Study of Phase Transformations and Hyperfine Interactions in Fe3O4 and Fe3O4@Au Nanoparticles
Abstract
1. Introduction
2. Experimental Part
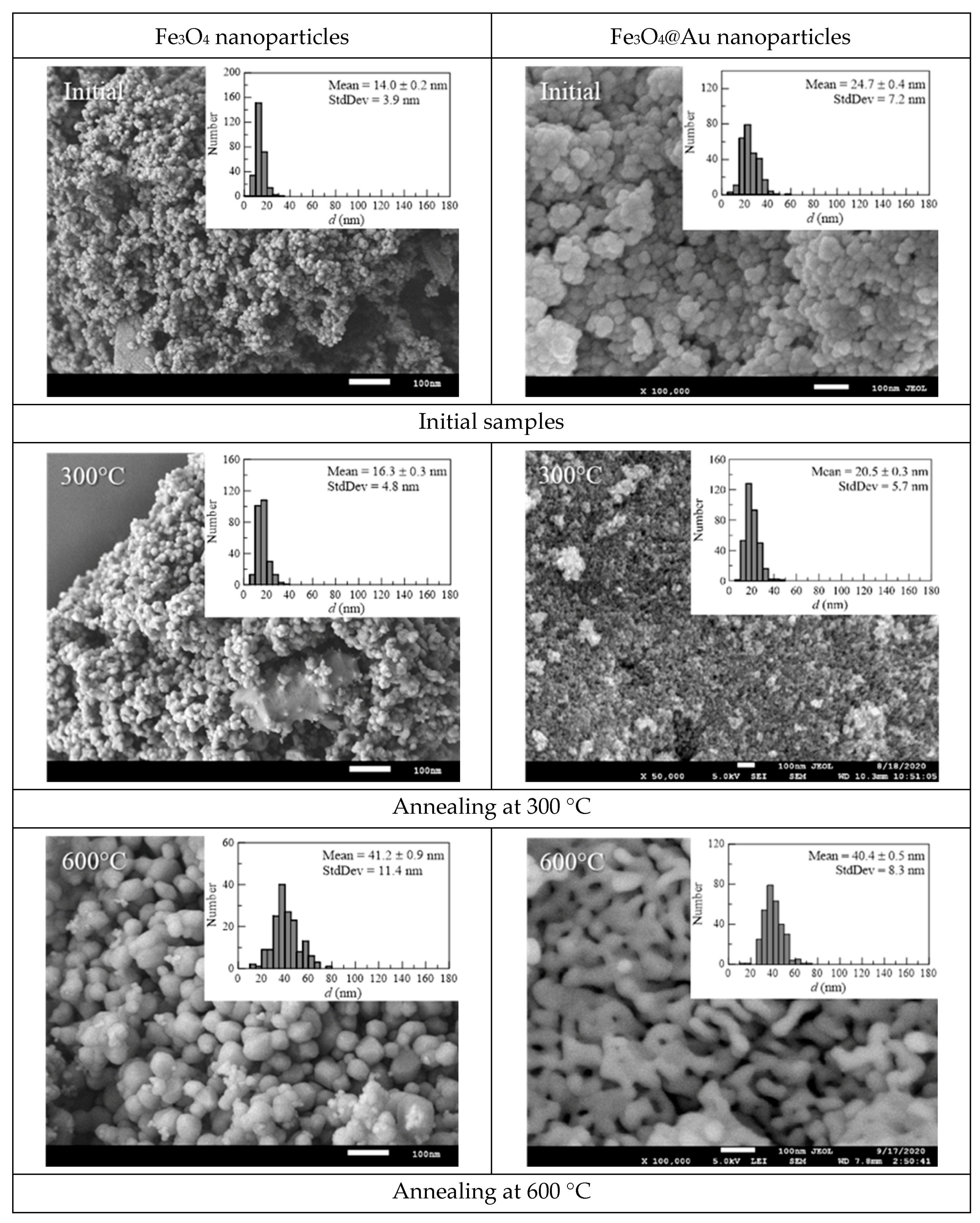
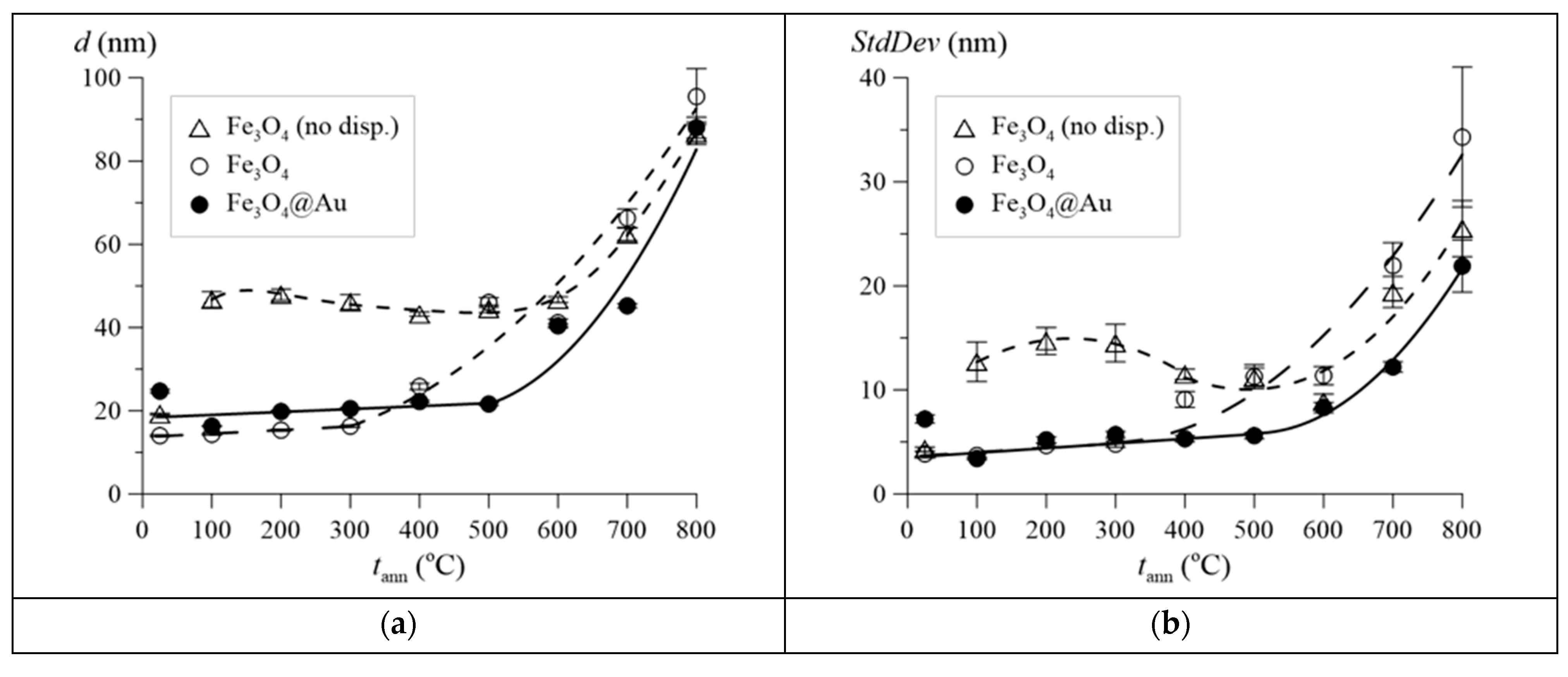
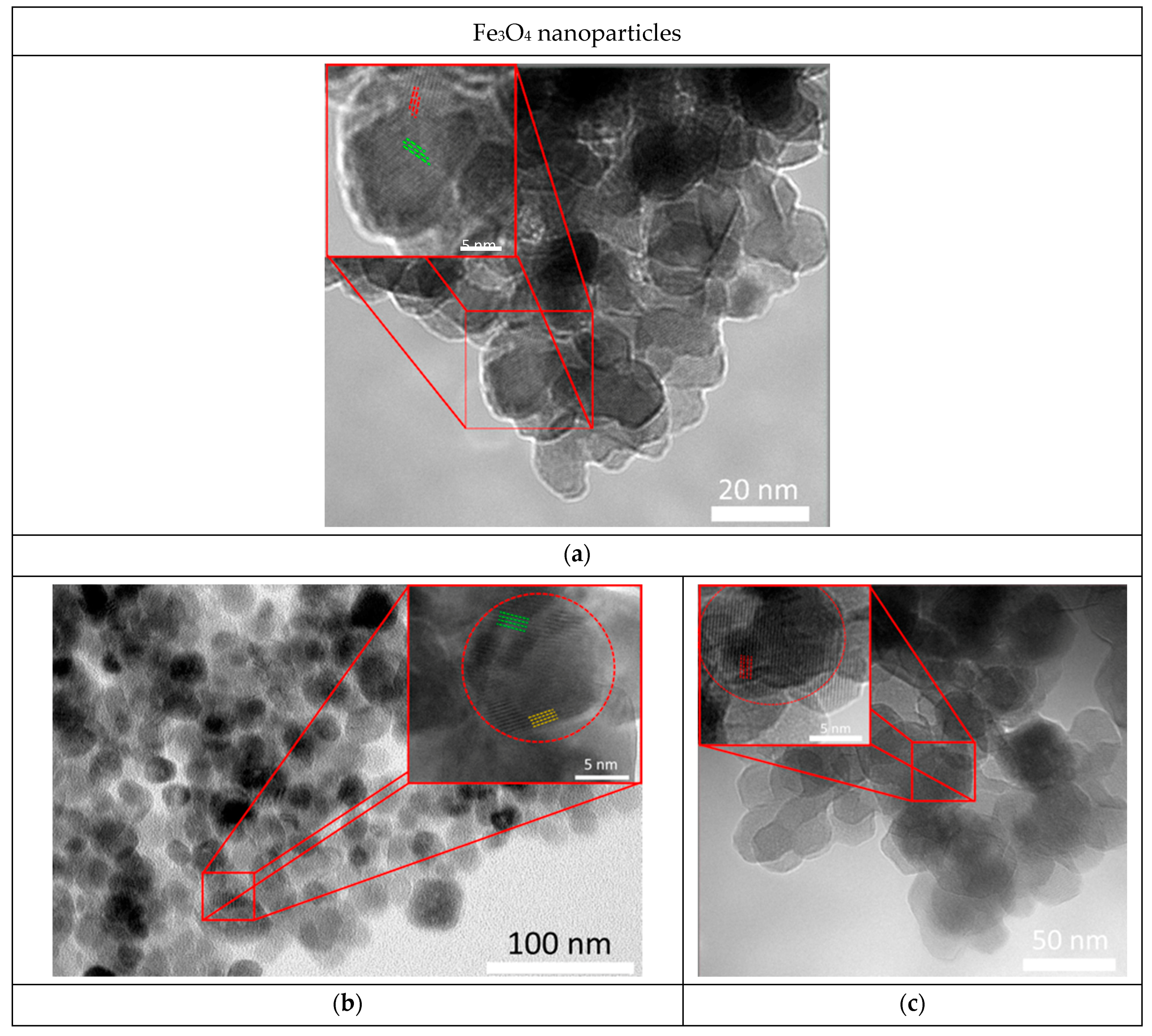
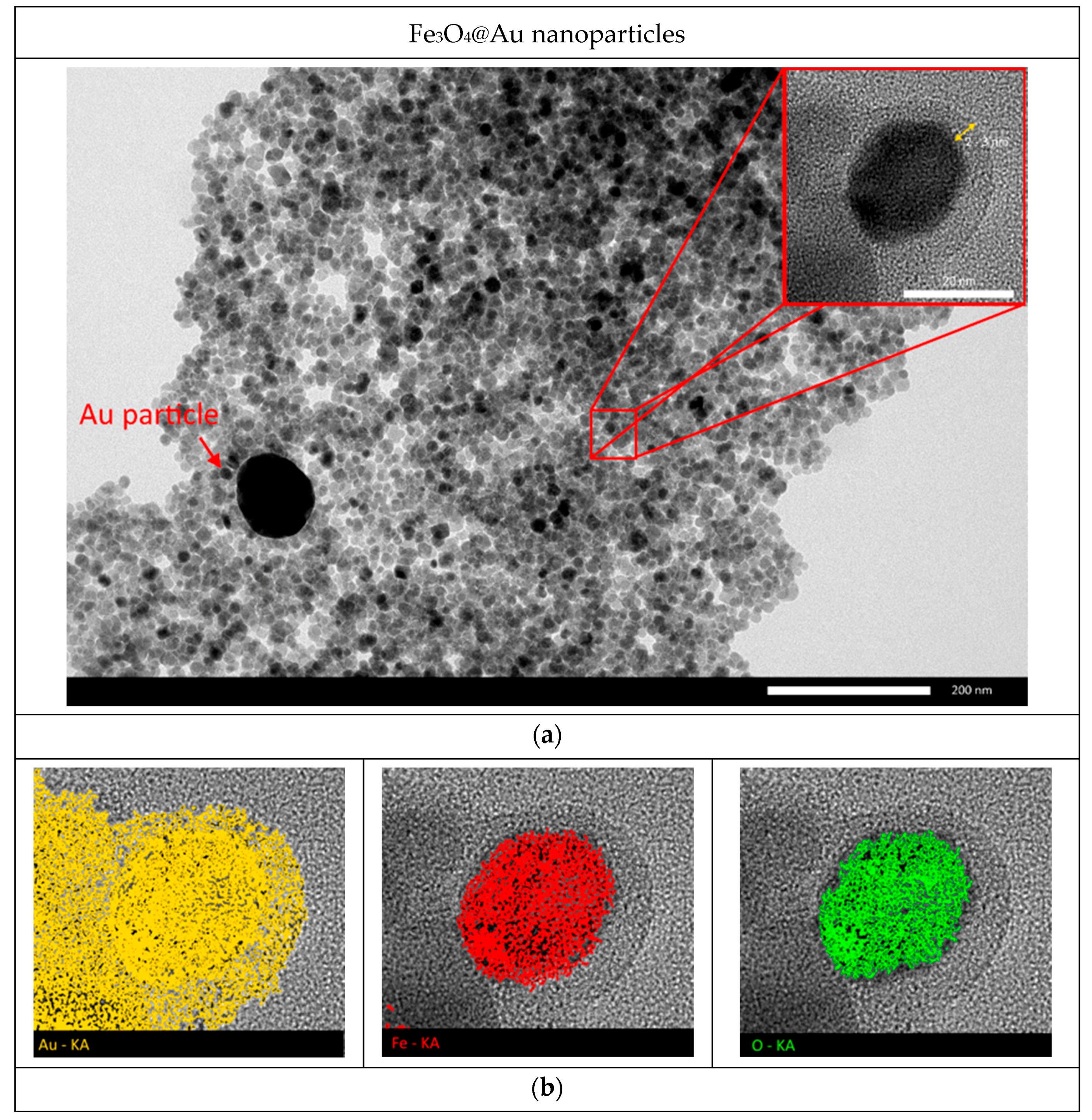
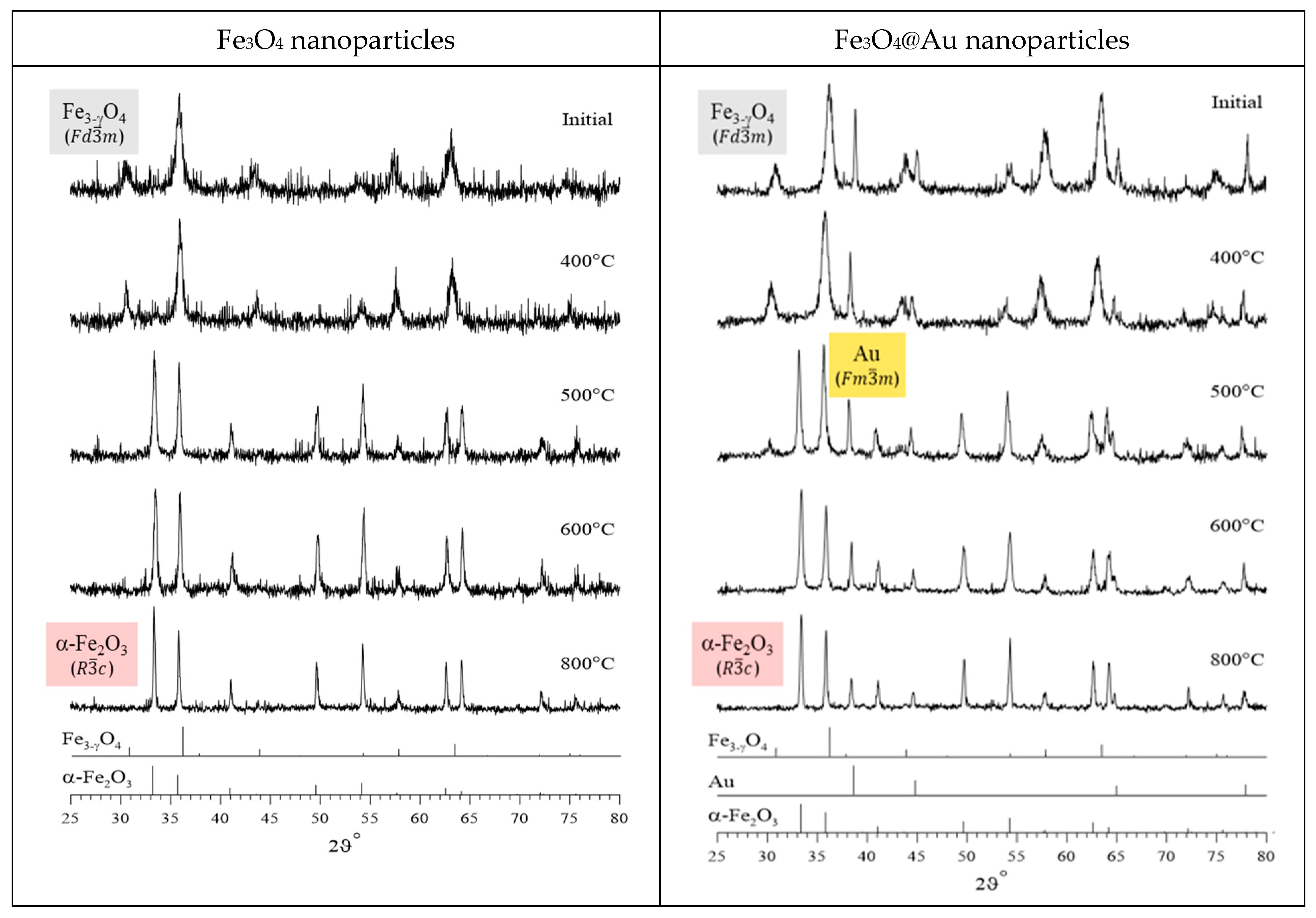
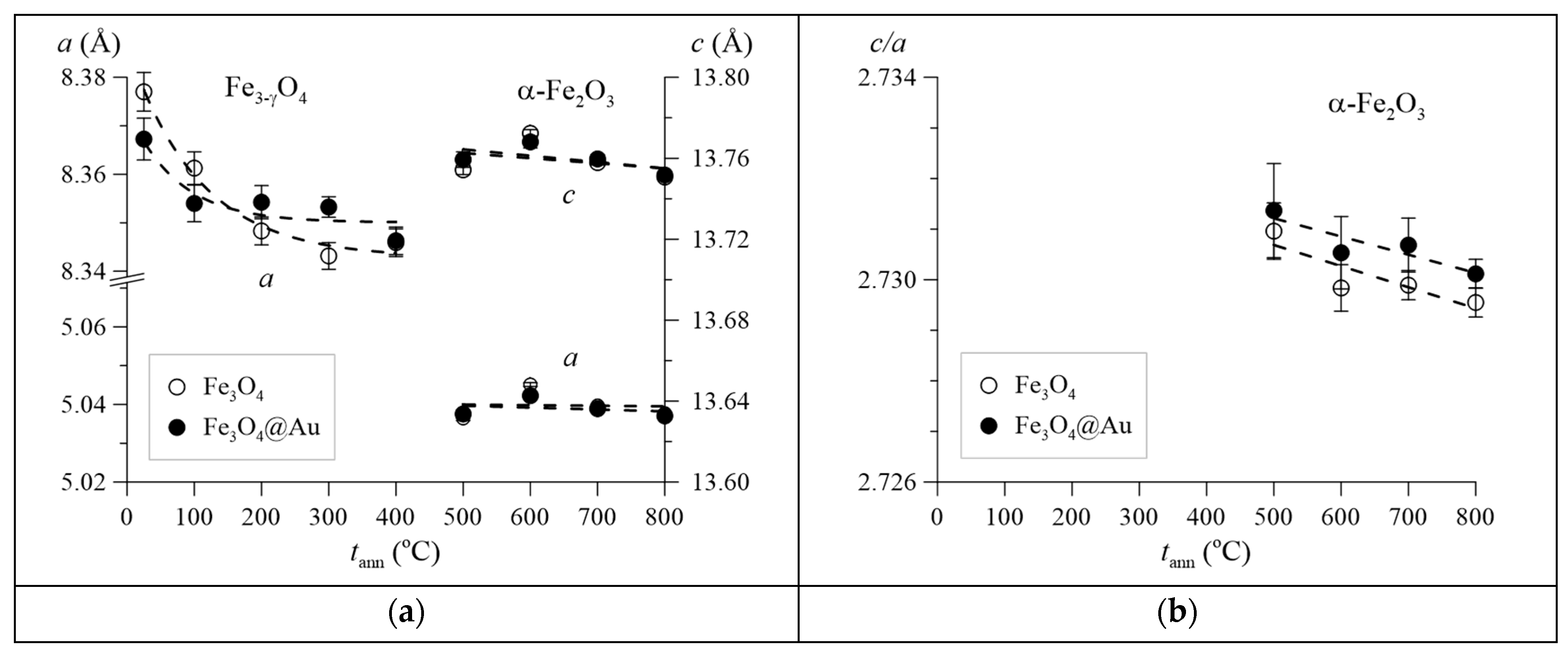
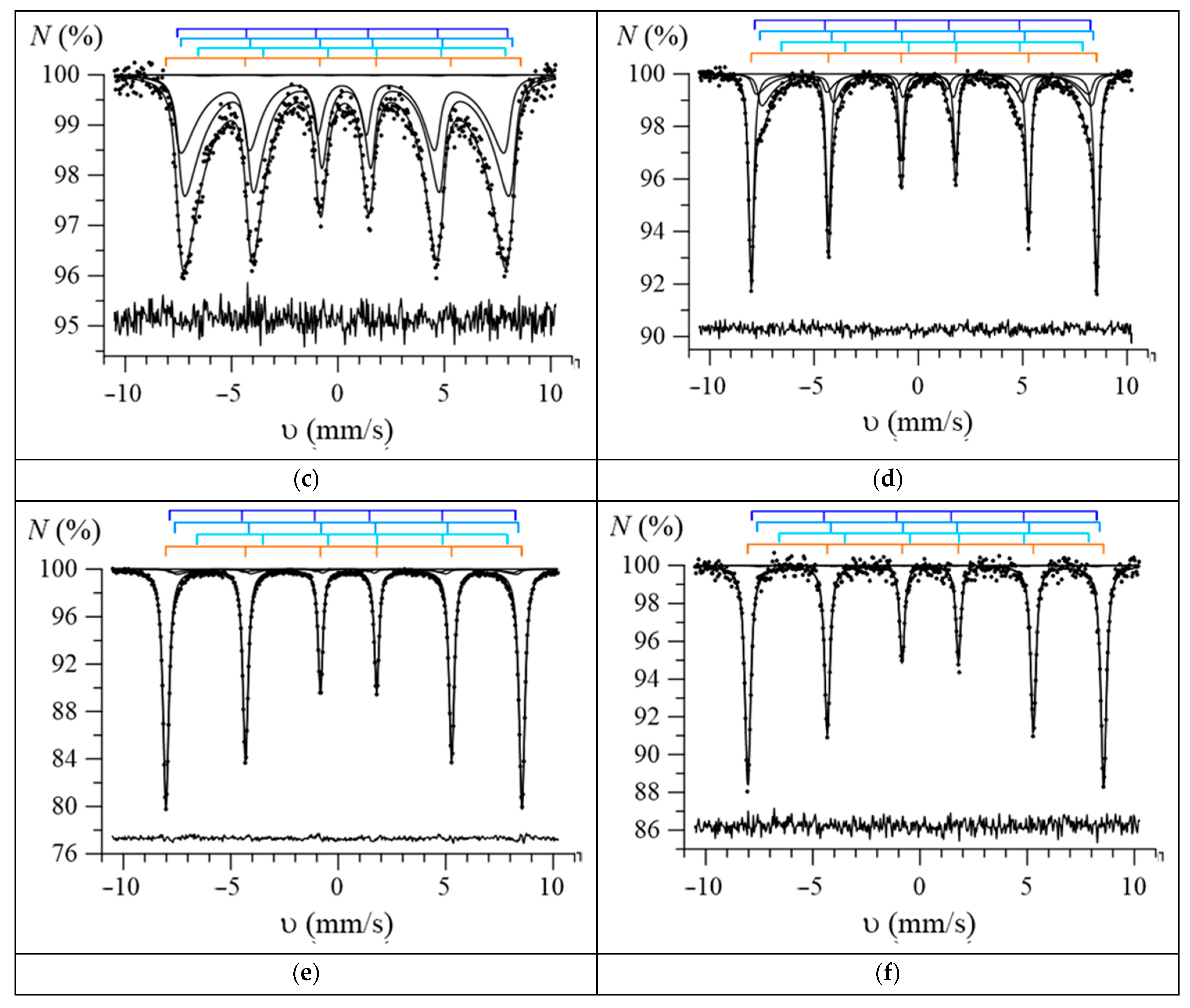
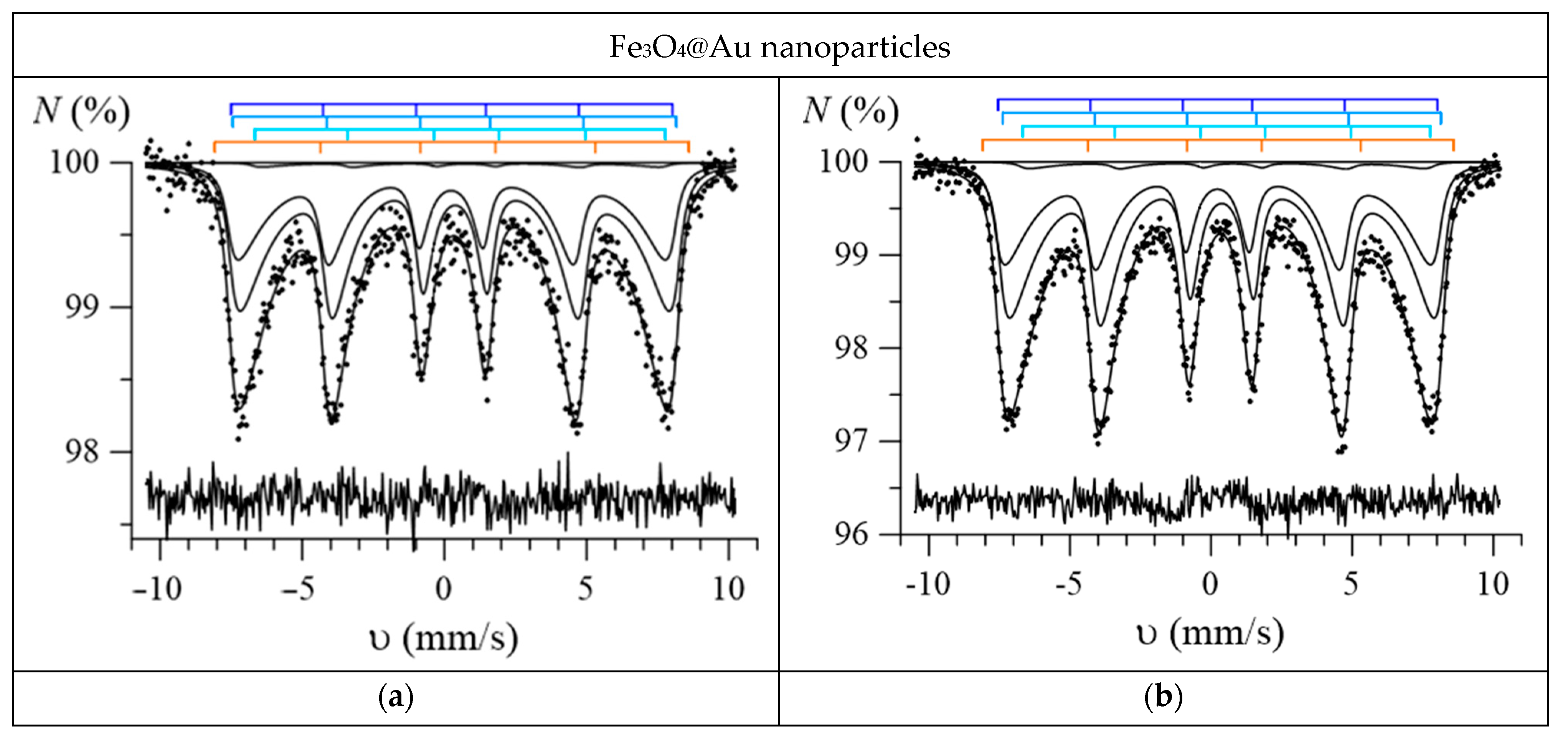
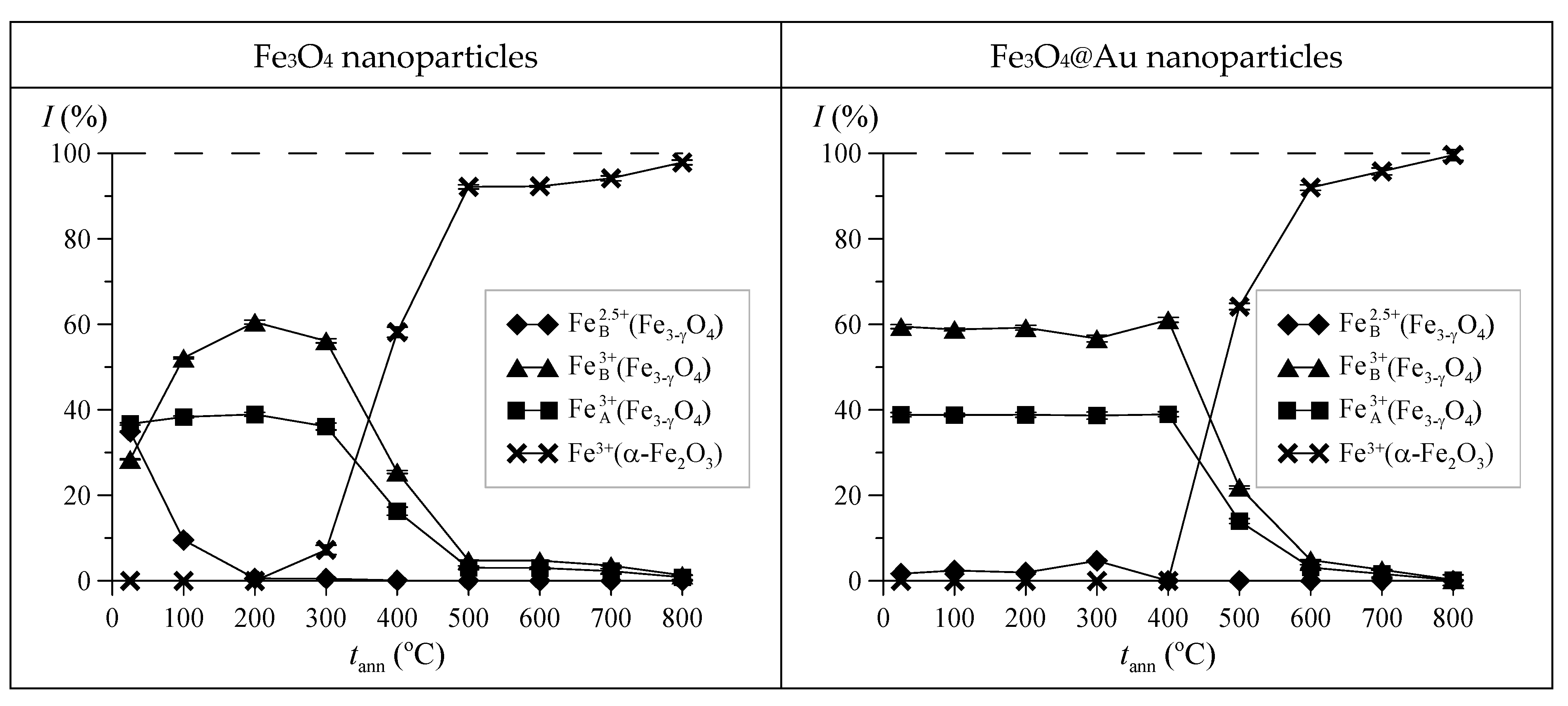
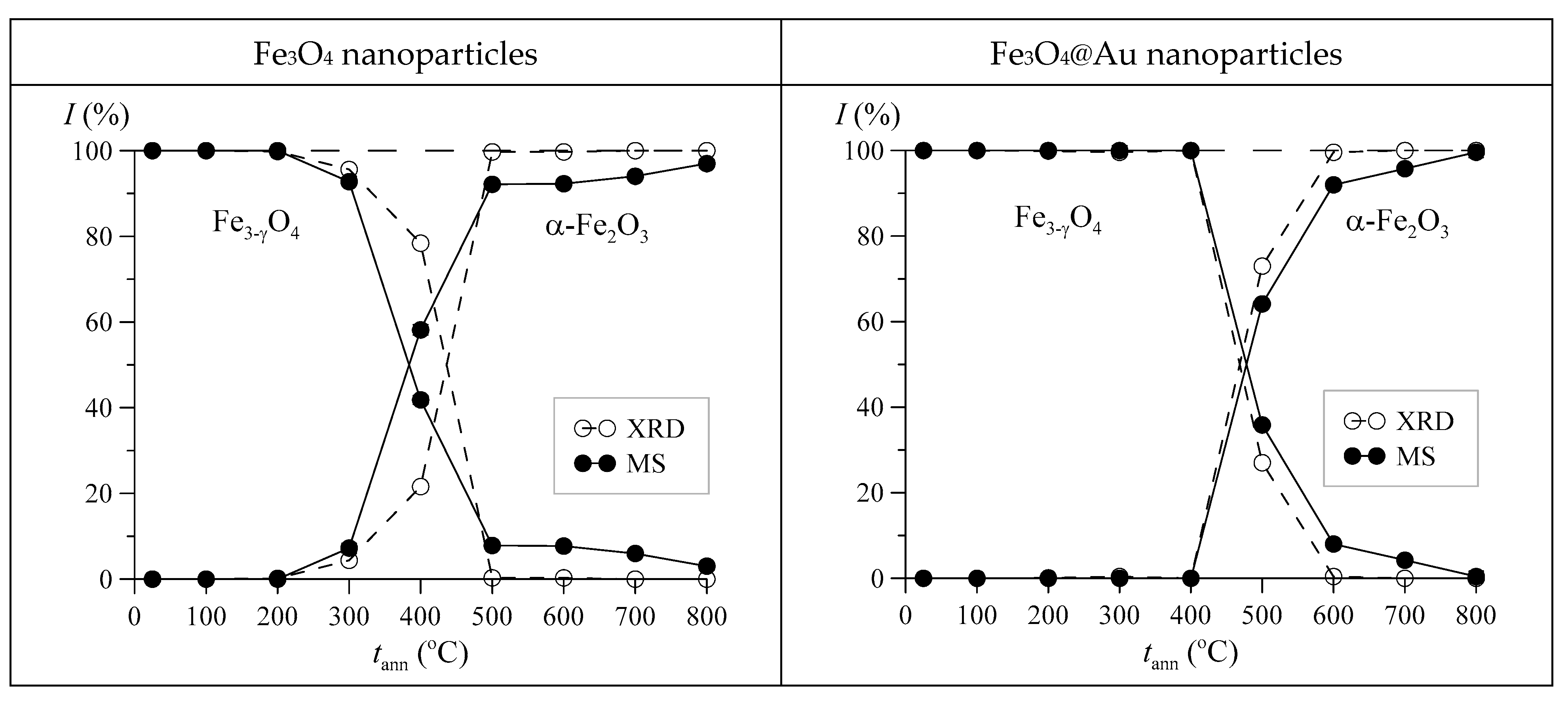
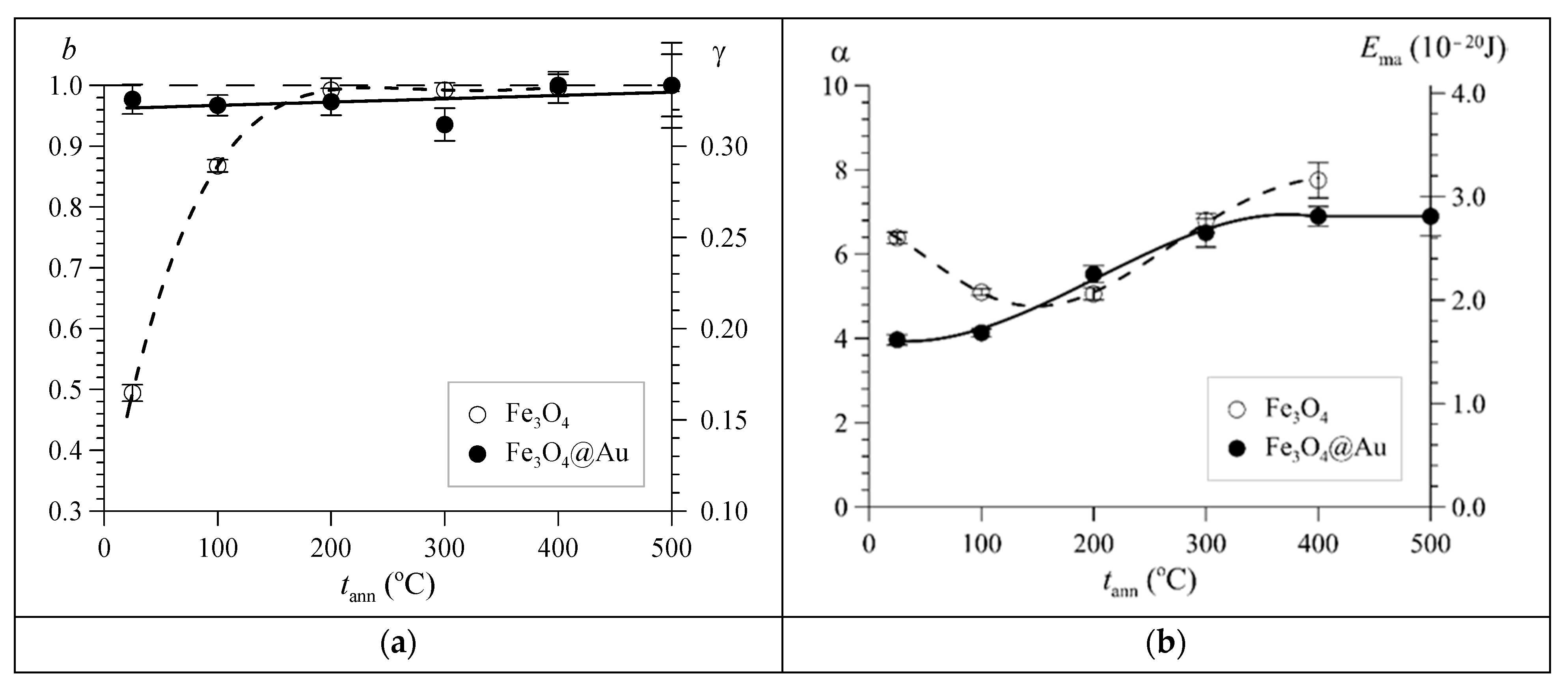
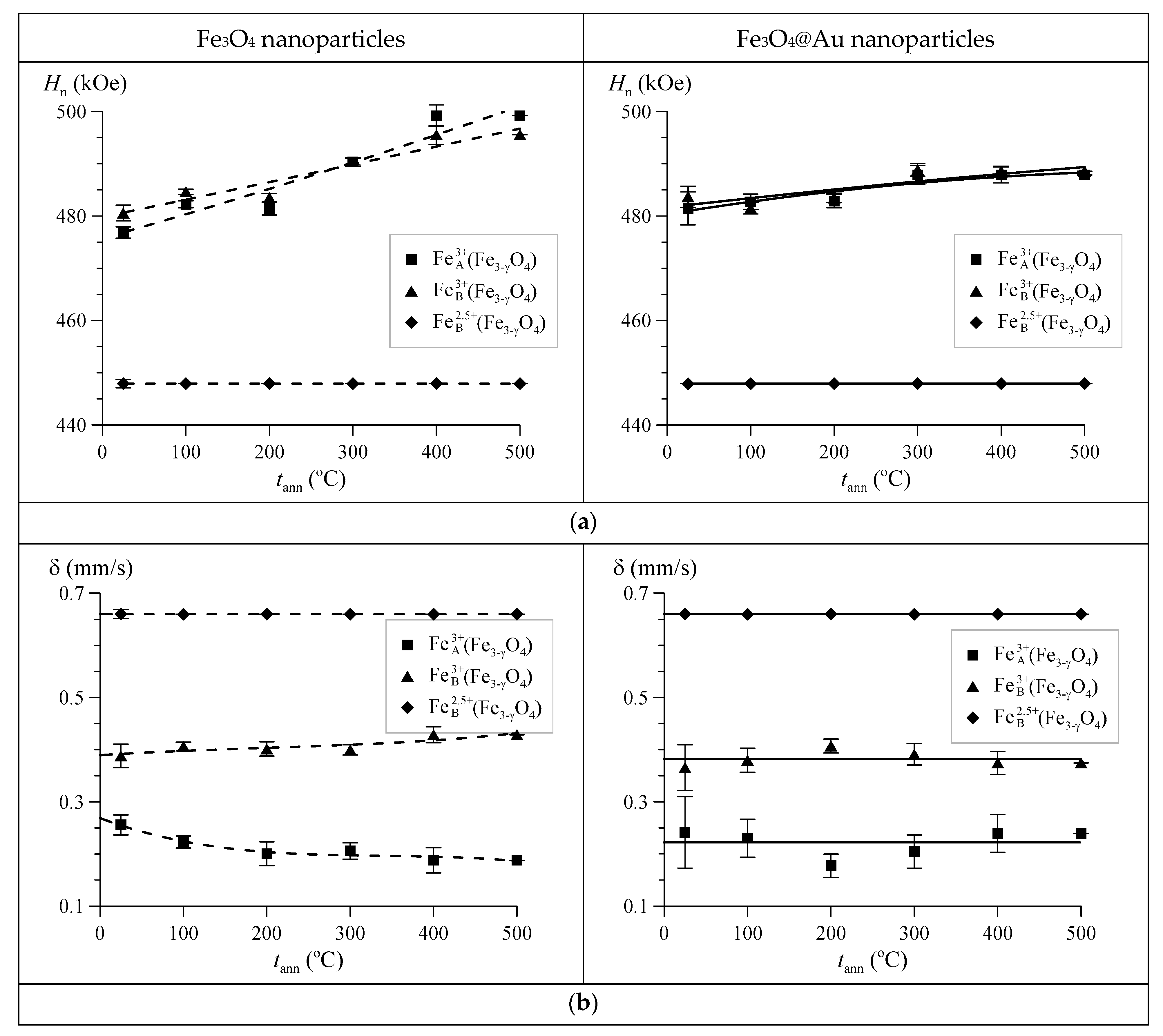
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Rui, Y. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khan, R.; Daverey, A. Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review. Environ. Technol. Innov. 2021, 24, 101924. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Ghorbani-Bidkorbeh, F.; Fallah, N.; Farnam, G.; Mortazavi, S.A.; Hamedi, M.H. Microfluidic platform for synthesis and optimization of chitosan-coated magnetic nanoparticles in cisplatin delivery. Carbohydr. Polym. 2021, 265, 118027. [Google Scholar] [CrossRef]
- Irfan, M.; Dogan, N.; Bingolbali, A.; Aliew, F. Synthesis and characterization of NiFe2O4 magnetic nanoparticles with different coating materials for magnetic particle imaging (MPI). J. Magn. Magn. Mater. 2021, 537, 168150. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S.; Solling, T.I. Application of magnetic nanoparticles in demulsification: A review on synthesis, performance, recyclability, and challenges. J. Pet. Sci. Eng. 2021, 196, 107680. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Zor, E.; Ozcelikay, G.; Ozkan, S.A. Magnetic nanoparticles in developing electrochemical sensors for pharmaceutical and biomedical applications. Talanta 2021, 226, 122108. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Azab, A.A.; Taha, T.A. Inducing lattice defects in calcium ferrite anode materials for improved electrochemical performance in lithium-ion batteries. Ceram. Int. 2022, 48, 12537–12548. [Google Scholar] [CrossRef]
- Golmohammad, M.; Mirhabibi, A.; Golestanifard, F.; Kelder, E.M. Optimizing synthesis of maghemite nanoparticles as an anode for Li-ion batteries by exploiting design of experiment. J. Electron. Mater. 2016, 45, 426–434. [Google Scholar] [CrossRef]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and aldehyde functionalized mesoporous silica on magnetic nanoparticles for enhanced lipase immobilization, biodiesel production, and facile separation. Fuel 2021, 291, 120126. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Li, R.N.; Da, X.H.; Li, X.; Lu, Y.S.; Gu, F.F.; Liu, Y. Functionalized magnetic nanoparticles for drug delivery in tumor therapy. Chin. Phys. B 2021, 30, 017502. [Google Scholar] [CrossRef]
- Keçili, R.; Ghorbani-Bidkorbeh, F.; Dolak, İ.; Canpolat, G.; Karabörk, M.; Hussain, C.M. Functionalized magnetic nanoparticles as powerful sorbents and stationary phases for the extraction and chromatographic applications. TrAC Trends Anal. Chem. 2021, 143, 116380. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374–390. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Nikitin, A.A.; Gabashvily, A.N.; Vishnevskiy, D.A.; Abakumov, M.A. Synthesis and intensive analysis of antibody labeled single core magnetic nanoparticles for targeted delivery to the cell membrane. J. Magn. Magn. Mater. 2021, 521, 167487. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Das, R.; Masa, J.A.; Phan, M.H.; Srikanth, H. Hybrid magnetic nanoparticles as efficient nanoheaters in biomedical applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef]
- Ilosvai, A.M.; Dojcsak, D.; Váradi, C.; Nagy, M.; Kristály, F.; Fiser, B.; Vanyorek, L. Sonochemical Combined Synthesis of Nickel Ferrite and Cobalt Ferrite Magnetic Nanoparticles and Their Application in Glycan Analysis. Int. J. Mol. Sci. 2022, 23, 5081. [Google Scholar] [CrossRef]
- Available online: https://www.webofscience.com/ (accessed on 20 September 2022).
- Kolosnjaj-Tabi, J.; Javed, Y.; Lartigue, L.; Volatron, J.; Elgrabli, D.; Marangon, I.; Gazeau, F. The one year fate of iron oxide coated gold nanoparticles in mice. ACS Nano 2015, 9, 7925–7939. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Szczepanowicz, K.; Kamyshny, A.; Magdassi, S. Metallic core-shell nanoparticles for conductive coatings and printing. Adv. Colloid Interface Sci. 2022, 299, 102578. [Google Scholar] [CrossRef]
- Bayles, A.; Tian, S.; Zhou, J.; Yuan, L.; Yuan, Y.; Jacobson, C.R.; Halas, N.J. Al@TiO2 Core–Shell Nanoparticles for Plasmonic Photocatalysis. ACS Nano 2022, 16, 5839–5850. [Google Scholar] [CrossRef]
- Ovchinnikov, O.V.; Smirnov, M.S.; Chevychelova, T.A.; Zvyagin, A.I.; Selyukov, A.S. Nonlinear absorption enhancement of Methylene Blue in the presence of Au/SiO2 core/shell nanoparticles. Dye. Pigment. 2022, 197, 109829. [Google Scholar] [CrossRef]
- Hodak, J.H.; Henglein, A.; Giersig, M.; Hartland, G.V. Laser-induced inter-diffusion in AuAg core−shell nanoparticles. J. Phys. Chem. B 2000, 104, 11708–11718. [Google Scholar] [CrossRef]
- Billen, A.; de Cattelle, A.; Jochum, J.K.; Van Bael, M.J.; Billen, J.; Seo, J.W.; Verbiest, T. Novel synthesis of superparamagnetic plasmonic core-shell iron oxide-gold nanoparticles. Phys. B Condens. Matter 2019, 560, 85–90. [Google Scholar] [CrossRef]
- Beik, J.; Asadi, M.; Khoei, S.; Laurent, S.; Abed, Z.; Mirrahimi, M.; Shakeri-Zadeh, A. Simulation-guided photothermal therapy using MRI-traceable iron oxide-gold nanoparticle. J. Photochem. Photobiol. B Biol. 2019, 199, 111599. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core–shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Llamosa, D.; Ruano, M.; Martínez, L.; Mayoral, A.; Roman, E.; García-Hernández, M.; Huttel, Y. The ultimate step towards a tailored engineering of core@ shell and core@shell@shell nanoparticles. Nanoscale 2014, 6, 13483–13486. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Q.; Zhu, J.; Sun, L.; Lin, H.; Guo, Z. Multifunctional composite core–shell nanoparticles. Nanoscale 2011, 3, 4474–4502. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L.; Karimi-Maleh, H.; Demirdögen, B. Magnetic iron oxide and iron oxide@gold nanoparticle anchored nitrogen and sulfur-functionalized reduced graphene oxide electrocatalyst for methanol oxidation. RSC Adv. 2015, 5, 26402–26409. [Google Scholar] [CrossRef]
- Ali Dheyab, M.; Aziz, A.A.; Jameel, M.S. Recent advances in inorganic nanomaterials synthesis using sonochemistry: A comprehensive review on iron oxide, gold and iron oxide coated gold nanoparticles. Molecules 2021, 26, 2453. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Fadeev, M.; Kozlovskiy, A.; Korolkov, I.; Egizbek, K.; Nazarova, A.; Chudoba, D.; Zdorovets, M. Iron oxide@gold nanoparticles: Synthesis, properties and potential use as anode materials for lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125178. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489, 178–185. [Google Scholar]
- Yang, J.B.; Zhou, X.D.; Yelon, W.B.; James, W.J.; Cai, Q.; Gopalakrishnan, K.V.; Nikles, D.E. Magnetic and structural studies of the Verwey transition in Fe3−δO4 nanoparticles. J. Appl. Phys. 2004, 95, 7540–7542. [Google Scholar] [CrossRef]
- Schmidbauer, E.; Keller, M. Magnetic hysteresis properties, Mossbauer spectra and structural data of spherical 250 nm particles of solid solutions Fe3O4−γ-Fe2O3. Magn. Magn. Mater. 2006, 297, 107–117. [Google Scholar] [CrossRef]
- Rozenberg, G.K.; Dubrovinsky, L.S.; Pasternak, M.P.; Naaman, O.; Le Bihan, T.; Ahuja, R. High-pressure structural studies of hematite Fe2O3. Phys. Rev. B 2002, 65, 064112. [Google Scholar] [CrossRef]
- Stary, O.; Malyshev, A.V.; Lysenko, E.N.; Petrova, A. Formation of magnetic properties of ferrites during radiation-thermal sintering. Eurasian Phys. Tech. J. 2021, 17, 6–10. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Electronic conduction of magnetite (Fe3O4) and its transition point at low temperatures. Nature 1939, 144, 327. [Google Scholar] [CrossRef]
- Sawatzky, G.A.; Van Der Woude, F.; Morrish, A.H. Recoilless-fraction ratios for Fe57 in octahedral and tetrahedral sites of a spinel and a garnet. Phys. Rev. 1969, 183, 383–386. [Google Scholar] [CrossRef]
- Jones, D.H.; Srivastava, K.K.P. Many-state relaxation model for the mossbauer spectra of superparamagnets. Phys. Rev. B 1986, 34, 7542–7548. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Kliava, J.; Bissey, J.C.; Baïetto, V. Magnetic resonance of superparamagnetic iron-containing nanoparticles in annealed glass. J. Appl. Phys. 2000, 87, 7389–7396. [Google Scholar] [CrossRef]
- Lin, C.R.; Chiang, R.K.; Wang, J.S.; Sung, T.W. Magnetic properties of monodisperse iron oxide nanoparticles. J. Appl. Phys. 2006, 99, 08N710. [Google Scholar] [CrossRef]
- Balaev, D.A.; Semenov, S.V.; Dubrovskiy, A.A.; Yakushkin, S.S.; Kirillov, V.L.; Martyanov, O.N. Superparamagnetic blocking of an ensemble of magnetite nanoparticles upon interparticle interactions. J. Magn. Magn. Mater. 2017, 440, 199–202. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusakov, V.S.; Kozlovskiy, A.L.; Fadeev, M.S.; Egizbek, K.B.; Nazarova, A.; Kadyrzhanov, K.K.; Shlimas, D.I.; Zdorovets, M.V. Study of Phase Transformations and Hyperfine Interactions in Fe3O4 and Fe3O4@Au Nanoparticles. Nanomaterials 2022, 12, 4121. https://doi.org/10.3390/nano12234121
Rusakov VS, Kozlovskiy AL, Fadeev MS, Egizbek KB, Nazarova A, Kadyrzhanov KK, Shlimas DI, Zdorovets MV. Study of Phase Transformations and Hyperfine Interactions in Fe3O4 and Fe3O4@Au Nanoparticles. Nanomaterials. 2022; 12(23):4121. https://doi.org/10.3390/nano12234121
Chicago/Turabian StyleRusakov, Vyacheslav S., Artem L. Kozlovskiy, Maxim S. Fadeev, Kamila B. Egizbek, Assel Nazarova, Kayrat K. Kadyrzhanov, Dmitriy I. Shlimas, and Maxim V. Zdorovets. 2022. "Study of Phase Transformations and Hyperfine Interactions in Fe3O4 and Fe3O4@Au Nanoparticles" Nanomaterials 12, no. 23: 4121. https://doi.org/10.3390/nano12234121
APA StyleRusakov, V. S., Kozlovskiy, A. L., Fadeev, M. S., Egizbek, K. B., Nazarova, A., Kadyrzhanov, K. K., Shlimas, D. I., & Zdorovets, M. V. (2022). Study of Phase Transformations and Hyperfine Interactions in Fe3O4 and Fe3O4@Au Nanoparticles. Nanomaterials, 12(23), 4121. https://doi.org/10.3390/nano12234121






