A Novel Metal-Containing Mesoporous Silica Composite for the Decolorization of Rhodamine B: Effect of Metal Content on Structure and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of MnxFeyS photocatalysts
2.3. Instruments
2.4. Experimental Procedure
3. Results and Discussion
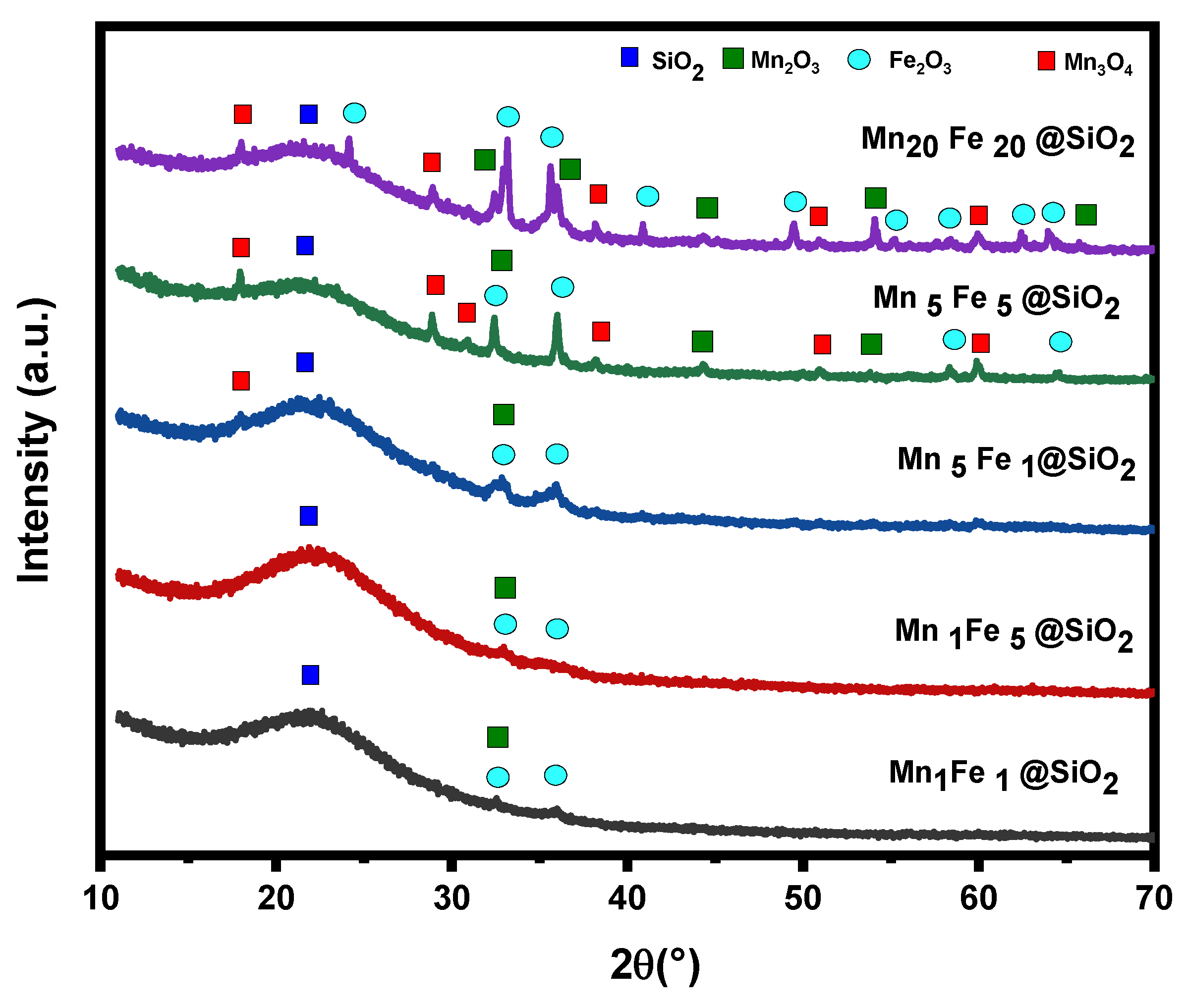
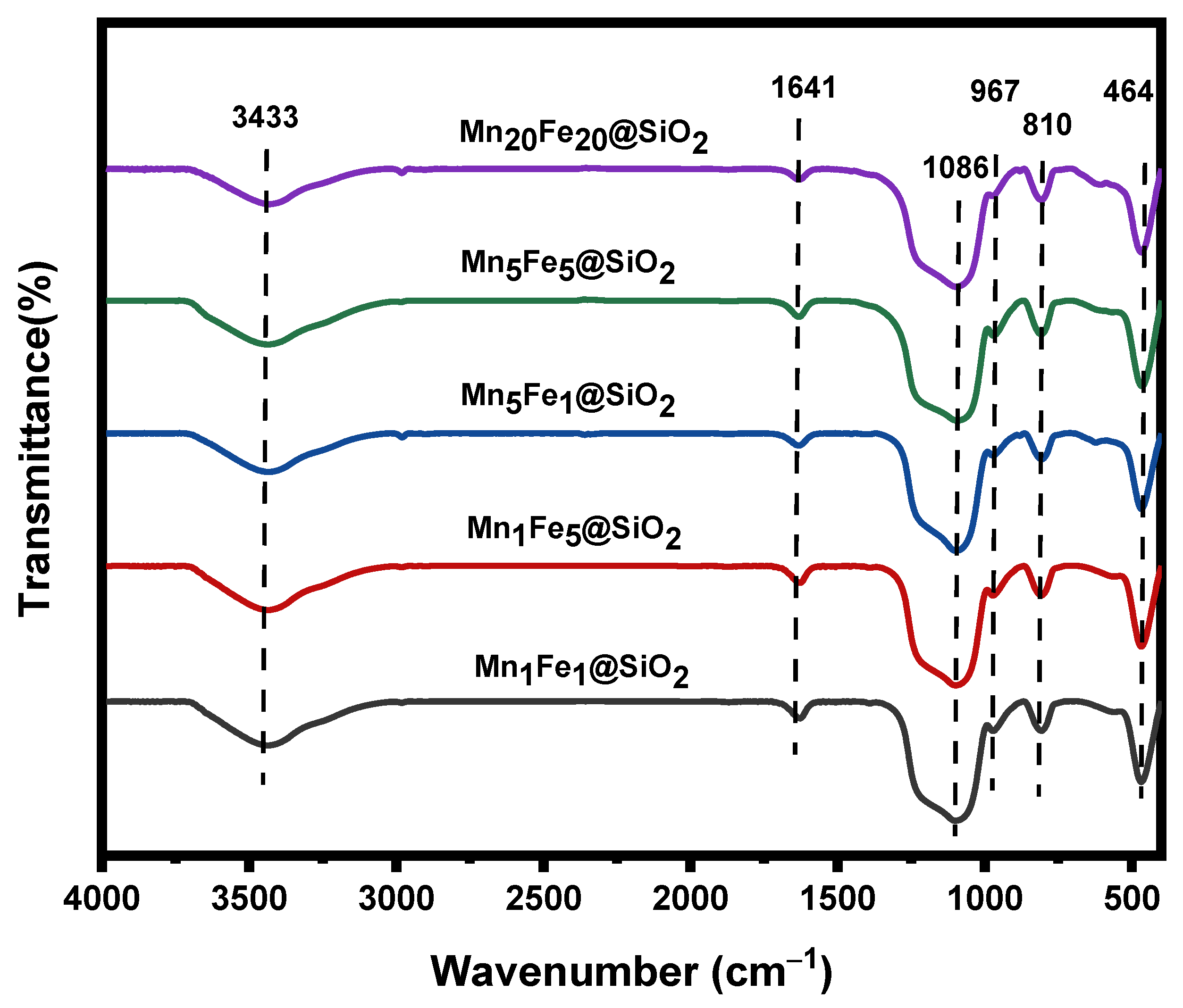
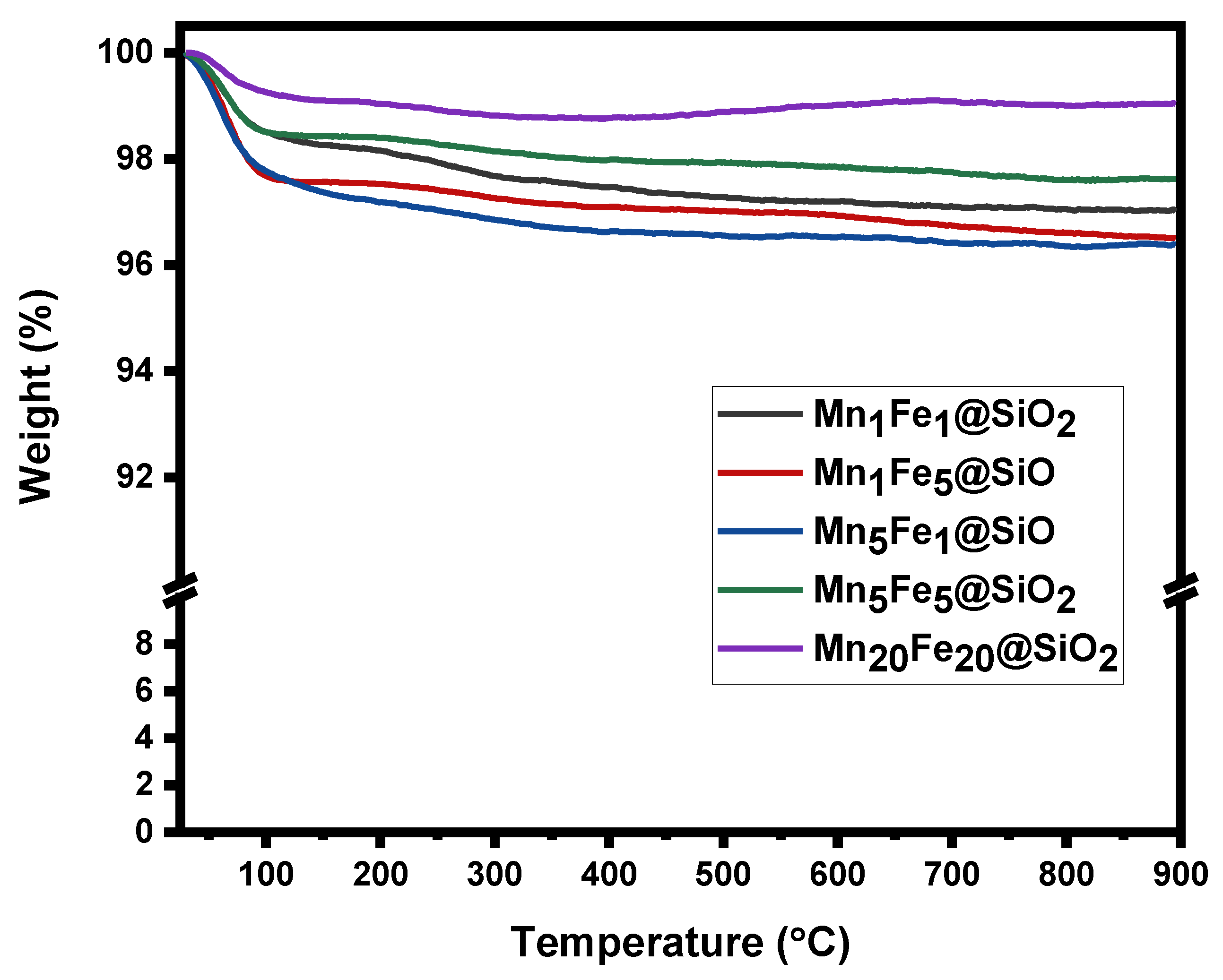
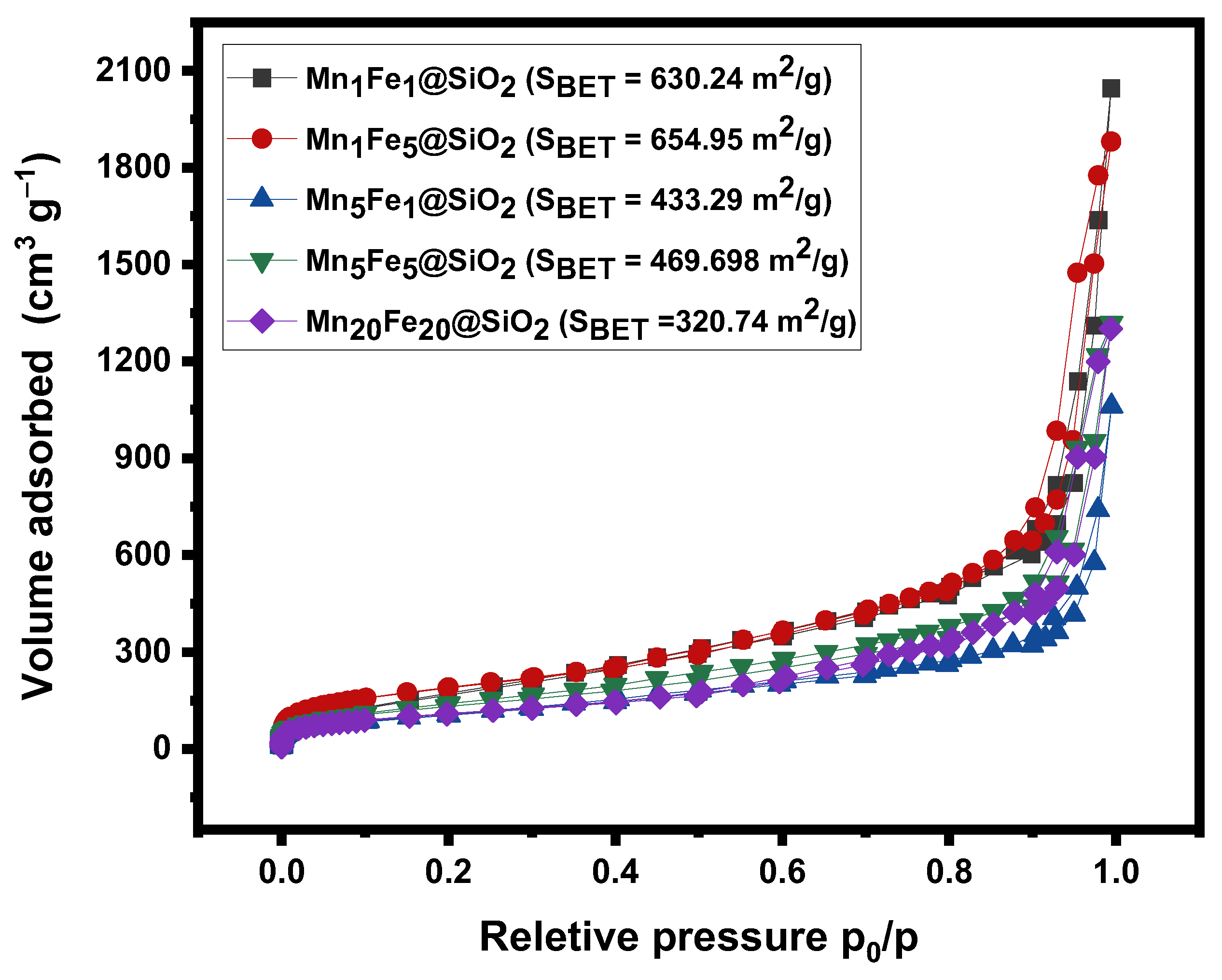
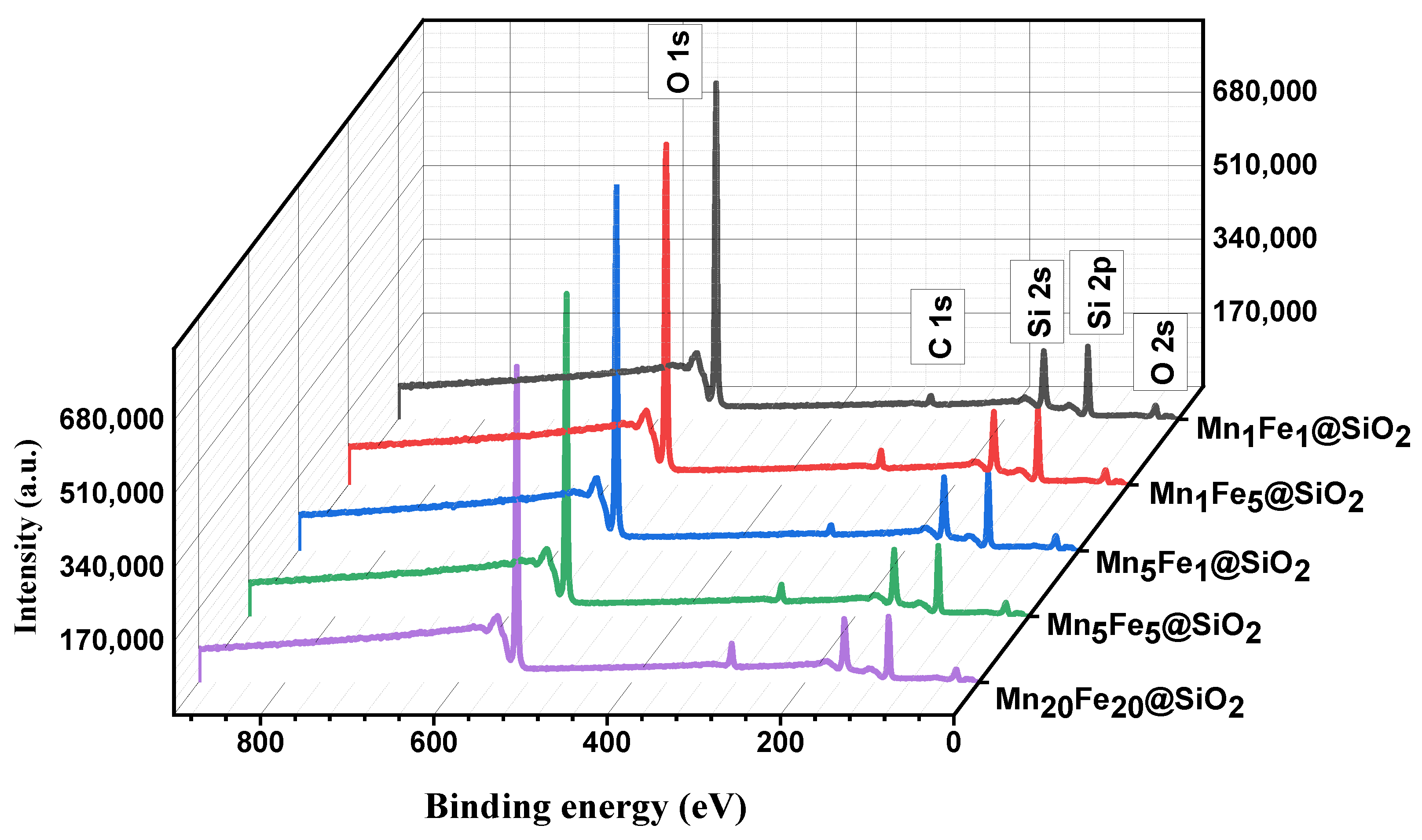
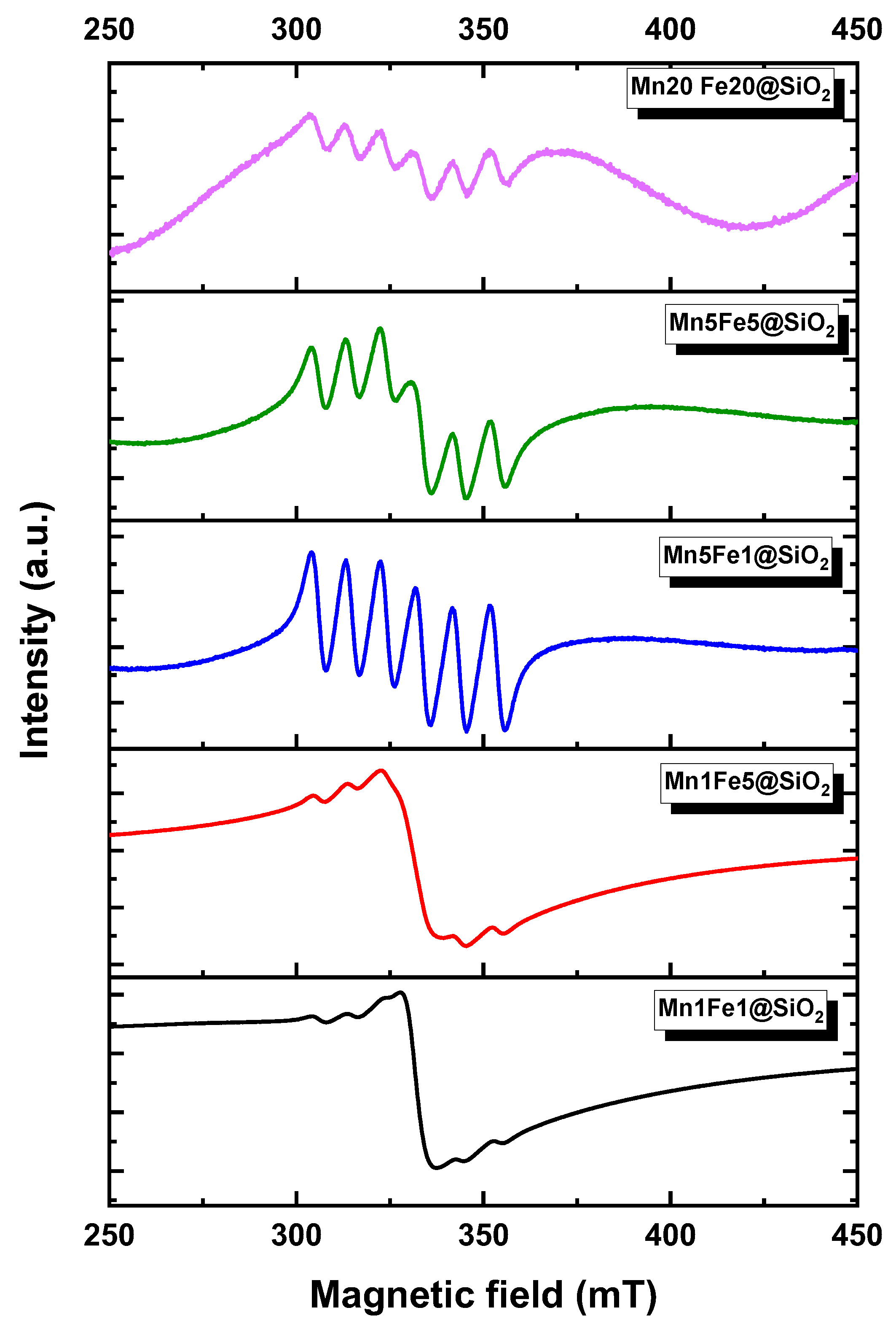
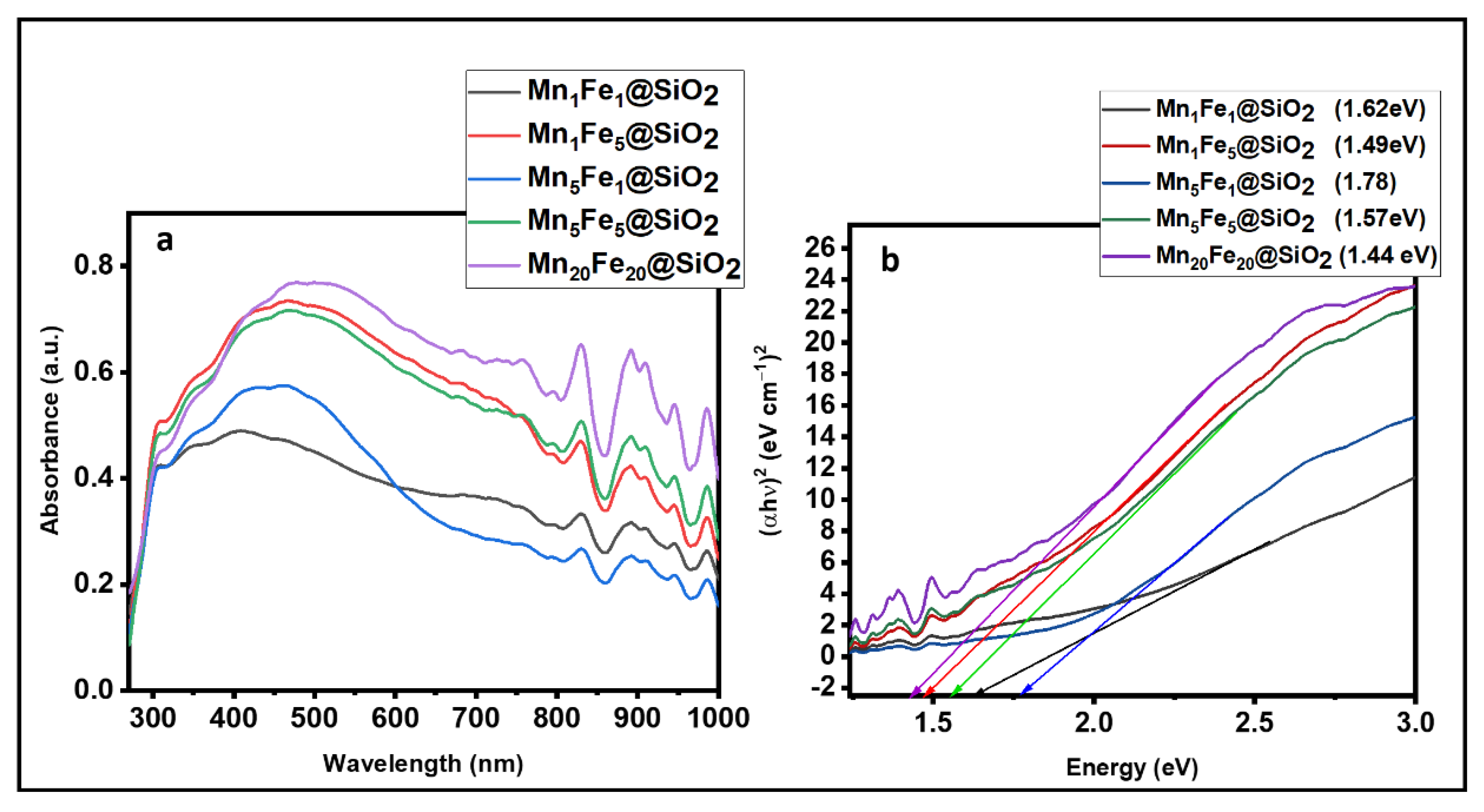
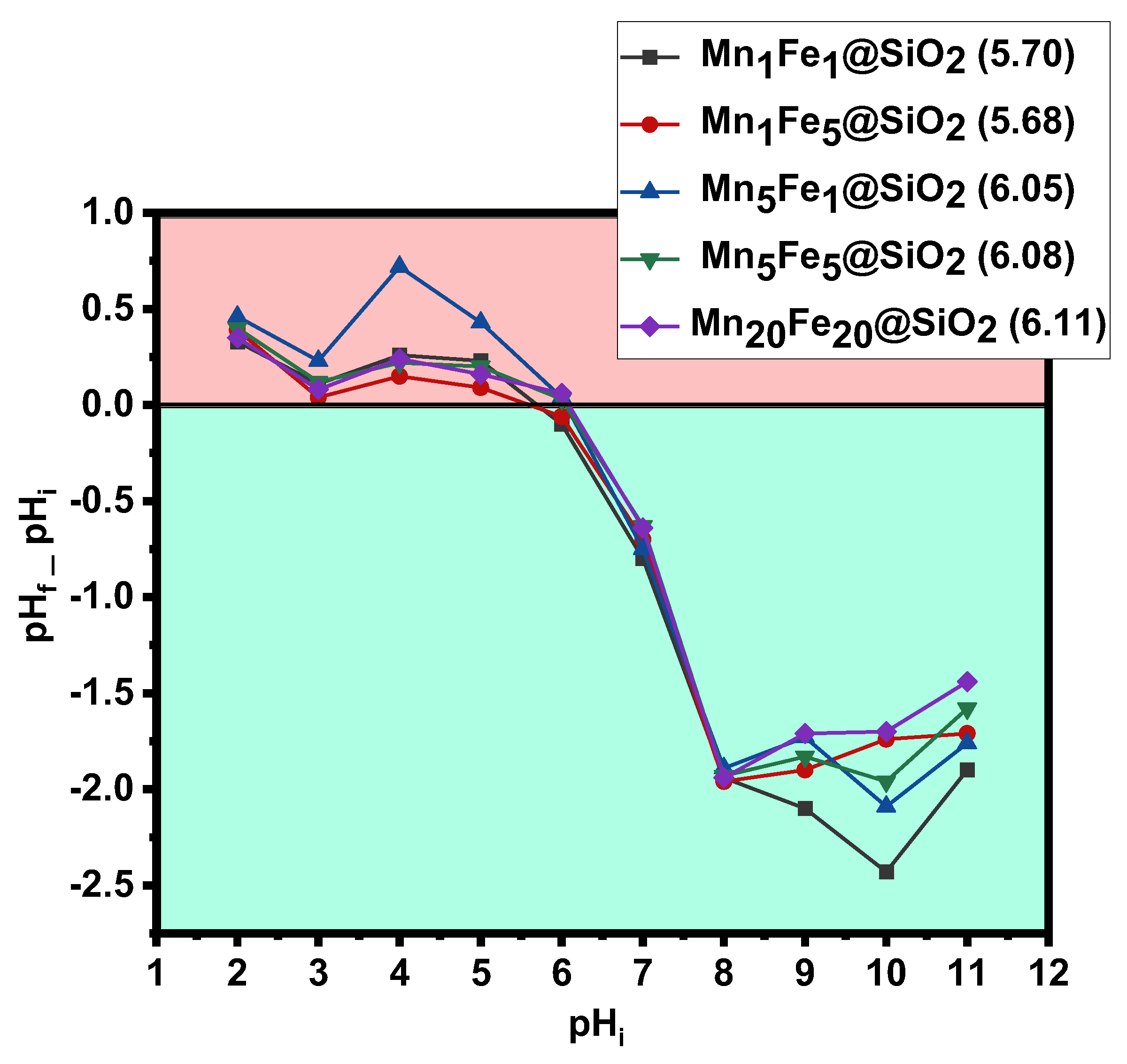
3.1. Physicochemical Characteristic of Nanocomposites
3.2. Dye Degradation Performance
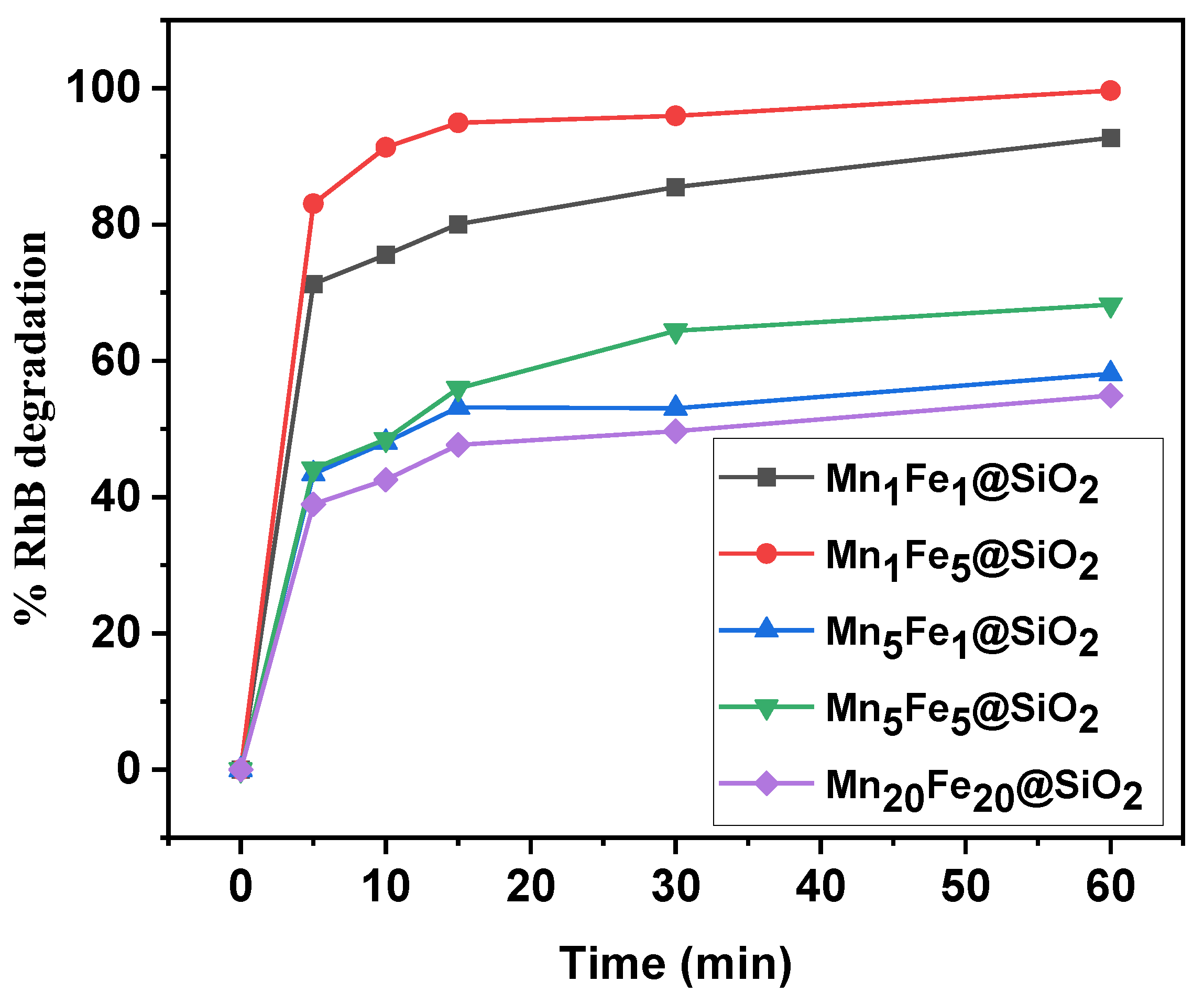
3.2.1. Effect of Metal Content on Performance
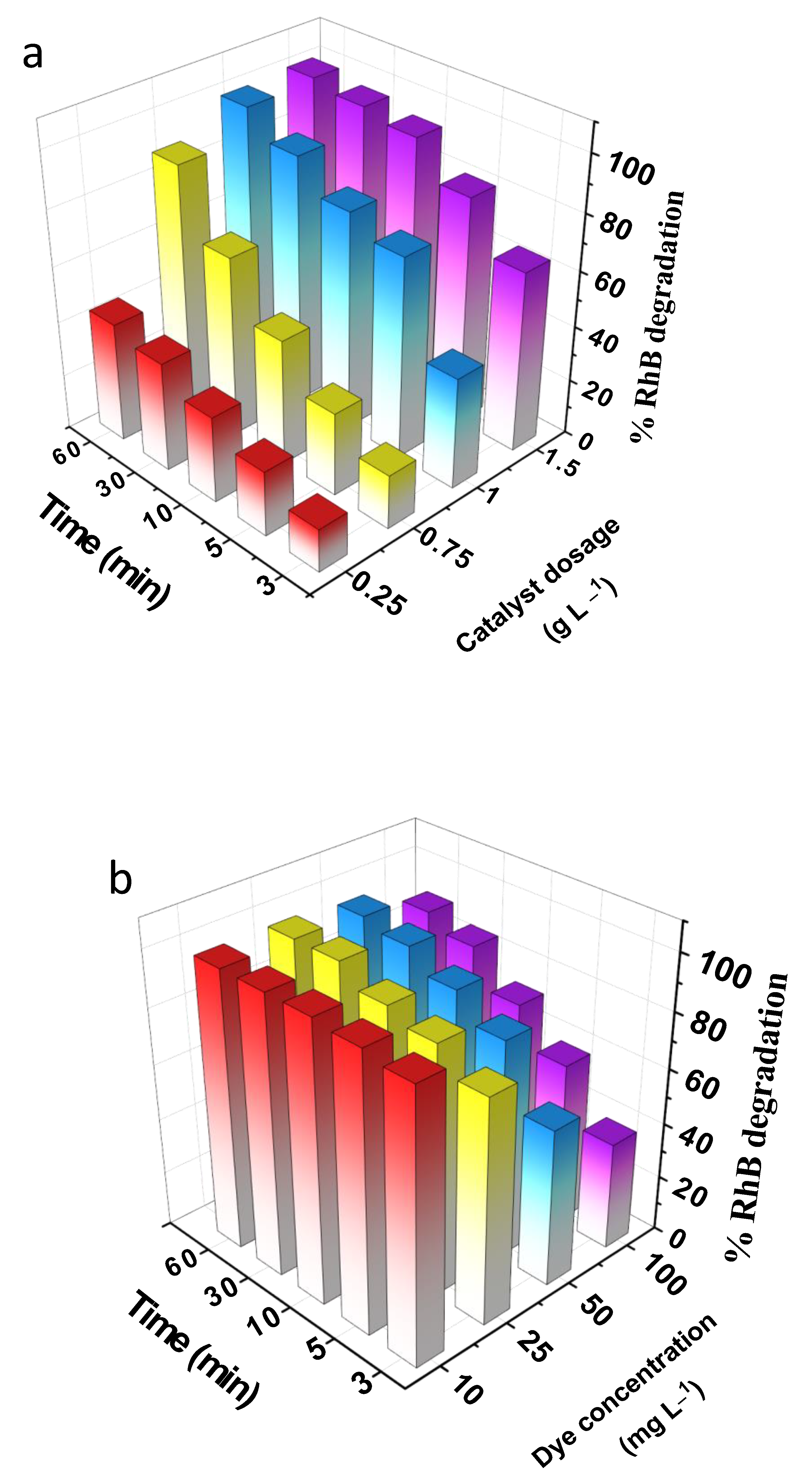
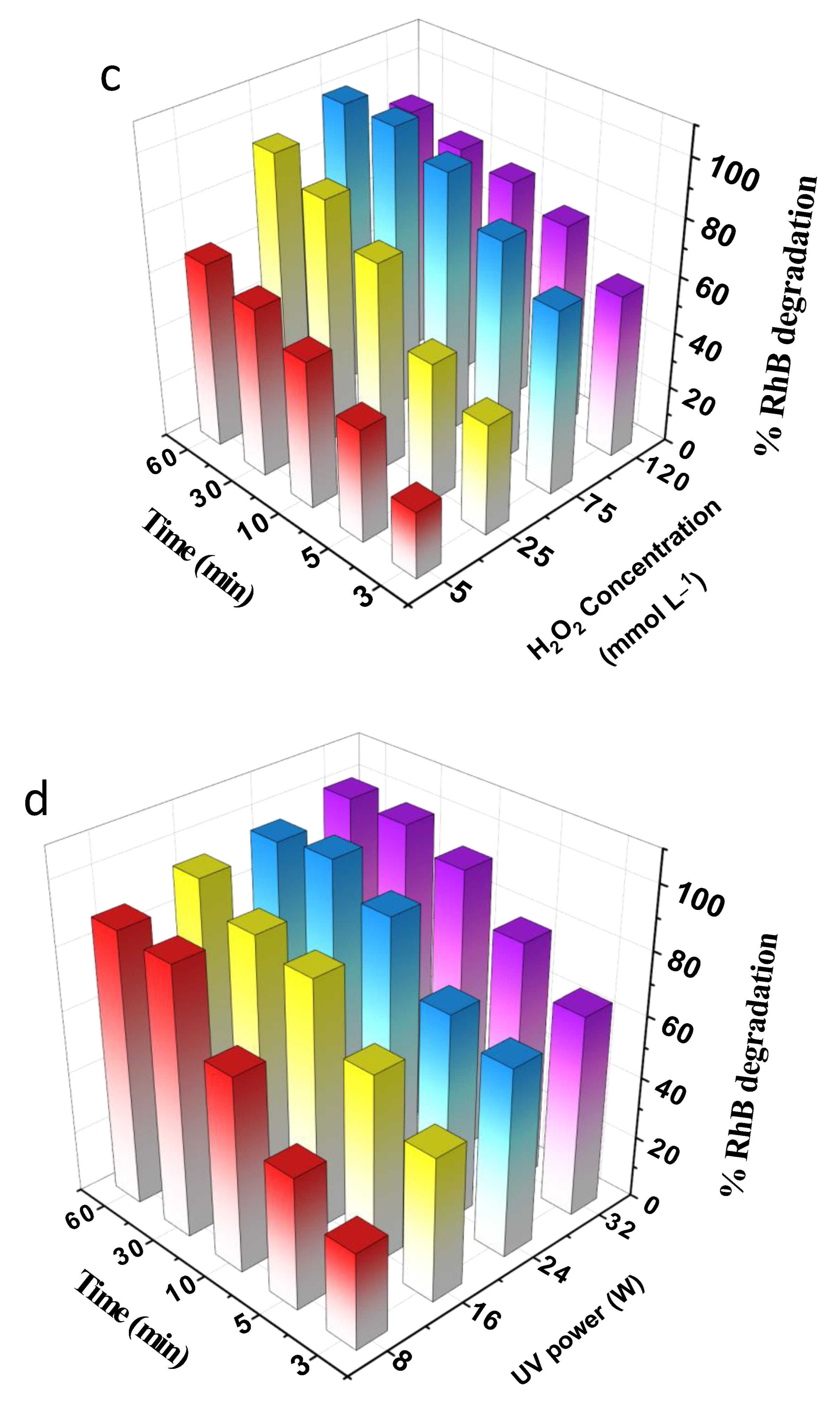
3.2.2. Effect of Experimental Parameters
3.2.3. Degradation Performance in the Optimum Condition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- San, N.O.; Celebioglu, A.; Tümtaş, Y.; Uyar, T.; Tekinay, T. Reusable bacteria immobilized electrospun nanofibrous webs for decolorization of methylene blue dye in wastewater treatment. RSC Adv. 2014, 4, 32249–32255. [Google Scholar] [CrossRef]
- Khan, N.A.; Bhadra, B.N.; Jhung, S.H. Heteropoly acid-loaded ionic liquid@metal-organic frameworks: Effective and reusable adsorbents for the desulfurization of a liquid model fuel. Chem. Eng. J. 2018, 334, 2215–2221. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Orimolade, B.O.; Masekela, D.; Mamba, B.; Mabuba, N. The application of photoelectrocatalysis in the degradation of rhodamine B in aqueous solutions: A review. RSC Adv. 2022, 12, 26176–26191. [Google Scholar] [CrossRef]
- Anandan, S.; Lee, G.-J.; Chen, P.-K.; Fan, C.; Wu, J.J. Removal of Orange II Dye in Water by Visible Light Assisted Photocatalytic Ozonation Using Bi2O3 and Au/Bi2O3 Nanorods. Ind. Eng. Chem. Res. 2010, 49, 9729–9737. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. Fabrication of sphere-like Bi2MoO6/ZnO composite catalyst with strong photocatalytic behavior for the detoxification of harmful organic dyes. Opt. Mater. 2020, 109, 110218. [Google Scholar] [CrossRef]
- Ali, I.; Kim, J.-O. Visible-light-assisted photocatalytic activity of bismuth-TiO2 nanotube composites for chromium reduction and dye degradation. Chemosphere 2018, 207, 285–292. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Liu, K.; Deng, S.; Wang, B.; Huang, J.; Wang, Y. Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework. Chem. Eng. J. 2017, 330, 157–165. [Google Scholar] [CrossRef]
- Farrokhi, A.; Jafarpour, M.; Alipour, M. Solar-driven advanced oxidation process catalyzed by metal–organic frameworks for water pollution. Polyhedron 2019, 170, 325–333. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- He, X.; Yang, D.-P.; Zhang, X.; Liu, M.; Kang, Z.; Lin, C.; Jia, N.; Luque, R. Waste eggshell membrane-templated CuO-ZnO nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem. Eng. J. 2019, 369, 621–633. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, X.; Le, Y.; Zhu, B.; Yu, J.; Ho, W. Hierarchically porous NiO–Al 2 O 3 nanocomposite with enhanced Congo red adsorption in water. Rsc Adv. 2016, 6, 10272–10279. [Google Scholar] [CrossRef]
- Nasseh, N.; Arghavan, F.S.; Rodriguez-Couto, S.; Hossein Panahi, A. Synthesis of FeNi3/SiO2/CuS magnetic nano-composite as a novel adsorbent for Congo Red dye removal. Int. J. Environ. Anal. Chem. 2020, 102, 2342–2362. [Google Scholar] [CrossRef]
- Chen, H.; Wageh, S.; Al-Ghamdi, A.A.; Wang, H.; Yu, J.; Jiang, C. Hierarchical C/NiO-ZnO nanocomposite fibers with enhanced adsorption capacity for Congo red. J. Colloid Interface Sci. 2019, 537, 736–745. [Google Scholar] [CrossRef]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.K.; Dan, A. Chitosan-graphene oxide hydrogels with embedded magnetic iron oxide nanoparticles for dye removal. ACS Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Laabd, M.; Ait Ahsaine, H.; El Jaouhari, A.; Bakiz, B.; Bazzaoui, M.; Ezahri, M.; Albourine, A.; Benlhachemi, A. Congo red removal by PANi/Bi2WO6 nanocomposites: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2016, 4, 3096–3105. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, D.; Zhang, Q.; Liu, Y.; Liu, Z.; Huang, H. Fe-Mn bi-metallic oxides loaded on granular activated carbon to enhance dye removal by catalytic ozonation. Environ. Sci. Pollut. Res. 2016, 23, 18800–18808. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Wu, K.; Wei, X.L.; Zhang, Y.; Chen, J.; Deng, C.X.; Jin, J.; Yu, Z.M.; Wang, L. Rectorite-supported nano-Fe3O4 composite materials as catalyst for P-chlorophenol degradation: Preparation, characterization, and mechanism. Appl. Clay Sci. 2019, 176, 66–77. [Google Scholar] [CrossRef]
- Li, X.; Pi, Y.; Wu, L.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-Iron terephthalate metal-organic framework for MB degradation. Appl. Catal. B Environ. 2017, 202, 653–663. [Google Scholar] [CrossRef]
- Arghavan, F.S.; Al-Musawi, T.J.; Rumman, G.A.; Pelalak, R.; Khataee, A.; Nasseh, N. Photocatalytic performance of a nickel ferrite/chitosan/bismuth(III) oxyiodide nanocomposite for metronidazole degradation under simulated sunlight illumination. J. Environ. Chem. Eng. 2021, 9, 105619. [Google Scholar] [CrossRef]
- Chang, S.; Harle, G.; Ma, J.; Yi, J. The effect of textural properties of CeO2-SiO2 mixed oxides on NH3-SCO activity of Pt/CeO2-SiO2 catalyst. Appl. Catal. A Gen. 2020, 604, 117775. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.-M. Nanomaterials via solution combustion synthesis: A step nearer to controllability. RSC Adv. 2014, 4, 58090–58100. [Google Scholar] [CrossRef]
- Ghaffari, Y.; Beak, S.; Bae, J.; Kim, S.; Saifuddin, M.; Kim, K.S. One-step fabrication of novel ultra porous Mn2O3-Fe2O3 @ SiO2: A versatile material for removal of organic pollutants from industrial wastewater at neutral pH. Sep. Purif. Technol. 2022, 285, 120259. [Google Scholar] [CrossRef]
- Ghaffari, Y.; Beak, S.; Bae, J.; Saifuddin, M.; Kim, K.S. Effect of UV Irradiation on the Structural Variation of Metal Oxide-Silica Nanocomposites for Enhanced Removal of Erythromycin at Neutral pH. Catalysts 2022, 12, 424. [Google Scholar] [CrossRef]
- Maddalena, R.; Hall, C.; Hamilton, A. Effect of silica particle size on the formation of calcium silicate hydrate [CSH] using thermal analysis. Thermochim. Acta 2019, 672, 142–149. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Development of CTAB modified ternary phase α-Fe2O3-Mn2O3-Mn3O4 nanocomposite as innovative super-adsorbent for Congo red dye adsorption. J. Environ. Chem. Eng. 2021, 9, 104827. [Google Scholar] [CrossRef]
- Jarvin, M.; Kumar, S.A.; Vinodhkumar, G.; Manikandan, E.; Inbanathan, S.S.R. Enhanced photocatalytic performance of Hausmannite Mn3O4-rGO nanocomposite in degrading methylene blue. Mater. Lett. 2021, 305, 130750. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Myint, M.T.Z.; Göthelid, M.A.; Dutta, J. CO Oxidation Efficiency and Hysteresis Behavior over Mesoporous Pd/SiO2 Catalyst. Catalysts 2021, 11, 131. [Google Scholar] [CrossRef]
- Christy, E.J.S.; Alagar, R.; Dhanu, M.; Pius, A. Porous nonhierarchical CeO2/SiO2 monolith for effective degradation of organic pollutants. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100365. [Google Scholar] [CrossRef]
- Khattak, A.K.; Afzal, M.; Saleem, M.; Yasmeen, G.; Ahmad, R. Surface modification of alumina by metal doping. Colloids Surf. A Physicochem. Eng. Asp. 2000, 162, 99–106. [Google Scholar] [CrossRef]
- Chen, G.; Nengzi, L.-C.; Gao, Y.; Zhu, G.; Gou, J.; Cheng, X. Degradation of tartrazine by peroxymonosulfate through magnetic Fe2O3/Mn2O3 composites activation. Chin. Chem. Lett. 2020, 31, 2730–2736. [Google Scholar] [CrossRef]
- Hazarika, K.K.; Goswami, C.; Saikia, H.; Borah, B.J.; Bharali, P. Cubic Mn2O3 nanoparticles on carbon as bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Mol. Catal. 2018, 451, 153–160. [Google Scholar] [CrossRef]
- Huang, Y.; Nengzi, L.-c.; Zhang, X.; Gou, J.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism. Chem. Eng. J. 2020, 388, 124274. [Google Scholar] [CrossRef]
- Wu, X.-L.; Shi, Y.; Zhong, S.; Lin, H.; Chen, J.-R. Facile synthesis of Fe3O4-graphene@mesoporous SiO2 nanocomposites for efficient removal of Methylene Blue. Appl. Surf. Sci. 2016, 378, 80–86. [Google Scholar] [CrossRef]
- Sambasivam, S.; Li, G.; Jeong, J.; Choi, B.; Lim, K.; Kim, S.; Song, T. Structural, optical, and magnetic properties of single-crystalline Mn3O4 nanowires. J. Nanoparticle Res. 2012, 14, 1138. [Google Scholar] [CrossRef]
- Cordischi, D.; Faticanti, M.; Minelli, G.; Occhiuzzi, M.; Porta, P. LaAl1−xMnxO3 perovskite-type oxide solid solutions: Structural, magnetic and electronic properties. Phys. Chem. Chem. Phys. 2003, 5, 1467–1473. [Google Scholar] [CrossRef]
- Ramasami, A.K.; Ravishankar, T.N.; Sureshkumar, K.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishnappa, T.; Balakrishna, G.R. Synthesis, exploration of energy storage and electrochemical sensing properties of hematite nanoparticles. J. Alloys Compd. 2016, 671, 552–559. [Google Scholar] [CrossRef]
- Xu, J.; Lan, X.; Cheng, J.; Zhou, X. Facile synthesis of g-C3N4/Ag2C2O4 heterojunction composite membrane with efficient visible light photocatalytic activity for water disinfection. Chemosphere 2022, 295, 133841. [Google Scholar] [CrossRef]
- Wang, H.; Xie, C.; Zhang, W.; Cai, S.; Yang, Z.; Gui, Y. Comparison of dye degradation efficiency using ZnO powders with various size scales. J. Hazard. Mater. 2007, 141, 645–652. [Google Scholar] [CrossRef]
- Akyol, A.; Yatmaz, H.; Bayramoglu, M. Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl. Catal. B Environ. 2004, 54, 19–24. [Google Scholar] [CrossRef]
- AlHamedi, F.H.; Rauf, M.A.; Ashraf, S.S. Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 2009, 239, 159–166. [Google Scholar] [CrossRef]
- Riga, A.; Soutsas, K.; Ntampegliotis, K.; Karayannis, V.; Papapolymerou, G. Effect of system parameters and of inorganic salts on the decolorization and degradation of Procion H-exl dyes. Comparison of H2O2/UV, Fenton, UV/Fenton, TiO2/UV and TiO2/UV/H2O2 processes. Desalination 2007, 211, 72–86. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Z.; Du, J.; Yin, R.; Zhou, X.; Jin, S.; Ren, N. Degradation of sulfadiazine in water by a UV/O3 process: Performance and degradation pathway. RSC Adv. 2016, 6, 57138–57143. [Google Scholar] [CrossRef]
- Kanjal, M.I.; Muneer, M.; Abdelhaleem, A.; Chu, W. Degradation of methotrexate by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism. Chin. J. Chem. Eng. 2020, 28, 2658–2667. [Google Scholar] [CrossRef]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.I.; Michael, C.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737–1746. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Zhang, Y.; Xu, Z.; Zhu, J.; Li, H. Preparation of Mn2O3 catalyst with core-shell structure via spray pyrolysis assisted with glucose. Res. Chem. Intermed. 2009, 35, 791–798. [Google Scholar] [CrossRef]
- Jung, J.-J.; Jang, J.-W.; Park, J.-W. Effect of generation growth on photocatalytic activity of nano TiO2-magnetic cored dendrimers. J. Ind. Eng. Chem. 2016, 44, 52–59. [Google Scholar] [CrossRef]


| Catalyst | Content | Color of Formed Gel |
|---|---|---|
| Mn1Fe1@SiO2 | Lowest metal content (Mn:1%, Fe:1%) | Yellow |
| Mn1Fe5@SiO2 | Higher Fe content (Mn:1%, Fe:5%) | Light brown |
| Mn5Fe1@SiO2 | Higher Mn content (Mn:5%, Fe:1%) | Yellow |
| Mn5Fe5@SiO2 | Medium metal content (Mn:5%, Fe:5%) | Light brown |
| Mn20Fe20@SiO2 | Highest metal content (Mn:20%, Fe:20%) | Dark brown |
| Adsorbent/Catalyst | Pollutant | Experimental Condition | Removal | References |
|---|---|---|---|---|
| TiO2 | [ATL]0 10 mg L−1 | pH3, Temperature = 25 °C, [catalyst] = 250 mg L−1, [H2O2] = 1.4 Mm, Reactiontime = 2 h, UVA9W (350 nm) | 70% | [45] |
| SBA-15 | [IBU]0 25 ug L−1 | pH5, Temperature=25 °C, [catalyst] = 0.3g L−1, [H2O2] = 10 Mm, Reaction time = 12 h | 93% | [46] |
| Mn2O3 | [MB]0 15 mg L−1 | pH not mentioned, Temperature = 25 °C, [catalyst] = 1g L−1, [H2O2] = 2 Ml (%30), Reaction time = 3 h | 50% | [47] |
| G0-TiO2 | Methyl orange 20 mg L−1 | pH6.2, Temperature = 25 °C, [catalyst] = 0.5g L−1, Reaction time = 2 h, UVC | 53% | [48] |
| Mn1Fe5@SiO2 | [RhB]0 50 mg L−1 | pH7,Temperature = 25 °C, [catalyst] = 1 g L–1, [H2O2] = 75 mmol L–1, Reactiontime = 1 h, UVC-32 W (254 nm) | 100% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffari, Y.; Saifuddin, M.; Kim, S.; Beak, S.; Bae, J.; Kim, K.S. A Novel Metal-Containing Mesoporous Silica Composite for the Decolorization of Rhodamine B: Effect of Metal Content on Structure and Performance. Nanomaterials 2022, 12, 4108. https://doi.org/10.3390/nano12234108
Ghaffari Y, Saifuddin M, Kim S, Beak S, Bae J, Kim KS. A Novel Metal-Containing Mesoporous Silica Composite for the Decolorization of Rhodamine B: Effect of Metal Content on Structure and Performance. Nanomaterials. 2022; 12(23):4108. https://doi.org/10.3390/nano12234108
Chicago/Turabian StyleGhaffari, Yasaman, Md Saifuddin, Suho Kim, Soyoung Beak, Jiyeol Bae, and Kwang Soo Kim. 2022. "A Novel Metal-Containing Mesoporous Silica Composite for the Decolorization of Rhodamine B: Effect of Metal Content on Structure and Performance" Nanomaterials 12, no. 23: 4108. https://doi.org/10.3390/nano12234108
APA StyleGhaffari, Y., Saifuddin, M., Kim, S., Beak, S., Bae, J., & Kim, K. S. (2022). A Novel Metal-Containing Mesoporous Silica Composite for the Decolorization of Rhodamine B: Effect of Metal Content on Structure and Performance. Nanomaterials, 12(23), 4108. https://doi.org/10.3390/nano12234108





