High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Bulk GO Samples Characterization
2.2. Reagents
2.2.1. Enzymes, Substrates, and Chemiluminescent Probes
2.2.2. Certified Reference Materials (CRMs)
2.3. Dialysis Setup Equipment and Operation
2.4. Other Instrumentation
2.5. Data Treatment
2.6. Procedures and Protocols
3. Results
3.1. Dialysis Dispersion Purification and Monitoring
3.1.1. Monitoring Metal Contents in Membrane during Preconditioning
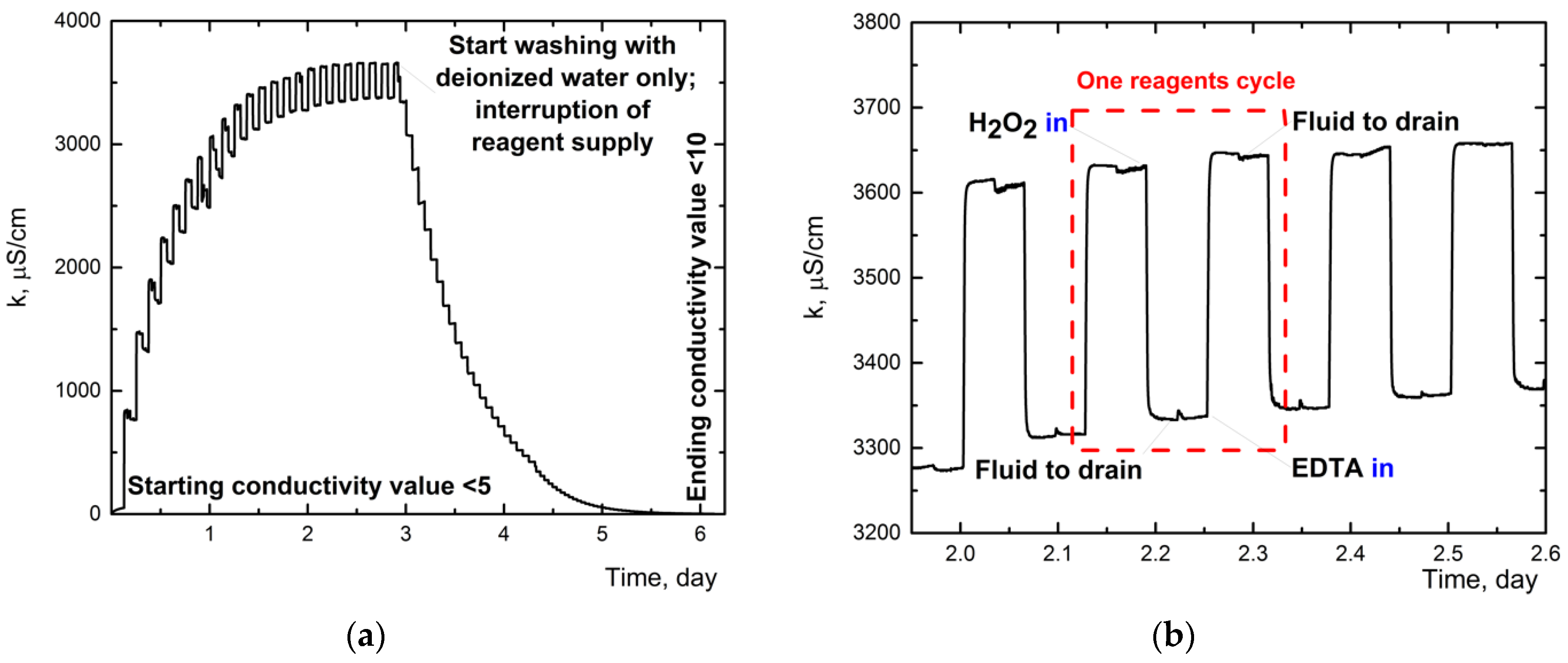
3.1.2. Conductivity Monitoring
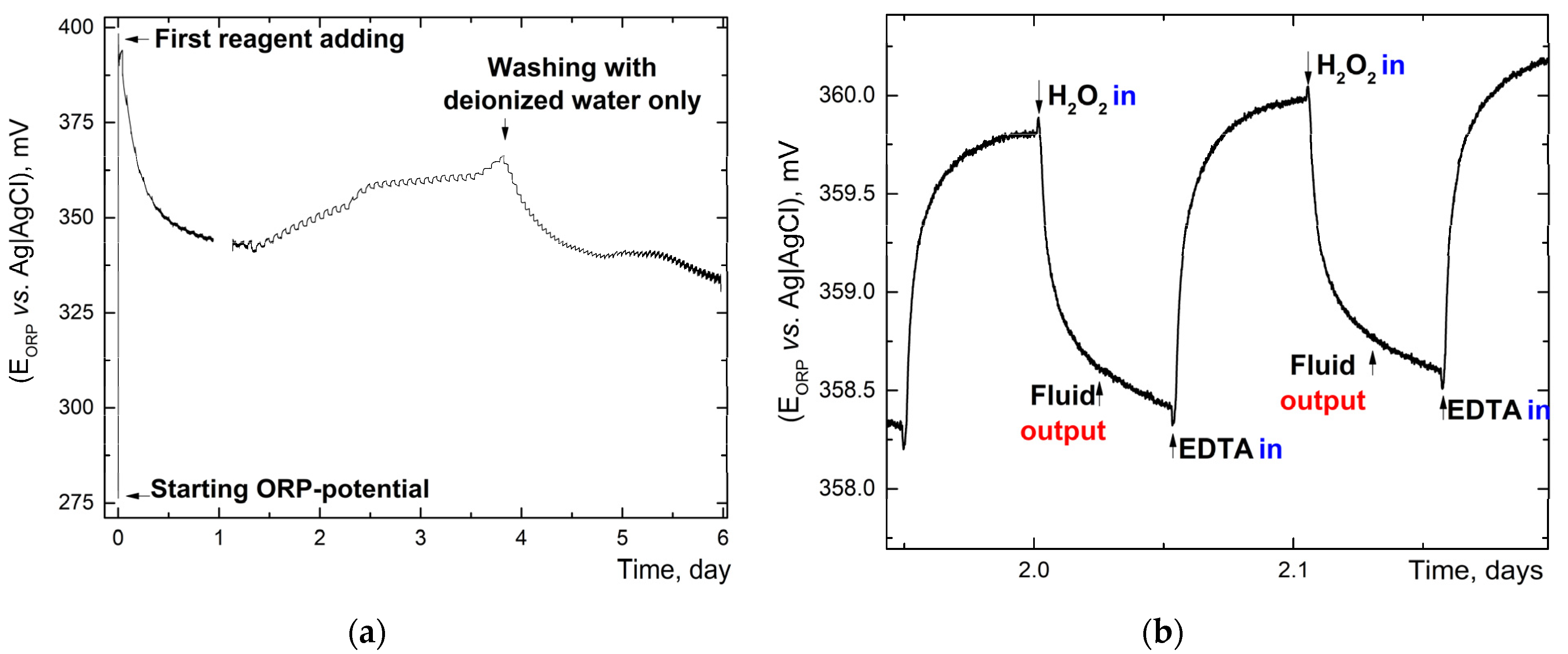
3.1.3. Oxidation-Reduction Potential Monitoring
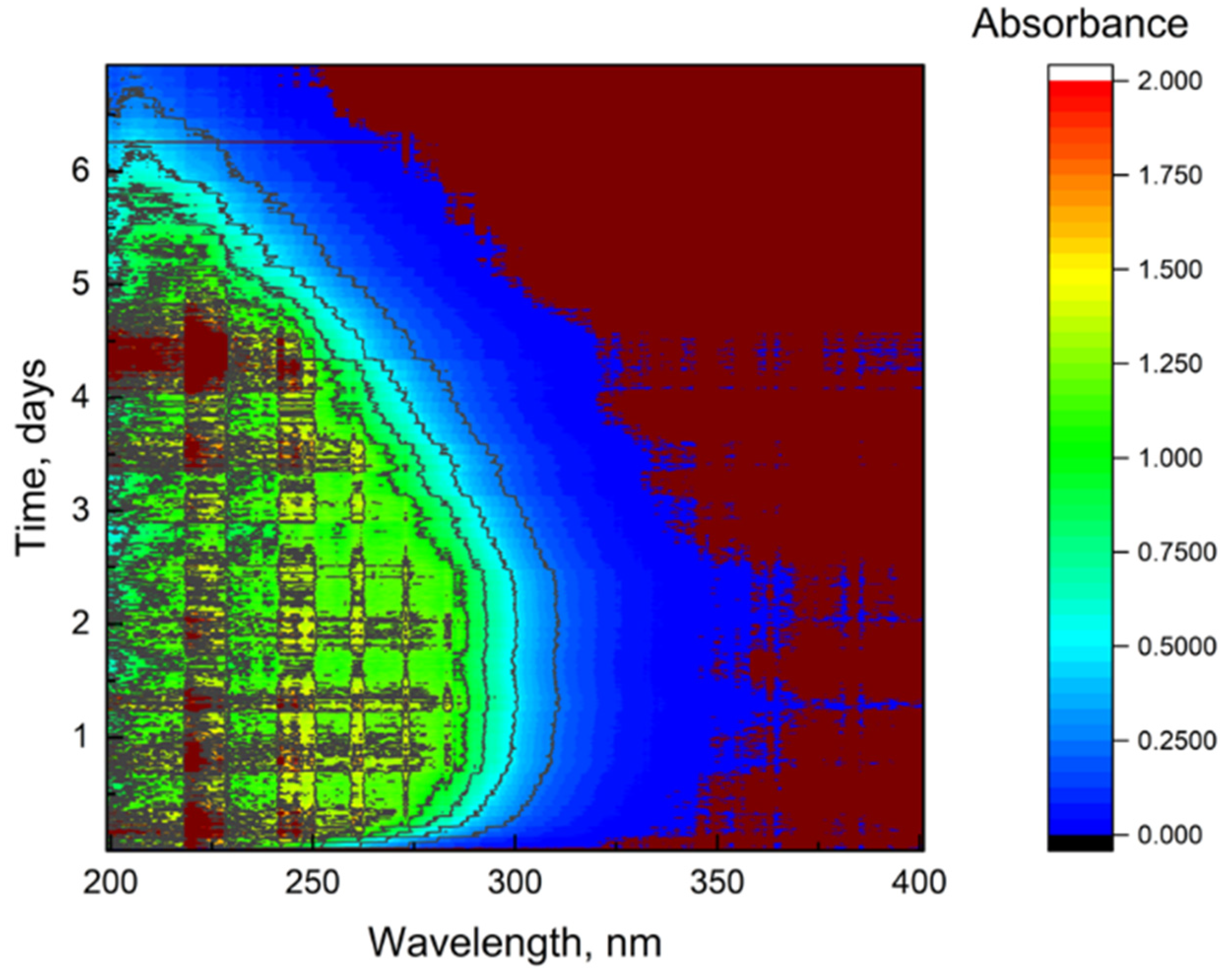
3.1.4. UV/vis Absorbance Spectra with Fiber Optical-Probe Monitoring
3.2. Properties of Purified Graphene Oxide Dispersions
3.2.1. Total Yield
3.2.2. Sample Stability Lateral Size and Zeta-Potential
3.2.3. pH and Conductivity
3.2.4. UV/Vis Absorption Spectra
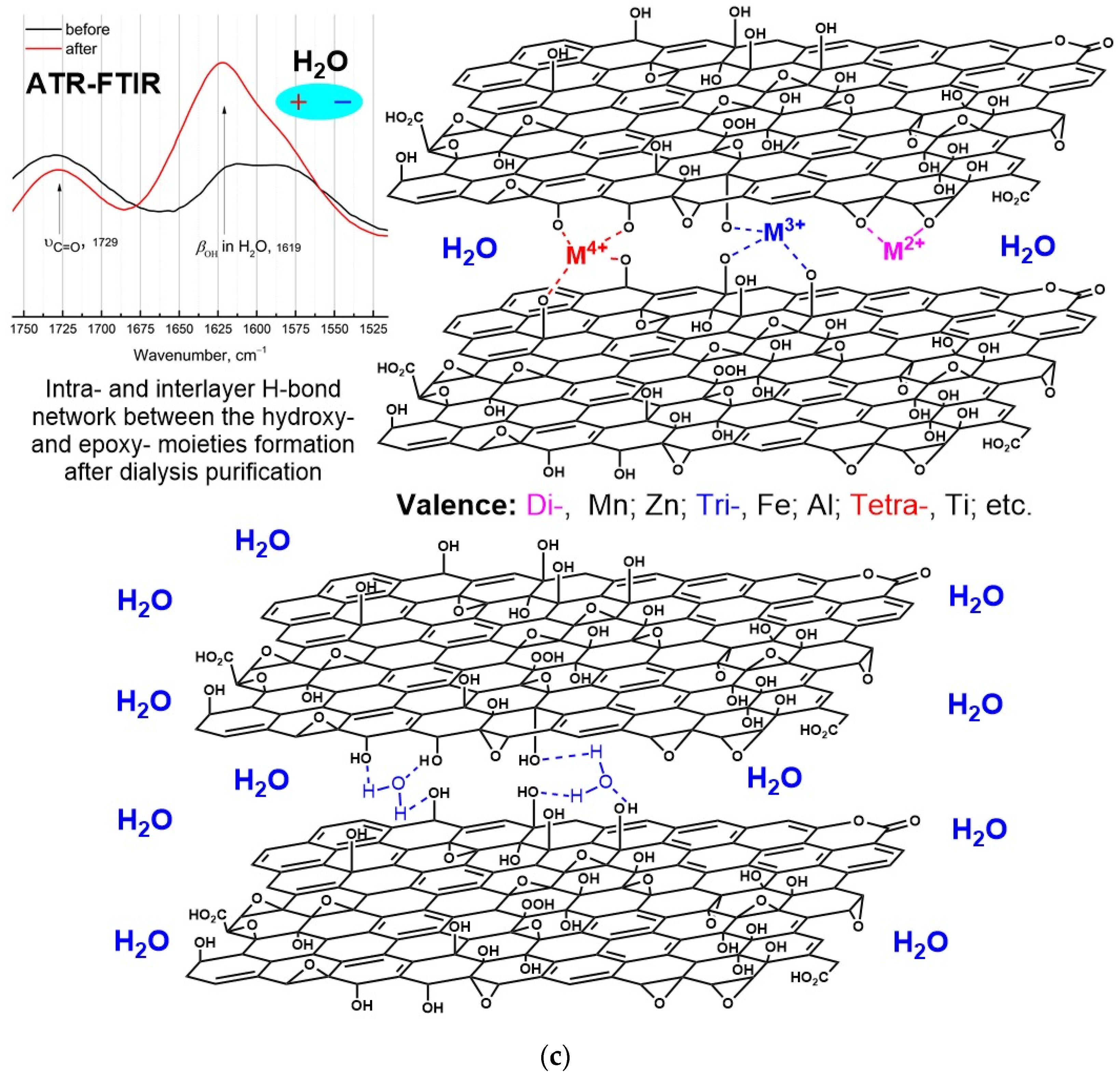
3.2.5. FTIR Absorption Spectra
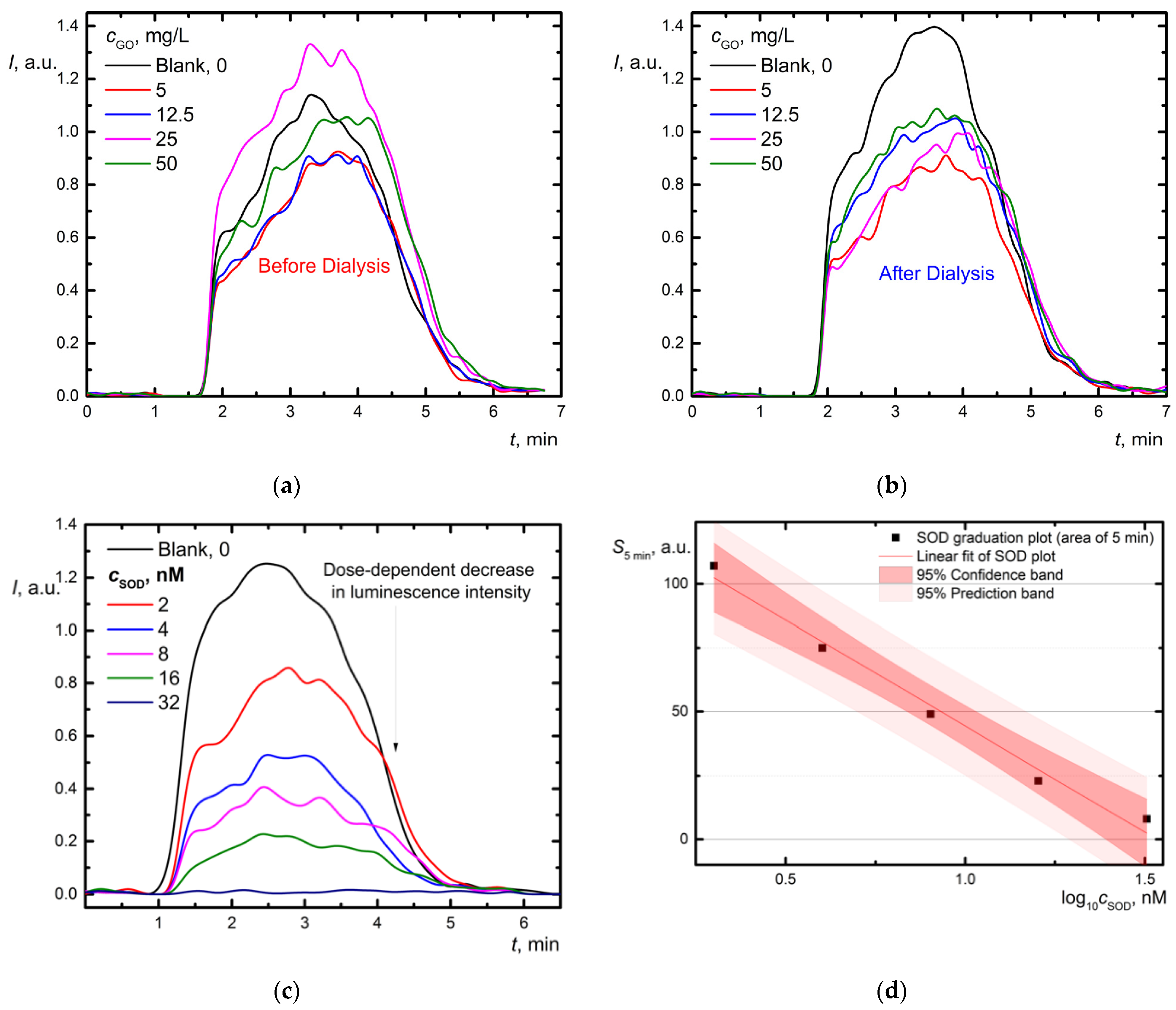
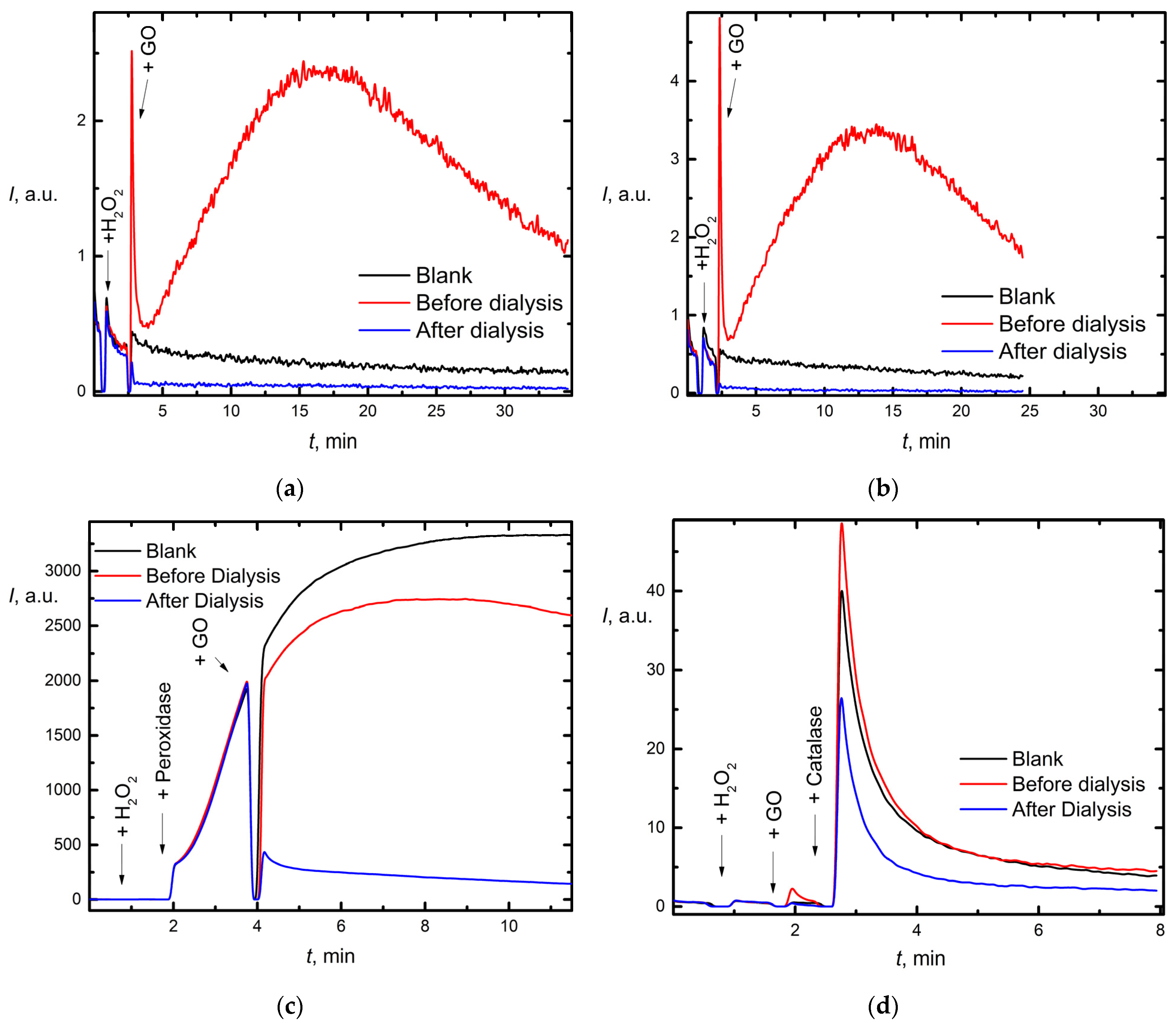
3.3. Chemiluminescence Assays
3.3.1. SOD-like Activity Using Lucigenin/Xanthine/Xanthine Oxidase System
3.3.2. Catalase/Peroxidase Activity
4. Discussion
4.1. Classification of Metal Impurities and Their Removal Mechanism
- For Fe: ; then ; logKf = 25.1; however, this does not rule out a Fenton’s catalytic pathway [74].
- For Ti [75]: ; then ; logKf = 21.3.
- For Mn: .
4.2. Purification Setup Requirements
4.2.1. Membrane (Bag)
4.2.2. Peristaltic Pump for Reproducible Reagent Dosing
4.2.3. Real-Time Online Signal Recording
4.3. Pros and Contras of Dialysis-Monitoring Methods
4.3.1. ORP Probe
4.3.2. UV/Vis Probe
4.4. Characterization of Purified GO Dispersions
4.5. Radical Scavenging and Nanozyme Activity of Purified GO Dispersions
4.5.1. Reactivity with Respect to the Superoxide Anion Radical
4.5.2. Reactivity with Respect to Hydrogen Peroxide
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef]
- Tene, T.; Usca, G.T.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Gomez, C.V. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.-M.; Ren, W. Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-A.; Ou, S.-M.; Lin, C.-C. Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation. Membranes 2022, 12, 152. [Google Scholar] [CrossRef]
- Karimi, K.; Rahsepar, M. Optimization of the Urea Removal in a Wearable Dialysis Device Using Nitrogen-Doped and Phosphorus-Doped Graphene. ACS Omega 2022, 7, 4083–4094. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Graphene-based composite membranes for nanofiltration: Performances and future perspectives. Emergent Mater. 2021, 5, 1429–1441. [Google Scholar] [CrossRef]
- Keramat, A.X.A.; Kadkhoda, J.; Farahzadi, R.; Fathi, E.; Davaran, S. The potential of Graphene Oxide and reduced Graphene Oxide in diagnosis and treatment of Cancer. Curr. Med. Chem. 2022, 29, 4529–4546. [Google Scholar] [CrossRef]
- Shafiee, A.; Iravani, S.; Varma, R.S. Graphene and graphene oxide with anticancer applications: Challenges and future perspectives. MedComm 2022, 3, e118. [Google Scholar] [CrossRef] [PubMed]
- Ozkan-Ariksoysal, D. Current Perspectives in Graphene Oxide-Based Electrochemical Biosensors for Cancer Diagnostics. Biosensors 2022, 12, 607. [Google Scholar] [CrossRef]
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.T.; Pinto, L.A.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zhihui, K.; Min, D. Application of Graphene Oxide-Based Hydrogels in Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 2849–2857. [Google Scholar] [CrossRef]
- Ricci, A.; Cataldi, A.; Zara, S.; Gallorini, M. Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials 2022, 15, 2229. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Ma, L.; Wu, P.; Liu, J. Graphene oxide as a photocatalytic nuclease mimicking nanozyme for DNA cleavage. Nano Res. 2020, 13, 455–460. [Google Scholar] [CrossRef]
- Sun, A.; Mu, L.; Hu, X. Graphene Oxide Quantum Dots as Novel Nanozymes for Alcohol Intoxication. ACS Appl. Mater. Interfaces 2017, 9, 12241–12252. [Google Scholar] [CrossRef]
- Ceriotti, G.; Romanchuk, A.Y.; Slesarev, A.S.; Kalmykov, S.N. Rapid method for the purification of graphene oxide. RSC Adv. 2015, 5, 50365–50371. [Google Scholar] [CrossRef]
- Muhmood, T.; Cai, Z.; Lin, S.; Xiao, J.; Hu, X.; Ahmad, F. Graphene/graphitic carbon nitride decorated with AgBr to boost photoelectrochemical performance with enhanced catalytic ability. Nanotechnology 2020, 31, 505602. [Google Scholar] [CrossRef] [PubMed]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Khan, M.A. Design of Graphene Nanoplatelet/Graphitic Carbon Nitride Heterojunctions by Vacuum Tube with Enhanced Photocatalytic and Electrochemical Response. Eur. J. Inorg. Chem. 2018, 2018, 1726–1732. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Kontos, A.G.; Moustakas, N.G.; Faria, J.L.; Doña-Rodríguez, J.M.; Falaras, P.; Silva, A.M. TiO2, surface modified TiO2 and graphene oxide-TiO2 photocatalysts for degradation of water pollutants under near-UV/Vis and visible light. Chem. Eng. J. 2013, 224, 17–23. [Google Scholar] [CrossRef]
- Brisebois, P.; Siaj, M. Harvesting graphene oxide—Years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Sun, L.; Song, H.; Chang, Y.; Hou, W.; Zhang, Y.; Li, H.; Han, G. Effective removal of manganese in graphene oxide via competitive ligands and the properties of reduced graphene oxide hydrogels and films. Diam. Relat. Mater. 2021, 114, 108314. [Google Scholar] [CrossRef]
- Kiciński, W.; Dyjak, S. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon 2020, 168, 748–845. [Google Scholar] [CrossRef]
- Chernova, E.; Petukhov, D.; Chumakov, A.; Kirianova, A.; Sadilov, I.; Kapitanova, O.; Boytsova, O.; Valeev, R.; Roth, S.; Eliseev, A.A. The role of oxidation level in mass-transport properties and dehumidification performance of graphene oxide membranes. Carbon 2021, 183, 404–414. [Google Scholar] [CrossRef]
- de Mendonça, J.P.A.; Lima, A.H.; Roldao, J.C.; Martins, J.D.S.; Junqueira, G.M.; Quirino, W.G.; Sato, F. The role of sulfate in the chemical synthesis of graphene oxide. Mater. Chem. Phys. 2018, 215, 203–210. [Google Scholar] [CrossRef]
- Bhunia, P.; Kumar, M.; De, S. Fast purification of graphene oxide solution by continuous counter current hollow fibre dialysis: A step towards large scale production. Can. J. Chem. Eng. 2019, 97, 1596–1604. [Google Scholar] [CrossRef]
- López-Díaz, D.; Merchán, M.D.; Velázquez, M.M.; Maestro, A. Understanding the Role of Oxidative Debris on the Structure of Graphene Oxide Films at the Air–Water Interface: A Neutron Reflectivity Study. ACS Appl. Mater. Interfaces 2020, 12, 25453–25463. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Jia, L.; Ma, X.; Zhu, L. Carbonaceous debris that resided in graphene oxide/reduced graphene oxide profoundly affect their electrochemical behaviors. Electrochem. Commun. 2012, 23, 94–97. [Google Scholar] [CrossRef]
- Rourke, J.P.; Pandey, P.A.; Moore, J.J.; Bates, M.; Kinloch, I.A.; Young, R.J.; Wilson, N.R. The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets. Angew. Chem. Int. Ed. 2011, 50, 3173–3177. [Google Scholar] [CrossRef]
- Jia, L.; Dong, L.; Zhu, L. Stripping voltammetry at graphene oxide: The negative effect of carbonaceous debris. Appl. Mater. Today 2017, 8, 26–30. [Google Scholar] [CrossRef]
- Martin-Folgar, R.; Esteban-Arranz, A.; Negri, V.; Morales, M. Toxicological effects of three different types of highly pure graphene oxide in the midge Chironomus riparius. Sci. Total Environ. 2022, 815, 152465. [Google Scholar] [CrossRef]
- Mrózek, O.; Melounková, L.; Smržová, D.; Machálková, A.; Vinklárek, J.; Němečková, Z.; Komárková, B.; Ecorchard, P. Salt-washed graphene oxide and its cytotoxicity. J. Hazard. Mater. 2020, 398, 123114. [Google Scholar] [CrossRef]
- Tölle, F.J.; Gamp, K.; Mülhaupt, R. Scale-up and purification of graphite oxide as intermediate for functionalized graphene. Carbon 2014, 75, 432–442. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Pei, X.; Shi, H.; Li, D.; Xu, Z.; Li, S.; Xue, Y.; Song, L. Free radical scavenging behavior of multidimensional nanomaterials in γ-irradiated epoxy resin and mechanical and thermal performance of γ-irradiated composites. Compos. Part C Open Access 2021, 4, 100095. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, B.; Han, G.; Liu, R.; Han, M.; Zhang, Z. Graphene Oxide: From Tunable Structures to Diverse Luminescence Behaviors. Adv. Sci. 2019, 6, 1900855. [Google Scholar] [CrossRef] [Green Version]
- Mazánek, V.; Luxa, J.; Matějková, S.; Kučera, J.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Ultrapure Graphene Is a Poor Electrocatalyst: Definitive Proof of the Key Role of Metallic Impurities in Graphene-Based Electrocatalysis. ACS Nano 2019, 13, 1574–1582. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, L.; Yang, Z.; Liu, Z.; Gu, J.; Bai, B.; Liu, J.; Xu, J.; Yang, H. Mechanisms of oxidative stress, apoptosis, and autophagy involved in graphene oxide nanomaterial anti-osteosarcoma effect. Int. J. Nanomed. 2018, ume 13, 2907–2919. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cao, H.-Y.; Wang, J.-Q.; Wu, G.-D.; Wang, L. Graphene Oxide and Reduced Graphene Oxide Exhibit Cardiotoxicity Through the Regulation of Lipid Peroxidation, Oxidative Stress, and Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2021, 9, 616888. [Google Scholar] [CrossRef]
- Mittal, S.; Kumar, V.; Dhiman, N.; Chauhan, L.K.S.; Pasricha, R.; Pandey, A.K. Physico-chemical properties based differential toxicity of graphene oxide/reduced graphene oxide in human lung cells mediated through oxidative stress. Sci. Rep. 2016, 6, 39548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, K.; Sundar, L.S.; Pereira, E.; Duarte, A. Graphene oxide induces cytotoxicity and oxidative stress in bluegill sunfish cells. J. Appl. Toxicol. 2018, 38, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Pattammattel, A.; Williams, C.L.; Pande, P.; Tsui, W.G.; Basu, A.K.; Kumar, C.V. Biological relevance of oxidative debris present in as-prepared graphene oxide. RSC Adv. 2015, 5, 59364–59372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazánek, V.; Matějková, S.; Sedmidubský, D.; Pumera, M.; Sofer, Z. One-Step Synthesis of B/N Co-doped Graphene as Highly Efficient Electrocatalyst for the Oxygen Reduction Reaction: Synergistic Effect of Impurities. Chem. Eur. J. 2018, 24, 928–936. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chee, S.Y.; Khezri, B.; Webster, R.D.; Sofer, Z.; Pumera, M. Metallic Impurities in Graphenes Prepared from Graphite Can Dramatically Influence Their Properties. Angew. Chem. Int. Ed. 2012, 51, 500–503. [Google Scholar] [CrossRef]
- Volkov, D.S.; Proskurnin, M.A.; Korobov, M.V. Elemental analysis of nanodiamonds by inductively-coupled plasma atomic emission spectroscopy. Carbon 2014, 74, 1–13. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Pirogova, M.O.; Usoltseva, L.O.; Uzhel, A.S.; Bolotnik, T.A.; Kareev, I.E.; Bubnov, V.P.; Lukonina, N.S.; Volkov, D.S.; Goryunkov, A.A.; et al. Green and rapid preparation of long-term stable aqueous dispersions of fullerenes and endohedral fullerenes: The pros and cons of an ultrasonic probe. Ultrason. Sonochem. 2021, 73, 105533. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, R.; Pratt, K. Standard reference materials: Primary standards and standard reference materials for electrolytic conductivity. NIST Spec. Publ. 2004, 260, 142. [Google Scholar]
- Baucke, F.G.K. New IUPAC recommendations on the measurement of pH—Background and essentials. Anal. Bioanal. Chem. 2002, 374, 772–777. [Google Scholar] [CrossRef]
- Krivoshein, P.K.; Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. FTIR Photoacoustic and ATR Spectroscopies of Soils with Aggregate Size Fractionation by Dry Sieving. ACS Omega 2022, 7, 2177–2197. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Sozarukova, M.M.; Izmailov, D.Y.; Kareev, I.E.; Proskurnina, E.V.; Proskurnin, M.A. Antioxidant Potential of Aqueous Dispersions of Fullerenes C60, C70, and Gd@C82. Int. J. Mol. Sci. 2021, 22, 5838. [Google Scholar] [CrossRef] [PubMed]
- Danzer, K.; Currie, L.A. Guidelines for calibration in analytical chemistry. Part I. Fundamentals and single component calibration (IUPAC Recommendations 1998). Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Sozarukova, M.M.; Proskurnina, E.V.; Kareev, I.E.; Proskurnin, M.A. Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules 2020, 25, 2506. [Google Scholar] [CrossRef] [PubMed]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon 2020, 168, 737–747. [Google Scholar] [CrossRef]
- Hall, L. The Origin of Ultrasonic Absorption in Water. Phys. Rev. 1948, 73, 775–781. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Light and Atmosphere Affect the Quasi-equilibrium States of Graphite Oxide and Graphene Oxide Powders. Small 2015, 11, 1266–1272. [Google Scholar] [CrossRef]
- Wang, C.; Huang, P.; Qiu, C.; Li, J.; Hu, S.; Sun, L.; Bai, Y.; Gao, F.; Li, C.; Liu, N.; et al. Occurrence, migration and health risk of phthalates in tap water, barreled water and bottled water in Tianjin, China. J. Hazard. Mater. 2021, 408, 124891. [Google Scholar] [CrossRef]
- Auld, D.S. [3] Metal-free dialysis tubing. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1988; Volume 158, pp. 13–14. [Google Scholar]
- Richmond, V.L.; Denis, R.S.; Cohen, E. Treatment of dialysis membranes for simultaneous dialysis and concentration. Anal. Biochem. 1985, 145, 343–350. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ionselective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Peacock, M.; Evans, C.D.; Fenner, N.; Freeman, C.; Gough, R.; Jones, T.G.; Lebron, I. UV-visible absorbance spectroscopy as a proxy for peatland dissolved organic carbon (DOC) quantity and quality: Considerations on wavelength and absorbance degradation. Environ. Sci. Process. Impacts 2014, 16, 1445–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of structural and electronic properties of graphene oxide. Appl. Phys. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef] [Green Version]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Mahmood, A. Fe-ZrO2 imbedded graphene like carbon nitride for acarbose (ACB) photo-degradation intermediate study. Adv. Powder Technol. 2018, 29, 3233–3240. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Liang, W.; Ge, L.; Hou, X.; Ren, X.; Yang, L.; Bunker, C.E.; Overton, C.M.; Wang, P.; Sun, Y.-P. Evaluation of Commercial “Carbon Quantum Dots” Sample on Origins of Red Absorption and Emission Features. C 2019, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Klímová, K.; Pumera, M.; Luxa, J.; Jankovský, O.; Sedmidubský, D.; Matějková, S.; Sofer, Z. Graphene Oxide Sorption Capacity toward Elements over the Whole Periodic Table: A Comparative Study. J. Phys. Chem. C 2016, 120, 24203–24212. [Google Scholar] [CrossRef]
- Amirov, R.R.; Shayimova, J.; Nasirova, Z.; Solodov, A.; Dimiev, A.M. Analysis of competitive binding of several metal cations by graphene oxide reveals the quantity and spatial distribution of carboxyl groups on its surface. Phys. Chem. Chem. Phys. 2018, 20, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Overton, T. Shriver and Atkins’ Inorganic Chemistry; OUP: Oxford, UK, 2010. [Google Scholar]
- Kharisov, B.I.; Kharissova, O.V.; Dimas, A.V.; De La Fuente, I.G.; Méndez, Y.P. Review: Graphene-supported coordination complexes and organometallics: Properties and applications. J. Co-ord. Chem. 2016, 69, 1125–1151. [Google Scholar] [CrossRef]
- Shayimova, J.; Amirov, R.R.; Iakunkov, A.; Talyzin, A.; Dimiev, A.M. Carboxyl groups do not play the major role in binding metal cations by graphene oxide. Phys. Chem. Chem. Phys. 2021, 23, 17430–17439. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, W. Speciation analysis in chemistry. ChemTexts 2021, 7, 7. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Accounts Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Mori, M.; Shibata, M.; Kyuno, E.; Ito, S. Reaction of Hydrogen Peroxide with Titanium (IV) at Different pH Values. Bull. Chem. Soc. Jpn. 1956, 29, 904–907. [Google Scholar] [CrossRef] [Green Version]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, T.; Ren, B.; Hursthouse, A.; Zhang, Y. Removal of Mn (II) by Sodium Alginate/Graphene Oxide Composite Double-Network Hydrogel Beads from Aqueous Solutions. Sci. Rep. 2018, 8, 10717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Sutariya, B.; Karan, S. A realistic approach for determining the pore size distribution of nanofiltration membranes. Sep. Purif. Technol. 2022, 293, 121096. [Google Scholar] [CrossRef]
- Anderegg, G. Critical Survey of Stability Constants of EDTA Complexes: Critical Evaluation of Equilibrium Constants in Solution: Stability Constants of Metal Complexes; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Matsumoto, I.; Sekiya, R.; Haino, T. A protocol for size separation of nanographenes. RSC Adv. 2019, 9, 33843–33846. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cui, R.; Chang, Y.; Guo, X.; Gu, W.; Huang, H.; Chen, K.; Lin, G.; Dong, J.; Xing, G.; et al. Adaption of the structure of carbon nanohybrids toward high-relaxivity for a new MRI contrast agent. RSC Adv. 2016, 6, 58028–58033. [Google Scholar] [CrossRef]
- Agar, J.W.M.; Barraclough, K.A. Water use in dialysis: Environmental considerations. Nat. Rev. Nephrol. 2020, 16, 556–557. [Google Scholar] [CrossRef]
- Abe, M.; Masakane, I.; Wada, A.; Nakai, S.; Nitta, K.; Nakamoto, H. Dialyzer surface area is a significant predictor of mortality in patients on hemodialysis: A 3-year nationwide cohort study. Sci. Rep. 2021, 11, 20616. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Development of a dialysis in vitro release method for biodegradable microspheres. AAPS PharmSciTech 2005, 6, E323–E328. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wang, X.; Ai, Y.; Liu, Y.; Li, J.; Ji, Y.; Wang, X. Coagulation Behavior of Graphene Oxide on Nanocrystallined Mg/Al Layered Double Hydroxides: Batch Experimental and Theoretical Calculation Study. Environ. Sci. Technol. 2016, 50, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Van Zomeren, A.; Van Der Weij-Zuiver, E.; Comans, R.N.J. Development of an automated system for isolation and purification of humic substances. Anal. Bioanal. Chem. 2008, 391, 2365–2370. [Google Scholar] [CrossRef] [Green Version]
- Love, J. Process Automation Handbook: A Guide to Theory and Practice; Springer: London, UK, 2007. [Google Scholar]
- Bhunia, P.; Kumar, M.; De, S. Rapid and efficient removal of ionic impurities from graphene oxide through hollow fiber diafiltration. Sep. Purif. Technol. 2019, 209, 103–111. [Google Scholar] [CrossRef]
- Ding, X.; Scieszka, D.; Watzele, S.; Xue, S.; Garlyyev, B.; Haid, R.W.; Bandarenka, A.S. A Systematic Study of the Influence of Electrolyte Ions on the Electrode Activity. ChemElectroChem 2022, 9, e202101088. [Google Scholar] [CrossRef]
- Brandariz, I.; Vilariño, T.; Alonso, P.; Herrero, R.; Fiol, S.; de Vicente, M.E.S. Effect of ionic strength on the formal potential of the glass electrode in various saline media. Talanta 1998, 46, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Bagshaw, E.A.; Wadham, J.L.; Tranter, M.; Beaton, A.D.; Hawkings, J.R.; Lamarche-Gagnon, G.; Mowlem, M.C. Measuring pH in low ionic strength glacial meltwaters using ion selective field effect transistor (ISFET) technology. Limnol. Oceanogr. Methods 2021, 19, 222–233. [Google Scholar] [CrossRef]
- Hauschild, W.; Lemke, E. High-Voltage Test and Measuring Techniques; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Texas Instruments. AN-1852 Designing with pH Electrodes; Texas Instruments: Dallas, TX, USA, 2008; pp. 1–7. [Google Scholar]
- Warner, T.B.; Schuldiner, S. Effects of Oxygen Absorbed in the Skin of a Platinum Electrode on the Determination of Carbon Monoxide Adsorption. J. Phys. Chem. 1965, 69, 4048–4049. [Google Scholar] [CrossRef]
- Valenzuela, A.C.; de la Rosa, J.F.V.; Salas, R.M.; Nájera, S.S.; Medina, L. Design of a faraday cage for biomedical measurements based on site electromagnetic field mapping. AIP Conf. Proc. 2021, 2348, 040008. [Google Scholar] [CrossRef]
- Katarzyński, J.; Olesz, M. Fault Loop Impedance Measurement in Circuits Fed by UPS and Principle of Safety Protection. Sustainability 2020, 12, 10126. [Google Scholar] [CrossRef]
- Winkler, I.; Gomes, A.T. Chapter 10—Countermeasures. In Advanced Persistent Security; Winkler, I., Gomes, A.T., Eds.; Syngress: Oxford, UK, 2017; pp. 105–130. [Google Scholar]
- Jeffery, P.G.; Hutchison, D.; Hutchison, D. Chemical Methods of Rock Analysis; Pergamon Press: Oxford, UK, 1981. [Google Scholar]
- Grieve, I.C. Determination of dissolved organic matter in streamwater using visible spectrophotometry. Earth Surf. Process. Landforms 1985, 10, 75–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of graphene oxide: Variations in reported approaches. Carbon 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Feng, R.; Yu, Y.; Shen, C.; Jiao, Y.; Zhou, C. Impact of graphene oxide on the structure and function of important multiple blood components by a dose-dependent pattern. J. Biomed. Mater. Res. Part A 2015, 103, 2006–2014. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef] [Green Version]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Prodan, D.; Moldovan, M.; Furtos, G.; Saroși, C.; Filip, M.; Perhaița, I.; Carpa, R.; Popa, M.; Cuc, S.; Varvara, S.; et al. Synthesis and Characterization of Some Graphene Oxide Powders Used as Additives in Hydraulic Mortars. Appl. Sci. 2021, 11, 11330. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Dékány, I. DRIFT study of deuterium-exchanged graphite oxide. Carbon 2005, 43, 3186–3189. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Lorenz-Fonfria, V.A. Infrared Difference Spectroscopy of Proteins: From Bands to Bonds. Chem. Rev. 2020, 120, 3466–3576. [Google Scholar] [CrossRef]
- Kempiński, M.; Florczak, P.; Jurga, S.; Śliwińska-Bartkowiak, M.; Kempiński, W. The impact of adsorption on the localization of spins in graphene oxide and reduced graphene oxide, observed with electron paramagnetic resonance. Appl. Phys. Lett. 2017, 111, 084102. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen Bond Networks in Graphene Oxide Composite Paper: Structure and Mechanical Properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef]
- David, R.; Tuladhar, A.; Zhang, L.; Arges, C.G.; Kumar, R. Effect of Oxidation Level on the Interfacial Water at the Graphene Oxide–Water Interface: From Spectroscopic Signatures to Hydrogen-Bonding Environment. J. Phys. Chem. B 2020, 124, 8167–8178. [Google Scholar] [CrossRef]
- Lian, B.; De Luca, S.; You, Y.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Smith, S.C.; Leslie, G.; Joshi, R.K. Extraordinary water adsorption characteristics of graphene oxide. Chem. Sci. 2018, 9, 5106–5111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larciprete, R.; Fabris, S.; Sun, T.; Lacovig, P.; Baraldi, A.; Lizzit, S. Dual Path Mechanism in the Thermal Reduction of Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 17315–17321. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L.L. Colloid Stability. In Emulsions, Foams, and Suspensions; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 117–154. [Google Scholar]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, D.; Whitcombe, M.; Davis, F.; Sharma, P.S.; Prasad, B.B. Electrochemical Detection of Uric Acid in Mixed and Clinical Samples: A Review. Electroanalysis 2011, 23, 305–320. [Google Scholar] [CrossRef]
- Tayade, U.S.; Borse, A.U.; Meshram, J.S. Green reduction of graphene oxide and its applications in band gap calculation and antioxidant activity. Green Mater. 2019, 7, 143–155. [Google Scholar] [CrossRef]
- Pelin, M.; Fusco, L.; Martín, C.; Sosa, S.; Frontiñán-Rubio, J.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E.; Prato, M.; Tubaro, A. Graphene and graphene oxide induce ROS production in human HaCaT skin keratinocytes: The role of xanthine oxidase and NADH dehydrogenase. Nanoscale 2018, 10, 11820–11830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmudzadeh, M.; Yari, H.; Ramezanzadeh, B.; Mahdavian, M. Urtica dioica extract as a facile green reductant of graphene oxide for UV resistant and corrosion protective polyurethane coating fabrication. J. Ind. Eng. Chem. 2019, 78, 125–136. [Google Scholar] [CrossRef]
- Pathipati, S.R.; Pavlica, E.; Treossi, E.; Palermo, V.; Bratina, G. The role of charge transfer at reduced graphene oxide/organic semiconductor interface on the charge transport properties. Org. Electron. 2020, 77, 105499. [Google Scholar] [CrossRef]
- Jalilov, A.S.; Nilewski, L.G.; Berka, V.; Zhang, C.; Yakovenko, A.A.; Wu, G.; Kent, T.A.; Tsai, A.-L.; Tour, J.M. Perylene Diimide as a Precise Graphene-like Superoxide Dismutase Mimetic. ACS Nano 2017, 11, 2024–2032. [Google Scholar] [CrossRef] [Green Version]
- Creighton, M.A.; Rangel-Mendez, J.R.; Huang, J.; Kane, A.B.; Hurt, R.H. Graphene-Induced Adsorptive and Optical Artifacts During In Vitro Toxicology Assays. Small 2013, 9, 1921–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Song, X.; Li, P.; Gao, X.J.; Gao, X. Origins of the peroxidase mimicking activities of graphene oxide from first principles. J. Mater. Chem. B 2020, 8, 9028–9034. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cazelles, R.; Liao, W.-C.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Mimicking Horseradish Peroxidase and NADH Peroxidase by Heterogeneous Cu2+-Modified Graphene Oxide Nanoparticles. Nano Lett. 2017, 17, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, W.; Liu, J.; Wu, X.; Wang, Y.; He, J. Luminol, horseradish peroxidase, and glucose oxidase ternary functionalized graphene oxide for ultrasensitive glucose sensing. Anal. Bioanal. Chem. 2018, 410, 543–552. [Google Scholar] [CrossRef]
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.-j.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J.; et al. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat. Commun. 2012, 3, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Lehninger Principles of Biochemistry; Macmillan: New York, NY, USA, 2008. [Google Scholar]
- Ren, C.; Hu, X.; Zhou, Q. Graphene Oxide Quantum Dots Reduce Oxidative Stress and Inhibit Neurotoxicity In Vitro and In Vivo through Catalase-Like Activity and Metabolic Regulation. Adv. Sci. 2018, 5, 1700595. [Google Scholar] [CrossRef]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.Y.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene Oxide Liquid Crystals. Angew. Chem. Int. Ed. 2011, 50, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Koltonow, A.R.; Luo, C.; Luo, J.; Huang, J. Graphene Oxide Sheets in Solvents: To Crumple or Not To Crumple? ACS Omega 2017, 2, 8005–8009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Ming, Z.; Cao, Y.; Feng, S.; Yang, H.; Chen, L.; Yang, S.-T. Influence of graphene oxide and reduced graphene oxide on the activity and conformation of lysozyme. Colloids Surf. B Biointerfaces 2017, 154, 96–103. [Google Scholar] [CrossRef]
- Song, L.; Huang, C.; Zhang, W.; Ma, M.; Chen, Z.; Gu, N.; Zhang, Y. Graphene oxide-based Fe2O3 hybrid enzyme mimetic with enhanced peroxidase and catalase-like activities. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 747–755. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Mercier, G.; Klechikov, A.; Hedenström, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Moons, E. Brodie vs. Hummers graphite oxides for preparation of multi-layered materials. Carbon 2017, 115, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tong, X.; Chen, Q.; Liu, H. Size effect of graphene oxide sheets on enantioseparation performances in membrane separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126464. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, W.; Li, C.; Cheng, M.; Su, Y.; Xu, L.; Chu, T.; Hou, S. l-Cysteine-Modified Graphene Oxide-Based Membrane for Chiral Selective Separation. ACS Appl. Mater. Interfaces 2021, 13, 49215–49223. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Guo, Y.; Yu, J.C. Graphene oxide–Fe2O3 hybrid material as highly efficient heterogeneous catalyst for degradation of organic contaminants. Carbon 2013, 60, 437–444. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hagiwara, S.; Takahashi, H. Automatic potentiometric titration of surface acidity of carbon black. Carbon 1976, 14, 163–167. [Google Scholar] [CrossRef]
- Sun, C.; Berg, J.C. A review of the different techniques for solid surface acid-base characterization. Adv. Colloid Interface Sci. 2003, 105, 151–175. [Google Scholar] [CrossRef]
- Langley, L.A.; Villanueva, E.D.; Fairbrother, D.H. Quantification of Surface Oxides on Carbonaceous Materials. Chem. Mater. 2006, 18, 169–178. [Google Scholar] [CrossRef]
- Szabó, T.; Tombácz, E.; Illés, E.; Dekany, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2004, 44, 537–545. [Google Scholar] [CrossRef]
- Long, C.M.; Nascarella, M.A.; Valberg, P.A. Carbon black vs. black carbon and other airborne materials containing elemental carbon: Physical and chemical distinctions. Environ. Pollut. 2013, 181, 271–286. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Flaherty, D.W. Modified potentiometric titration method to distinguish and quantify oxygenated functional groups on carbon materials by pKa and chemical reactivity. Carbon 2020, 166, 436–445. [Google Scholar] [CrossRef]
- Linstorm, P. Nist chemistry webbook, nist standard reference database number 69. J. Phys. Chem. Ref. Data Monogr. 1998, 9, 1–1951. [Google Scholar]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal Suspensions of Highly Reduced Graphene Oxide in a Wide Variety of Organic Solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, U.; König, E. Untersuchungen über Graphitoxyd. Z. Für Anorg. Und Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Seop Kim, H. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [Green Version]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Sengupta, I.; Sharat Kumar, S.S.S.; Pal, S.K.; Chakraborty, S. Characterization of structural transformation of graphene oxide to reduced graphene oxide during thermal annealing. J. Mater. Res. 2020, 35, 1197–1204. [Google Scholar] [CrossRef]


| Type of GO Dispersion | Type Reagent for Purification, and Experiment Duration | Concentration, ppb * | Brief Comment | ||||
|---|---|---|---|---|---|---|---|
| Mn | Ti | Zn | Fe | Al | |||
| Pristine I series | Not applicable | 18,050 ± 900 | 2450 ± 120 | 100 ± 10 | 1050 ± 100 | 230 ± 10 | |
| After 3.5 kDa, dialysis bag width 18 mm | Only H2O, 7 d | 25 ± 3 | 480 ± 35 | 60 ± 10 | 220 ± 15 | 15 ± 3 | Almost complete purification from Mn and Al; removes 80% Ti and Fe; does not get rid of Zn, approx. 40% |
| EDTA 50 mM, H2O2 3% w/v, 7 d | <2 | 420 ± 30 | 65 ± 10 | 170 ± 20 | 40 ± 5 | Complete purification from Mn; up to 80% reduced Ti, Fe and Al, and 35% Zn content | |
| EDTA 50 mM, H2O2 3% w/v, 7 d (membrane second usage) | 150 ± 15 | 420 ± 30 | 45 ± 7 | 155 ± 20 | <2 | Repeated dialysis bag usage allows washing out Mn almost completely; Al, completely, decreases Ti and Fe up to 85%; Zn is reduced up to 55%. | |
| Pristine II series | Not applicable | 16,080 ± 800 | 940 ± 70 | 35 ± 5 | 750 ± 75 | 95 ± 10 | |
| After 14 kDa, 25 mm | EDTA 50 mM, H2O2 3% w/v, 7 d | <2 | 135 ± 15 | 100 ± 15 | 125 ± 15 | <2 | The resulting dispersion contains less Ti due to the replacement of the ultrasonic probe with a new one. The use of a membrane with a higher threshold (MWCO 14 kDa) also completely removes Mn and Al, reduces Ti and Fe contents up to 90%, while the purification increases Zn content up to 300%. |
| After 14 kDa, 75 mm | EDTA 50 mM, H2O2 3% w/v, 7 d | <2 | 145 ± 15 | 20 ± 3 | 135 ± 15 | <2 | Complete removal of Mn and Al, reduces Ti and Fe contents up to 85%. and Zn content up to 45%. |
| EDTA 50 mM, H2O2 3% w/v, 7 d (membrane second usage) | <2 | 30 ± 5 | 30 ± 5 | 75 ± 10 | <2 | Complete removal of Mn and Al, reduces Ti and Fe contents up to 97%, and Zn content remains the same. | |
| Type of GO Dispersion | TOC, ppm | Yield, % | pH | , ppm ** | , ppm | Lateral Size, nm | ζ, mV | PDI | κ, µS/cm |
|---|---|---|---|---|---|---|---|---|---|
| Pristine I | 2030 ± 100 | Not applicable | 2.3 ± 0.2 | 150 ± 25 | 400 ± 40 | 1100 ± 120 | −36.8 ± 0.5 | 0.464 | 2800 ± 140 |
| Pristine, 0.45 µm PTFE filtering | 8.0 ± 0.9 | 0.39 | 4.4 ± 0.2 | n/m | n/m | 130 ± 15 | −27.5 ± 0.8 | 0.273 | n/m * |
| 3.5 kDa, only H2O, 7 d | 1710 ± 80 | 84.2 | 4.0 ± 0.2 | 2.0 ± 0.5 | 78 ± 20 | 1300 ± 150 | −30.4 ± 0.5 | 0.354 | 400 ± 40 |
| 3.5 kDa, 7 d purification (with reagents) | 1650 ± 70 | 81.3 | 4.1 ± 0.2 | <2.0 | 45 ± 10 | 1250 ± 120 | −32.6 ± 1.5 | 0.320 | 380 ± 40 |
| Reuse of 3.5 kDa, 7 d purification (with reagents) | 1320 ± 75 | 65.0 | 4.2 ± 0.2 | <2.0 | 40 ± 10 | 1050 ± 120 | −30.6 ± 1.2 | 0.370 | 360 ± 45 |
| Boiled membrane preconditioning, 3.5 kDa, 7 d purification (with reagents) | 700 ± 50 | 34.5 | 4.6 ± 0.2 | <2.0 | 55 ± 10 | 1270 ± 150 | −29.5 ± 1.2 | 0.370 | 230 ± 20 |
| Pristine II | 1820 ± 120 | Not applicable | 2.0 ± 0.2 | 130 ± 15 | 350 ± 35 | 1250 ± 130 | −37.5 ± 0.5 | 0.480 | 2650 ± 120 |
| 14 kDa, 7 d purification (with reagents, 25 mm) | 877 ± 95 | 48.2 | 4.5 ± 0.2 | <2.0 | 15 ± 3 | 1450 ± 140 | −32.5 ± 0.5 | 0.380 | 320 ± 45 |
| 14 kDa, 7 d purification (with reagents, 75 mm) | 1030 ± 130 | 56.7 | 4.6 ± 0.2 | <2.0 | 18 ± 4 | 1375 ± 135 | −34.3 ± 0.5 | 0.390 | 300 ± 35 |
| Reuse of 14 kDa, 7 d purification (with reagents, 25 mm) | 833 ± 80 | 45.8 | 4.8 ± 0.2 | <2.0 | 10 ± 2 | 1550 ± 130 | −31.8 ± 0.8 | 0.370 | 315 ± 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikheev, I.V.; Byvsheva, S.M.; Sozarukova, M.M.; Kottsov, S.Y.; Proskurnina, E.V.; Proskurnin, M.A. High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis. Nanomaterials 2022, 12, 4159. https://doi.org/10.3390/nano12234159
Mikheev IV, Byvsheva SM, Sozarukova MM, Kottsov SY, Proskurnina EV, Proskurnin MA. High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis. Nanomaterials. 2022; 12(23):4159. https://doi.org/10.3390/nano12234159
Chicago/Turabian StyleMikheev, Ivan V., Sofiya M. Byvsheva, Madina M. Sozarukova, Sergey Yu. Kottsov, Elena V. Proskurnina, and Mikhail A. Proskurnin. 2022. "High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis" Nanomaterials 12, no. 23: 4159. https://doi.org/10.3390/nano12234159
APA StyleMikheev, I. V., Byvsheva, S. M., Sozarukova, M. M., Kottsov, S. Y., Proskurnina, E. V., & Proskurnin, M. A. (2022). High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis. Nanomaterials, 12(23), 4159. https://doi.org/10.3390/nano12234159






