Effect of Silica-Based Nanomaterials on Seed Germination and Seedling Growth of Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Si-Based NMs
2.2. Seed Germination
2.3. Plant Growth and Treatment
2.4. Chlorophyll Content
2.5. Antioxidant System
2.5.1. POD Activity
2.5.2. SOD Activity
2.5.3. CAT Activity
2.5.4. MDA Content
2.6. Mineral Element Content Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Si-Based NMs on Seed Germination
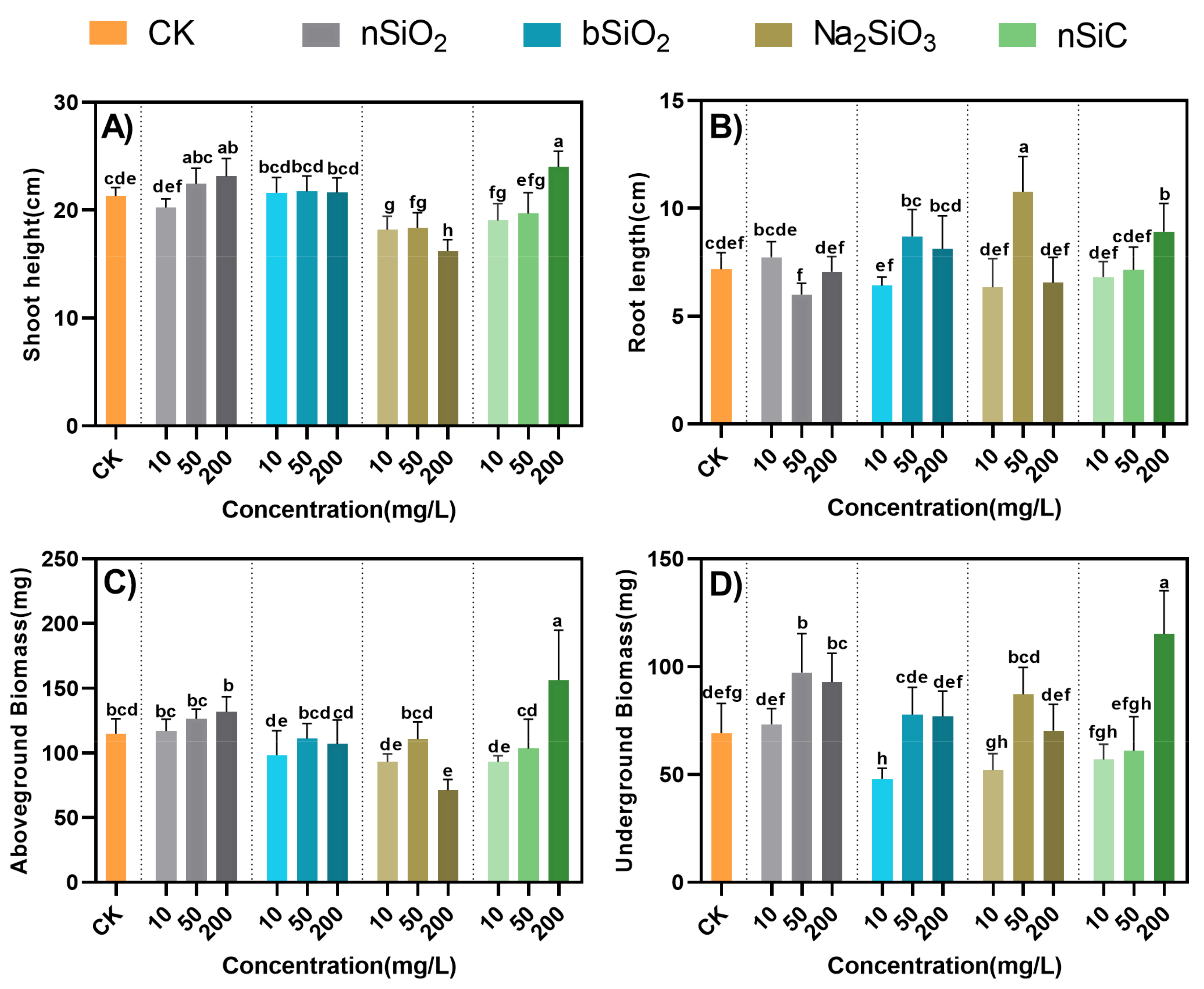
3.2. Effects of Si-Based NMs on the Growth of Rice Seedlings
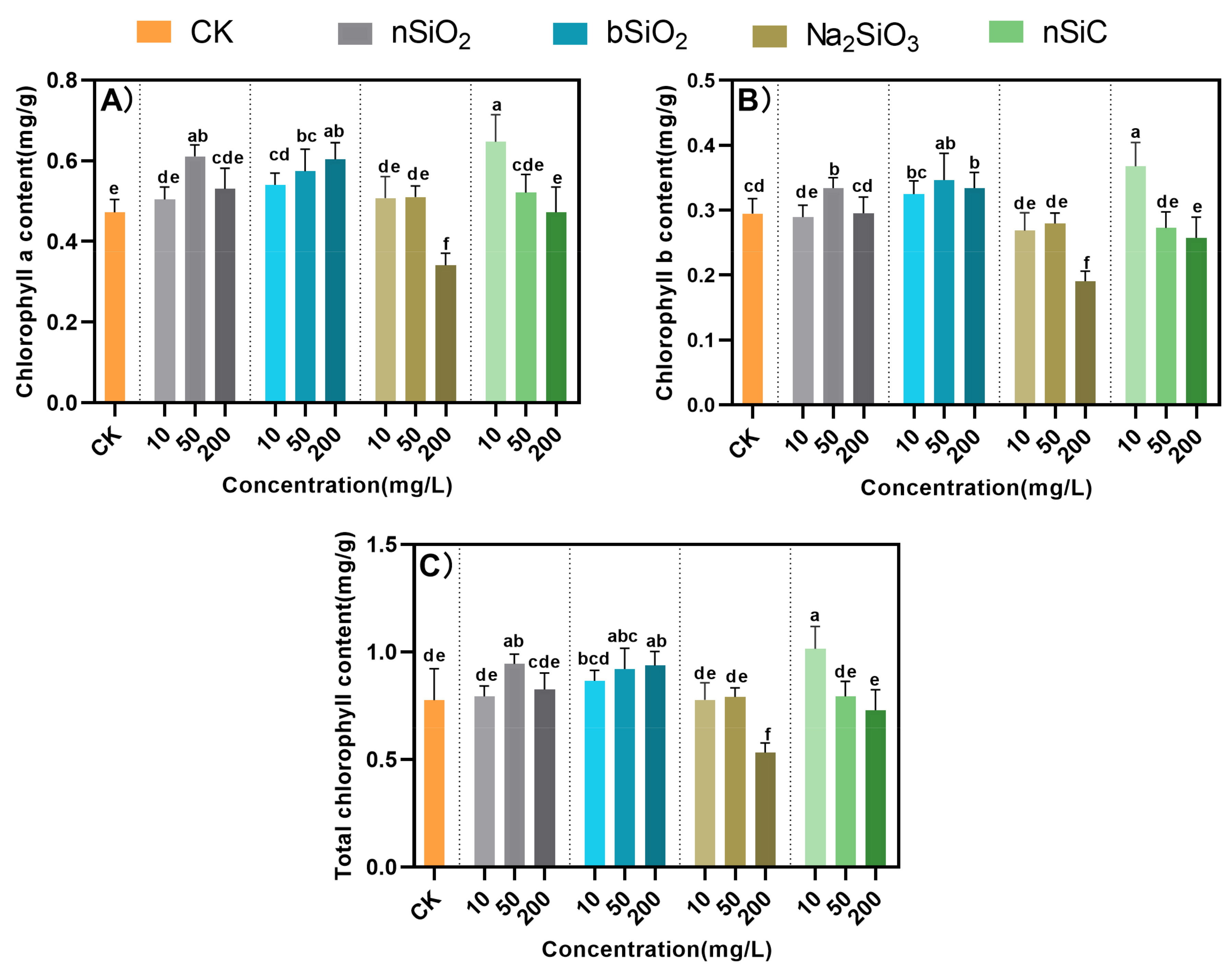
3.3. Effects of Si-Based NMs on Chlorophyll Content in Rice Seedlings
3.4. Effects of Si-Based NMs on the Antioxidant System of Rice Seedlings
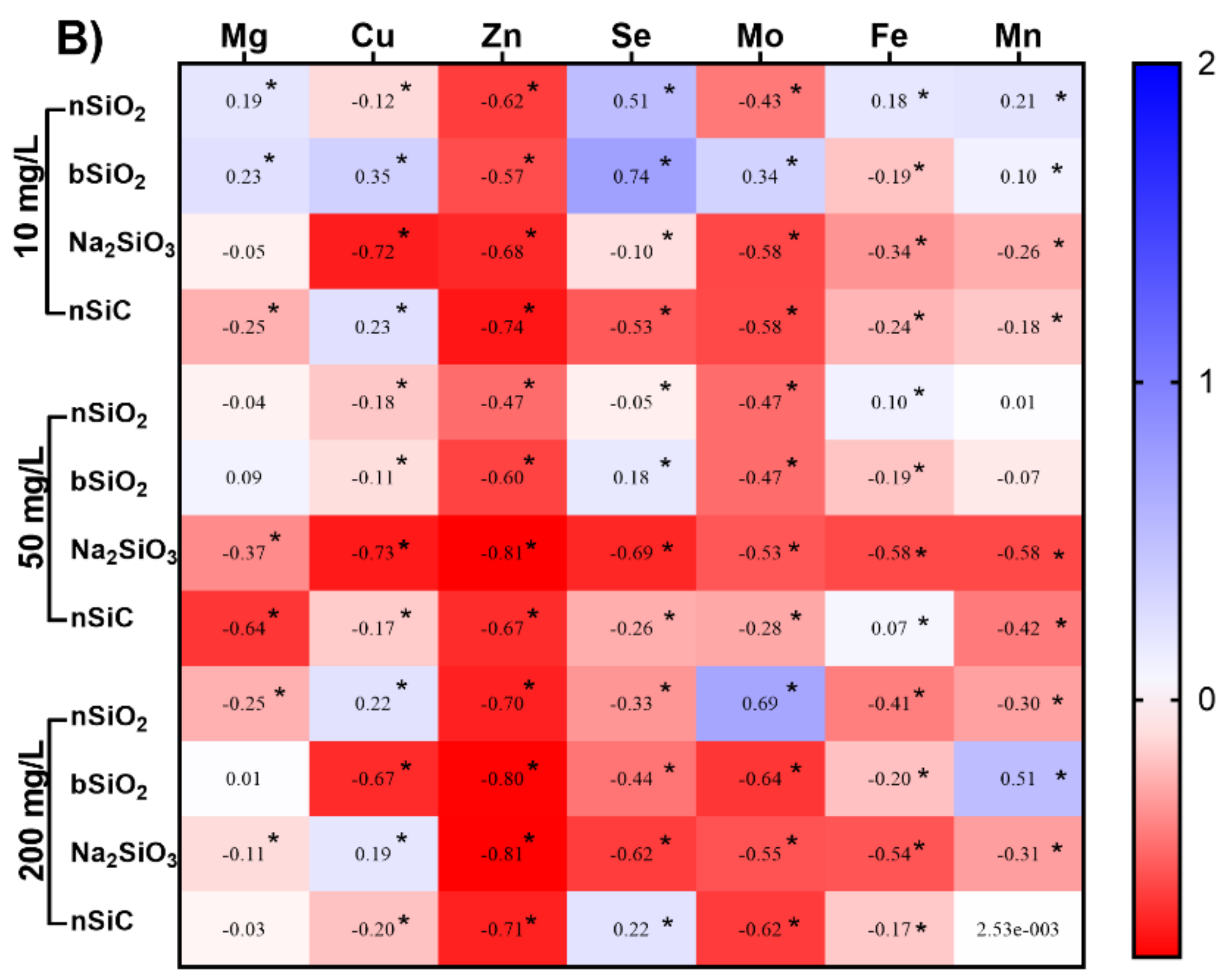
3.5. Effects of Si-Based NMs on Mineral Element Content in Rice Seedling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouman, B.; Barker, R.; Humphreys, E.; Tuong, T.P.; Atlin, G.N.; Bennett, J.; Dawe, D.; Dittert, K.; Dobermann, A.; Facon, T.; et al. Rice: Feeding the billion. In Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Earthscan: Oxford, UK, 2007; pp. 515–549. [Google Scholar]
- Wang, L.; Lu, Q.; Wen, X.; Lu, C. Enhanced Sucrose Loading Improves Rice Yield by Increasing Grain Size. Plant Physiol. 2015, 169, 2848–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, D.N. Nanotechnology and Nanomaterials in Everyday Life. J. Res. Sci. Eng. 2017, 6, 1560–1562. [Google Scholar]
- Jiang, Y.; Zhou, P.; Zhang, P.; Adeel, M.; Shakoor, N.; Li, Y.; Li, M.; Guo, M.; Zhao, W.; Lou, B.; et al. Green synthesis of metal-based nanoparticles for sustainable agriculture. Environ. Pollut. 2022, 309, 119755. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Chapter 4—Applications of Nanotechnology in Daily Life. In Interface Science and Technology; Nasrollahzadeh, M., Sajadi, S.M., Sajjadi, M., Issaabadi, Z., Atarod, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–143. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Bacchu, M.S.; Ali, M.R.; Khan, M.Z.H. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef] [PubMed]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A Review of Silicon in Soils and Plants and Its Role in US Agriculture: History and Future Perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- El-Ramady, H.; Verma, K.K.; Rajput, V.D.; Minkina, T.; Elbehery, F.; Elbasiony, H.; Elsakhawy, T.; El-Dein Omara, A.; Amer, M. Chapter 1—Sources of silicon and nano-silicon in soils and plants. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Etesami, H., Al Saeedi, A.H., El-Ramady, H., Fujita, M., Pessarakli, M., Hossain, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–15. [Google Scholar]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Swaminathan, S.; Edward, B.S.; Kurpad, A.V. Micronutrient deficiency and cognitive and physical performance in Indian children. Eur. J. Clin. Nutr. 2013, 67, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Bao-Shan, L.; Shao-Qi, D.; Chun-Hui, L.; Li-Jun, F.; Shu-Chun, Q.; Min, Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 1399. [Google Scholar] [CrossRef]
- Younis, A.; Khattab, H.; Emam, M. Impacts of silicon and silicon nanoparticles on leaf ultrastructure and TaPIP1 and TaNIP2 gene expressions in heat stressed wheat seedlings. Biol. Plant. 2020, 64, 343–352. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wu, X.; Guo, Z.; Yang, X.; Hu, X.; Lynch, I. Stress Response and Nutrient Homeostasis in Lettuce (Lactuca sativa) Exposed to Graphene Quantum Dots Are Modulated by Particle Surface Functionalization. Adv. Biol. 2021, 5, e2000778. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhou, P.; Ma, T.; Adeel, M.; Shakoor, N.; Li, Y.; Li, M.; Guo, M.; Rui, Y. Effects of two Mn-based nanomaterials on soybean antioxidant system and mineral element homeostasis. Environ. Sci. Pollut. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Rui, Y.-K.; Qu, L.-C.; Kong, X.-B. Effects of soil use along Yellow River basin on the pollution of soil by heavy metals. Spectrosc. Spectr. Anal. 2008, 28, 934–936. [Google Scholar]
- Nath, A.; Molnár, M.A.; Albert, K.; Das, A.; Bánvölgyi, S.; Márki, E.; Vatai, G. Chapter Nine—Agrochemicals from nanomaterials—Synthesis, mechanisms of biochemical activities and applications. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–312. [Google Scholar]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Mao, J.; Chen, B. Effects of biochar nanoparticles on seed germination and seedling growth. Environ. Pollut. 2019, 256, 113409. [Google Scholar] [CrossRef]
- Feist, B.; Sitko, R. Method for the determination of Pb, Cd, Zn, Mn and Fe in rice samples using carbon nanotubes and cationic complexes of batophenanthroline. Food Chem. 2018, 249, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2020, 269, 116134. [Google Scholar] [CrossRef] [PubMed]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, Y.; Liu, X.; Wei, Q.; Qi, Z.; Yang, S.; Liao, L. A general non-rectangular hyperbola equation for photosynthetic light response curve of rice at various leaf ages. Sci. Rep. 2019, 9, 9909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Xie, W.; Xing, H.; Yan, J.; Meng, X.; Li, X.; Fu, X.; Xu, J.; Lian, X.; Yu, S.; et al. Genetic Architecture of Natural Variation in Rice Chlorophyll Content Revealed by a Genome-Wide Association Study. Mol. Plant 2015, 8, 946–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, A.K.; Mandal, K.G.; Mohanty, R.K.; Ambast, S.K. Rice root growth, photosynthesis, yield and water productivity improvements through modifying cultivation practices and water management. Agric. Water Manag. 2018, 206, 67–77. [Google Scholar] [CrossRef]
- Xiong, H.; Hua, L.; Reyna-Llorens, I.; Shi, Y.; Chen, K.M.; Smirnoff, N.; Kromdijk, J.; Hibberd, J.M. Photosynthesis-independent production of reactive oxygen species in the rice bundle sheath during high light is mediated by NADPH oxidase. Proc. Natl. Acad. Sci. USA 2021, 118, e2022702118. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Zhou, P.; Long, B.; Wang, R.; Jiang, Y.; Zhao, W.; Li, Y.; Li, M.; Guo, Z.; Zhang, P.; Rui, Y.; et al. Increase in the active ingredients of traditional Chinese medicine Isatis indigotica through iron nanoparticles supplementation versus carbon nanotubes: A comparative study. Environ. Sci. Nano 2022, 9, 2966–2978. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xu, G.; Qian, H.; Liu, P.; Zhao, P.; Hu, Y. Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ. Pollut. 2013, 176, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef] [PubMed]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient balance and micronutrient amounts through growth and development. Ital. J. Pediatr. 2021, 47, 109. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Sadeghzadeh, B.; Rengel, Z. Zinc in Soils and Crop Nutrition. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 335–375. [Google Scholar]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Mantadakis, E.; Chatzimichael, E.; Zikidou, P. Iron Deficiency Anemia in Children Residing in High and Low-Income Countries: Risk Factors, Prevention, Diagnosis and Therapy. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020041. [Google Scholar] [CrossRef]
- Motyka, O.; Štrbová, K.; Zinicovscaia, I. Chlorophyll Content in Two Medicinal Plant Species Following Nano-TiO2 Exposure. Bull. Environ. Contam. Toxicol. 2020, 104, 373–379. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Hayat, S.; Alam, P. Foliar Application of Copper Oxide Nanoparticles Increases the Photosynthetic Efficiency and Antioxidant Activity in Brassica juncea. J. Food Qual. 2022, 2022, 5535100. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Yang, J.; Li, M.; Li, Y.; Zhou, P.; Wang, Q.; Sun, Y.; Zhu, G.; Wang, Q.; Zhang, P.; et al. Effect of Silica-Based Nanomaterials on Seed Germination and Seedling Growth of Rice (Oryza sativa L.). Nanomaterials 2022, 12, 4160. https://doi.org/10.3390/nano12234160
Jiang Y, Yang J, Li M, Li Y, Zhou P, Wang Q, Sun Y, Zhu G, Wang Q, Zhang P, et al. Effect of Silica-Based Nanomaterials on Seed Germination and Seedling Growth of Rice (Oryza sativa L.). Nanomaterials. 2022; 12(23):4160. https://doi.org/10.3390/nano12234160
Chicago/Turabian StyleJiang, Yaqi, Jie Yang, Mingshu Li, Yuanbo Li, Pingfan Zhou, Quanlong Wang, Yi Sun, Guikai Zhu, Qibin Wang, Peng Zhang, and et al. 2022. "Effect of Silica-Based Nanomaterials on Seed Germination and Seedling Growth of Rice (Oryza sativa L.)" Nanomaterials 12, no. 23: 4160. https://doi.org/10.3390/nano12234160






