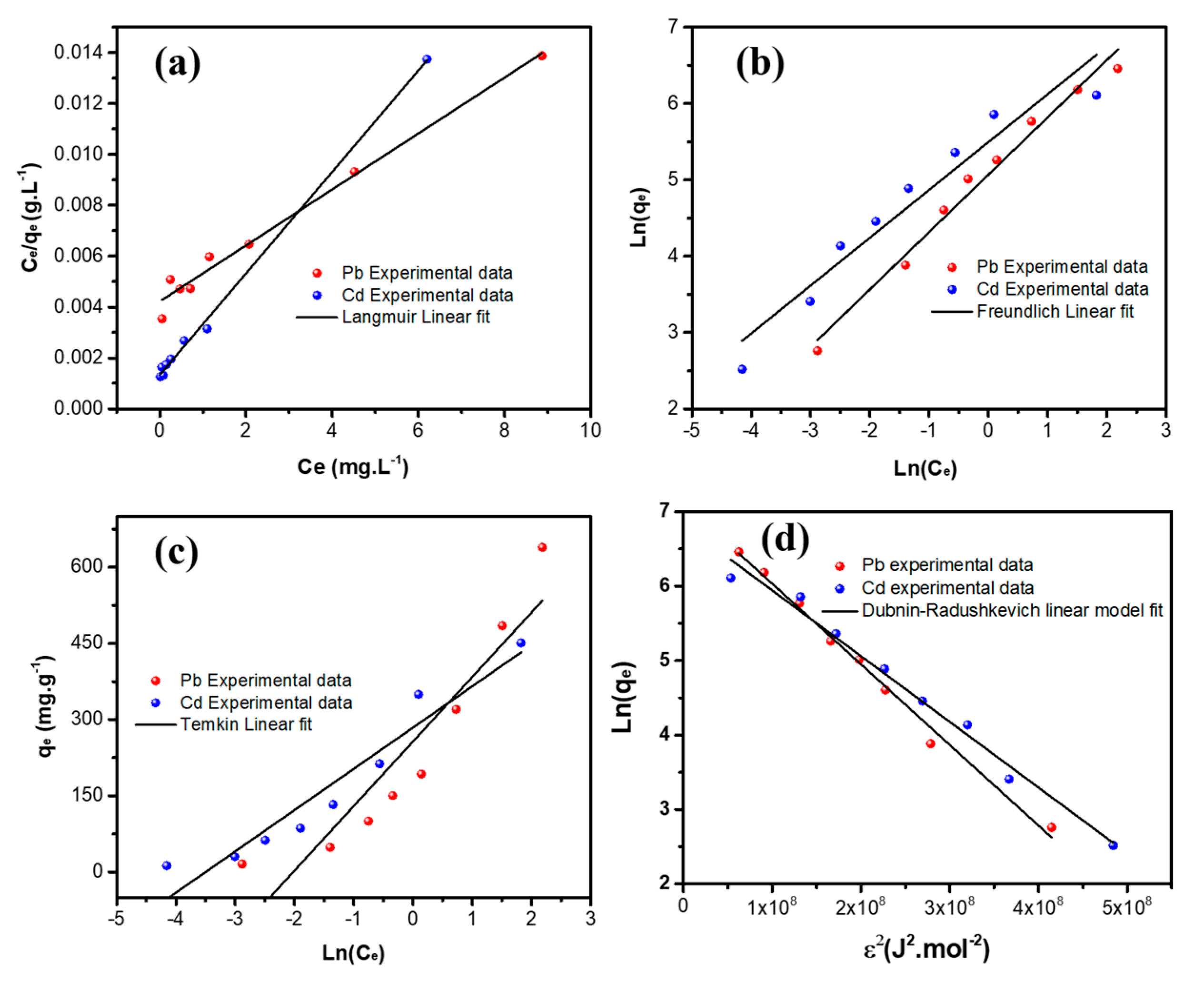
3.2.5. Adsorption Isotherms
| Equilibrium Model | Linear Form | Non-Linear Form |
|---|---|---|
| Langmuir [53] | ||
| Freundlich [54] | = | |
| Temkin [55] | ||
| Dubnin-Radushkevich [53] | , |
| Equilibrium Model | Parameters | Pb++ | Cd++ |
| Langmuir | qm (mg g−1) | 927.81 | 511.55 |
| KL (mg g−1) | 0.247 | 1.467 | |
| RL (L mg−1) | 0.0043 | 0.0013 | |
| r2 | 0.9989 | 0.9909 | |
| Freundlich | n | 1.71 | 2.57 |
| KF (L mg−1) | 186.34 | 239.24 | |
| R2 | 0.9867 | 0.9168 | |
| Temkin | B (J mol−1) | 19.51 | 30.51 |
| KT (L mg−1) | 7.55 | 33.14 | |
| r2 | 0.8879 | 0.9367 | |
| Dubnin–Radishkevich | qm (mg g−1) | 1234 | 922 |
| K (mol kJ−1)2 | 1.08 × 10−8 | 8.83 × 10−9 | |
| E (kJ mol−1) | 6.795 | 7.525 | |
| r2 | 0.9924 | 0.9852 |
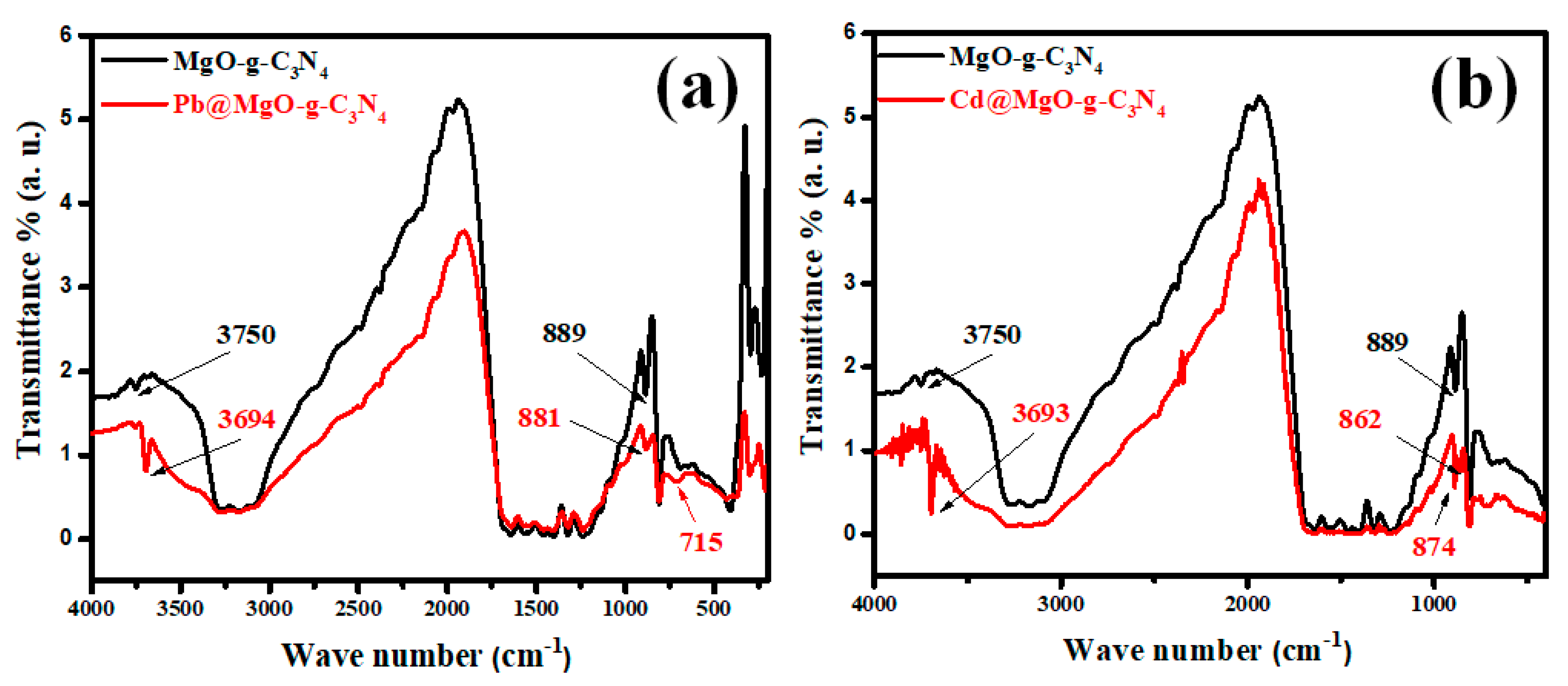
3.3. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vajedi, F.; Dehghani, H. The characterization of TiO2-reduced graphene oxide nanocomposites and their performance in electrochemical determination for removing heavy metals ions of cadmium(II), lead(II) and copper(II). Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 243, 189–198. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Conventional and Current Methods of Toxic Metals Removal from Water Using g-C3N4-Based Materials. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1419–1442. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Jin, X.Y.; Lu, X.Q.; Chen, Z.L. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Agents classified by the IARC monographs. Igarss 2012, 1–105, 1–5. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Skyllberg, U.; Drott, A. Competition between disordered iron sulfide and natural organic matter associated thiols for mercury(II)—An EXAFS study. Environ. Sci. Technol. 2010, 44, 1254–1259. [Google Scholar] [CrossRef]
- Melita, L.; Popescu, M. Removal of Cr (VI) from industrial water effluents and surface waters using activated composite membranes. J. Memb. Sci. 2008, 312, 157–162. [Google Scholar] [CrossRef]
- Lu, X.; Huangfu, X.; Zhang, X.; Wang, Y.; Ma, J. Removal of trace mercury (II) from aqueous solution by in situ MnOx combined with poly-aluminum chloride. J. Water Health 2015, 13, 383–393. [Google Scholar] [CrossRef]
- Farrukh, A.; Akram, A.; Ghaffar, A.; Hanif, S.; Hamid, A.; Duran, H.; Yameen, B. Design of polymer-brush-grafted magnetic nanoparticles for highly efficient water remediation. ACS Appl. Mater. Interfaces 2013, 5, 3784–3793. [Google Scholar] [CrossRef]
- Teekayuttasakul, P.; Annachhatre, A.P. Lead removal and toxicity reduction from industrial wastewater through biological sulfate reduction process. J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2008, 43, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process. Sci. Total Environ. 2019, 674, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Mondal, S.; Sahoo, L.; Gautam, U.K. Ni-Fe-layered double hydroxide/N-doped graphene oxide nanocomposite for the highly efficient removal of Pb(II) and Cd(II) ions from water. J. Solid State Chem. 2019, 280, 120963. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Dallas, P.; Dimotikali, D.; Trapalis, C. Novel torus shaped g-C3N4 photocatalysts. Appl. Catal. B Environ. 2020, 268, 118733. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef]
- Li, J.; Liu, E.; Ma, Y.; Hu, X.; Wan, J.; Sun, L.; Fan, J. Synthesis of MoS2/g-C3N4 nanosheets as 2D heterojunction photocatalysts with enhanced visible light activity. Appl. Surf. Sci. 2016, 364, 694–702. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Modwi, A.; Idriss, H.; Aldaghri, O.; Ismail, M.; Ibnaouf, K.H. Detoxification of Pb (II) from aquatic media via CaMgO2@g-C3N4 nanocomposite. Mater. Lett. 2022, 322, 132501. [Google Scholar] [CrossRef]
- Toghan, A.; El-Lateef, H.M.A.; Taha, K.K.; Modwi, A. Mesoporous TiO2@g-C3N4 composite: Construction, characterization, and boosting indigo carmine dye destruction. Diam. Relat. Mater. 2021, 118, 108491. [Google Scholar] [CrossRef]
- Yin, R.; Luo, Q.; Wang, D.; Sun, H.; Li, Y.; Li, X.; An, J. SnO2/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity. J. Mater. Sci. 2014, 49, 6067–6073. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Qian, Q.; Machida, M.; Tatsumoto, H. Effect of ZnO loading to activated carbon on Pb(II) adsorption from aqueous solution. Carbon N. Y. 2006, 44, 195–202. [Google Scholar] [CrossRef]
- Di, Z.C.; Ding, J.; Peng, X.J.; Li, Y.H.; Luan, Z.K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Ghoniem, M.; Nguyen-Tri, P.; Baaloudj, O.; Guesmi, A.; AlGethami, F.; Amer, M.; Assadi, A. Superior removal of dyes by mesoporous MgO/g-C3N4 fabricated through ultrasound method: Adsorption mechanism and process modeling. Environ. Res. 2022, 205, 112543. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, K.; Xu, W.; Chen, D.; Fang, J.; Zhu, X.; Sun, J.; Liang, Y.; Hu, X.; Li, R.; et al. Adsorption-photocatalysis synergistic removal of contaminants under antibiotic and Cr(VI) coexistence environment using non-metal g-C3N4 based nanomaterial obtained by supramolecular self-assembly method. J. Hazard. Mater. 2021, 404, 124171. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Barathi, D.; Meyvel, S.; Sathya, P. S-scheme Ag2CrO4/g-C3N4 photocatalyst for effective degradation of organic pollutants under visible light. Inorg. Chem. Commun. 2021, 132, 108849. [Google Scholar] [CrossRef]
- Vuggili, S.B.; Khanth, S.K.; Kadiya, K.; Gaur, U.K.; Sharma, M. Improvement in visible light stimulated photocatalysis by the inducement of magnesium dopant inside graphitic carbon nitride frameworks. J. Environ. Chem. Eng. 2019, 7, 103440. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Taha, K.K.; Idriss, H. Flower Buds Like MgO Nanoparticles: From Characterisation to Indigo Carmine Elimination. Zeitschrift fur Naturforsch.—Sect. A J. Phys. Sci. 2018, 73, 975–983. [Google Scholar] [CrossRef]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Ghosh, S. Visible-light-induced nitrogen photofixation ability of g-C3N4 nanosheets decorated with MgO nanoparticles. J. Ind. Eng. Chem. 2020, 84, 185–195. [Google Scholar] [CrossRef]
- Suresh, J.; Yuvakkumar, R.; Sundrarajan, M.; Hong, S.I. Green synthesis of magnesium oxide nanoparticles. Adv. Mater. Res. 2014, 952, 141–144. [Google Scholar] [CrossRef]
- Imani, M.M.; Safaei, M. Optimized Synthesis of Magnesium Oxide Nanoparticles as Bactericidal Agents. J. Nanotechnol. 2019, 2019, 6063832. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Ramadass, K.; Benzigar, M.R.; Lakhi, K.S.; Yang, J.-H.; Ravon, U.; Albahily, K.; Vinu, A. Controlled synthesis of three dimensional mesoporous C3N4 with ordered porous structure for room temperature Suzuki coupling reaction. ScienceDirect 2019, 477, 110548. [Google Scholar] [CrossRef]
- Jiang, J.; Ou-Yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon N. Y. 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Yang, N.-N.; Chen, Z.-G.; Zhao, Z.-G.; Cui, Y. Electrochemical fabrication of ultrafine g-C3N4 quantum dots as a catalyst for the hydrogen evolution reaction. New Carbon Mater. 2022, 37, 392–401. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Pham, M.-T.; Tran, D.P.H.; Bui, X.-T.; You, S.-J. Rapid Fabrication of MgO@g-C3N4 Heterojunction for Photocatalytic Nitric Oxide degradation under Visible Light. Beilstein Arch. 2022, 2022, 30. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Xu, S.; Li, P.; Yang, C.; Jin, Y.; Sun, Q.; Su, H. Superior adsorption performance of graphitic carbon nitride nanosheets for both cationic and anionic heavy metals from wastewater. Chin. J. Chem. Eng. 2019, 27, 305–313. [Google Scholar] [CrossRef]
- Kumar, M.R.A.; Nagaswarupa, H.P.; Ravikumar, C.R.; Prashantha, S.C.; Nagabhushana, H.; Bhatt, A.S. Green engineered nano MgO and ZnO doped with Sm3+: Synthesis and a comparison study on their characterization, PC activity and electrochemical properties. J. Phys. Chem. Solids 2019, 127, 127–139. [Google Scholar] [CrossRef]
- Mao, N.; Jiang, J.X. MgO/g-C3N4 nanocomposites as efficient water splitting photocatalysts under visible light irradiation. Appl. Surf. Sci. 2019, 476, 144–150. [Google Scholar] [CrossRef]
- Toghan, A.; Modwi, A. Boosting unprecedented indigo carmine dye photodegradation via mesoporous MgO@g-C3N4 nanocomposite. J. Photochem. Photobiol. A Chem. 2021, 419, 113467. [Google Scholar] [CrossRef]
- Sharma, M.; Poddar, M.; Gupta, Y.; Nigam, S.; Avasthi, D.; Adelung, R.; Abolhassani, R.; Fiutowski, J.; Joshi, M.; Mishra, Y. Solar light assisted degradation of dyes and adsorption of heavy metal ions from water by CuO–ZnO tetrapodal hybrid nanocomposite. Mater. Today Chem. 2020, 17, 100336. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Chii, Y.Y.; Siddique, B.M. Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination 2009, 247, 636–646. [Google Scholar] [CrossRef]
- Adarakatti, P.S.; Gangaiah, V.K.; Banks, C.E.; Siddaramanna, A. One-pot synthesis of Mn3O4/graphitic carbon nanoparticles for simultaneous nanomolar detection of Pb(II), Cd(II) and Hg(II). J. Mater. Sci. 2018, 53, 4961–4973. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kebir, M.; Khezami, L.; Amrane, A.; Assadi, A.A. A comparative study of ceramic nanoparticles synthesized for antibiotic removal: Catalysis characterization and photocatalytic performance modeling. Environ. Sci. Pollut. Res. 2020, 28, 13900–13912. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Mondal, N.K.; Basu, S. Potentiality of waste human hair towards removal of chromium(VI) from solution: Kinetic and equilibrium studies. Appl. Water Sci. 2019, 9, 49. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Xie, J.; Wang, S.; Cao, Y. A solid-state chemical method for synthesizing MgO nanoparticles with superior adsorption properties. RSC Adv. 2019, 9, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Razavi, M.M. Water reuse: Brackish water desalination using Prosopis juliflora. Environ. Technol. Innov. 2020, 17, 100614. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Khezami, L.; Modwi, A.; Ghiloufi, I.; Taha, K.K.; Bououdina, M.; ElJery, A.; El Mir, L. Effect of aluminum loading on structural and morphological characteristics of ZnO nanoparticles for heavy metal ion elimination. Environ. Sci. Pollut. Res. 2020, 27, 3086–3099. [Google Scholar] [CrossRef]
- Khezami, L.; Taha, K.K.; Amami, E.; Ghiloufi, I.; El Mir, L. Removal of cadmium (II) from aqueous solution by zinc oxide nanoparticles: Kinetic and thermodynamic studies. Desalin. Water Treat. 2017, 62, 346–354. [Google Scholar] [CrossRef]
- Mustafa, B.; Modwi, A.; Ismail, M.; Makawi, S.; Hussein, T.; Abaker, Z.; Khezami, L. Adsorption performance and Kinetics study of Pb(II) by RuO2–ZnO nanocomposite: Construction and Recyclability. Int. J. Environ. Sci. Technol. 2021, 19, 327–340. [Google Scholar] [CrossRef]
- Li, C.; Yan, W.; Xin, Q. Interaction of methane with surface of alumina studied by FT-IR spectroscopy. Catal. Lett. 1994, 24, 249–256. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Unravelling the Environmental Application of Biochar as Low-Cost Biosorbent: A Review. Appl. Sci. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Bhukkal, C.; Ahlawat, R. Cu2+–Mn2+-Co-doped CdO nanocrystallites: Comprehensive research on phase, morphology and optoelectronic properties. Res. Chem. Intermed. 2020, 46, 4211–4232. [Google Scholar] [CrossRef]
- Arulmozhi, K.T.; Mythili, N. Studies on the chemical synthesis and characterization of lead oxide nanoparticles with different organic capping agents. AIP Adv. 2013, 3, 122122. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, H.; Zhang, Q.; Jiang, G.; Cai, W.; Hu, W. Adsorption of cadmium(II) in wastewater by magnesium oxide modified biochar. Arab. J. Chem. 2022, 15, 104059. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.-M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Baniamerian, H.; Teimoori, M.; Saberi, M. Fe2O3/TiO2/Activated Carbon Nanocomposite with Synergistic Effect of Adsorption and Photocatalysis. Chem. Eng. Technol. 2021, 44, 130–139. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Sheetan, K.M. Development of a polymeric nanocomposite as a high performance adsorbent for Pb(II) removal from water medium: Equilibrium, kinetic and antimicrobial activity. J. Hazard. Mater. 2021, 407, 124816. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-coated magnetic nanocomposites for Pb2+ removal from aqueous solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Sun, Y.; Zhou, X.; Baig, S.A.; Xu, X. Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2016, 369, 267–276. [Google Scholar] [CrossRef]




| Kinetics Model | Kinetic Equation |
|---|---|
| Pseudo-first order [49] | |
| Pseudo-second order [50] | |
| Elovich [50] | |
| Intra-particle Diffusion [50] |
| Pseudo-Second Order Model | ||||||
|---|---|---|---|---|---|---|
| Metal ion | qe(Exp)a (mg g−1) | t1/2 (min) | h0 (mg g−1 min−1) | qe(Cal)b (mg g−1) | K2 × 102 (g mg−1 min−1) | r2 |
| Pb++ | 114 | 0.84 | 133.34 | 112.4 | 1.06 | 0.9999 |
| Cd++ | 90 | 0.75 | 112.36 | 84.8 | 1.56 | 0.9999 |
| Pseudo-First Order model | Elovich’s model | |||||
| Metal ion | qe(Cal) b (mg g−1) | K1 (min−1) | r2 | kb (L g−1) | α × 102 | r2 |
| Pb++ | 3.3 | 1 × 10−3 | 0.7880 | 1.252 | 5.8 | 0.9864 |
| Cd++ | 5.1 | 2 × 10−5 | 0.8649 | 1.245 | 1.0 | 0.6744 |
| Intra-particle diffusion/transport model | ||||||
| Metal ion | kdif1 (mg g−1 min−1/2) | C1 | r2 | kdif2 (mg g−1 min−1/2) | C2 | r2 |
| Pb++ | 59.92 | 23.13 | 0.9941 | 0.225 | 109.4 | 0.9731 |
| Cd++ | 50.80 | 29.7 | 0.9818 | 0.005 | 84.83 | 0.9527 |
| Adsorbents | Surface Area (m2/g) | qe (mg g−1) | Removal Efficiency (%) | Optimum pH and Initial Concentration Ci | Reference |
|---|---|---|---|---|---|
| g-C3N4 | 111.2 | Cd: 123.205 | 80% | Not mentioned Ci = 20 mg L−1 | [41] |
| MgO | Not mentioned | Cd: 135 | 74.1% | Not mentioned Ci = Not mentioned 100 mg dosage | [66] |
| Inorganic nanocomposites with different iron concentration | 649–680 | Cd: 1.12 | 90–92% | 6.5 Ci = 30 mg/L | [67] |
| Modified orange peel | Not mentioned | Cd: 13.7 | 85% | 5 Ci = 20 mg L−1 | [6] |
| NiFe-CO3-LDH-NGO composite | 151 | Cd: 971 | >95% | 5 Ci = 10–1000 mg L−1 | [14] |
| Natural kaolinite clay | 3.7 | Cd: Not mentioned | 94% | 7 Ci = 20 mg L−1 | [4] |
| MGCN | 84.37 | Cd: 511.55 | 97% | 5 Ci = 5–200 mg L−1 | This paper |
| Fe2O3/TiO2 | 130 | Pb: Not mentioned | 94% | 6.5 Ci = 35 mg L−1 | [68] |
| CSt-ZnO nanocomposite | 185.21 | Pb: 256.4 | 68% | 6 Ci = 19.97 mg L−1 | [69] |
| g-C3N4 | 111.2 | Pb: 136.571 | 80% | Not mentioned Ci = 20 mg L−1 | [41] |
| MgO | Not mentioned | Pb: 148.6 | 72.7% | Not mentioned Ci = not mentioned 100 mg dosage | [66] |
| Silica-Coated Magnetic Nanocomposites | 271.0 m2 | Pb: 14.9 | Not mentioned | 4–6 Ci = 2–120 mg L−1 | [70] |
| Modified orange peel | Not mentioned | Pb: 73.53 | 96% | 5 Ci = 20 mg L−1 | [6] |
| Fe3O4@SiO2-EDTA | 24.07 | Pb: 125.24 | Not mentioned | 5.3 ± 0.1 Ci = 100 mg L−1 | [71] |
| MGCN | 84.37 | Pb: 927.81 | 95.6% | 3 Ci = 5–200 mg L−1 | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AbuMousa, R.A.; Khezami, L.; Ismail, M.; Ben Aissa, M.A.; Modwi, A.; Bououdina, M. Efficient Mesoporous MgO/g-C3N4 for Heavy Metal Uptake: Modeling Process and Adsorption Mechanism. Nanomaterials 2022, 12, 3945. https://doi.org/10.3390/nano12223945
AbuMousa RA, Khezami L, Ismail M, Ben Aissa MA, Modwi A, Bououdina M. Efficient Mesoporous MgO/g-C3N4 for Heavy Metal Uptake: Modeling Process and Adsorption Mechanism. Nanomaterials. 2022; 12(22):3945. https://doi.org/10.3390/nano12223945
Chicago/Turabian StyleAbuMousa, Rasha A., Lotfi Khezami, Mukhtar Ismail, Mohamed Ali Ben Aissa, Abueliz Modwi, and Mohamed Bououdina. 2022. "Efficient Mesoporous MgO/g-C3N4 for Heavy Metal Uptake: Modeling Process and Adsorption Mechanism" Nanomaterials 12, no. 22: 3945. https://doi.org/10.3390/nano12223945
APA StyleAbuMousa, R. A., Khezami, L., Ismail, M., Ben Aissa, M. A., Modwi, A., & Bououdina, M. (2022). Efficient Mesoporous MgO/g-C3N4 for Heavy Metal Uptake: Modeling Process and Adsorption Mechanism. Nanomaterials, 12(22), 3945. https://doi.org/10.3390/nano12223945








