Plasma Ag-Modified α-Fe2O3/g-C3N4 Self-Assembled S-Scheme Heterojunctions with Enhanced Photothermal-Photocatalytic-Fenton Performances
Abstract
:1. Introduction
2. Experimental Section
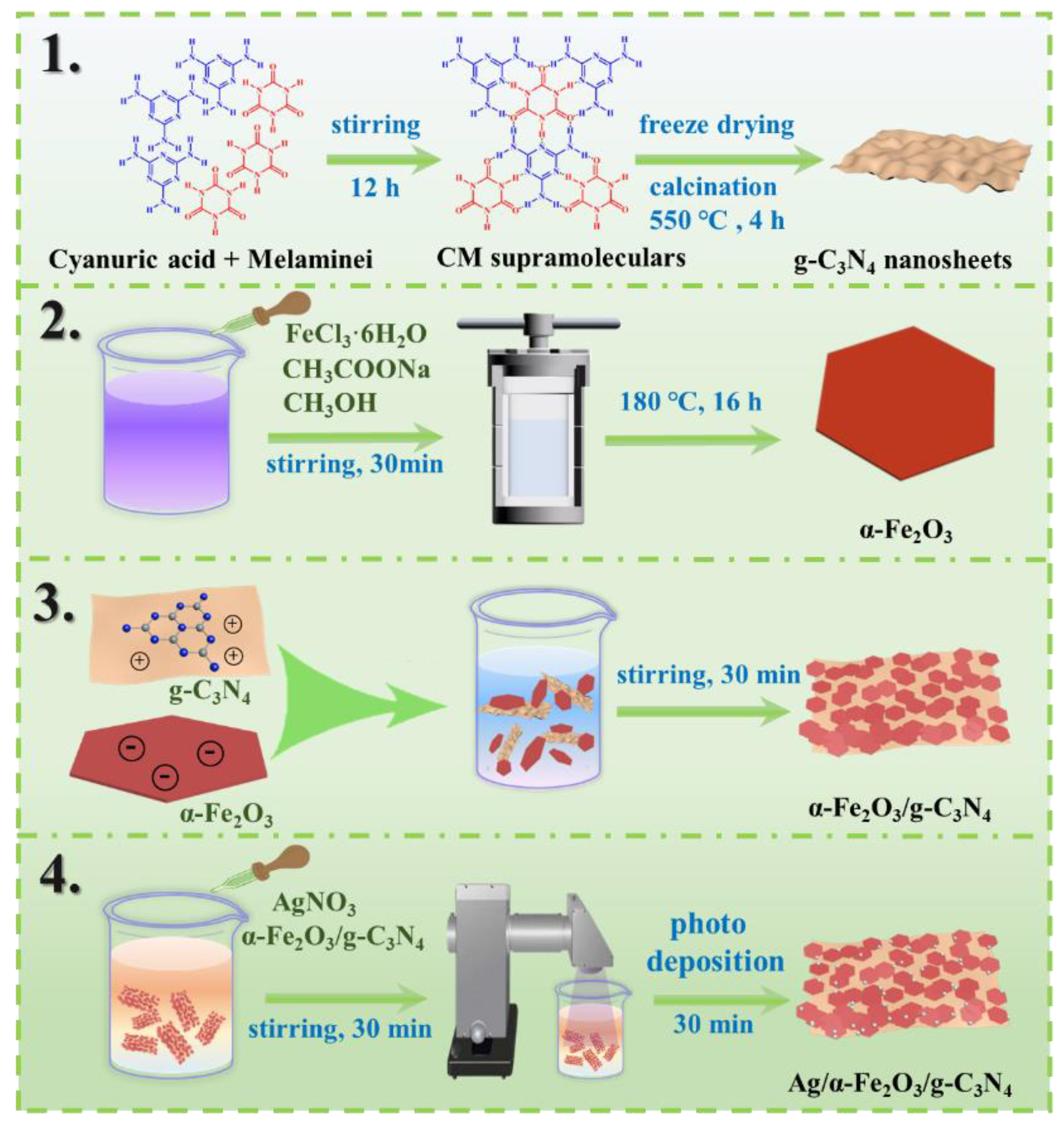
2.1. Preparation of g-C3N4 ultra-Thin Nanosheets
2.2. Preparation of Hexagonal α-Fe2O3 Nanosheets
2.3. Preparation of α-Fe2O3/g-C3N4 Heterojunction Photocatalyst
2.4. Preparation of Ag/α-Fe2O3/g-C3N4 Ternary Composite Photocatalyst
2.5. Experiment Characterizations
2.6. Photothermal Test
2.7. Photocatalytic Performance Assessment
3. Results and Discussion
3.1. Physical Phase Composition and Energy Band Structure
3.2. Morphological and Structural Analysis
3.3. Analysis of Charge Transfer Mechanism
3.4. Photothermal Effect Evaluation
3.5. Photocatalytic Hydrogen Production and Pollutant Degradation Testing
3.6. Photoelectrochemical Performance Analysis
3.7. Mechanistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Kosco, J.; Gonzalez, S.; Howells, C.T.; Fei, T.; Dong, Y.; Sougrat, R.; Harrison, G.T.; Firdaus, Y.; Sheelamanthula, R.; Purushothaman, B.; et al. Generation of long-lived charges in organic semiconductor heterojunction nanoparticles for efficient photocatalytic hydrogen evolution. Nat. Energy 2022, 7, 340–351. [Google Scholar] [CrossRef]
- Song, L.; Wang, W.; Yue, J.P.; Jiang, Y.X.; Wei, M.K.; Zhang, H.P.; Yan, S.S.; Liao, L.L.; Yu, D.G. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 2022, 5, 823–838. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.J.; Wang, C.; Gan, L.Y.; Chen, X.J.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.H.; Zhou, X.Y.; et al. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Y.; Qu, B.; Bai, L.; Liu, Y.; Yang, Z.-D.; Zhang, W.; Jing, L.; Fu, H. Construction of six-oxygen-coordinated single Ni sites on g-C3N4 with boron-oxo species for photocatalytic water-activation-induced CO2 reduction. Adv. Mater. 2021, 33, 2105482. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, L.; Jiang, B.; Li, Q.; Tian, C.; Xie, Y.; Li, W.; Fu, H. Ultrathin porous carbon nitride bundles with an adjustable energy band structure toward simultaneous solar photocatalytic water splitting and selective phenylcarbinol oxidation. Angew. Chem. Int. Ed. 2021, 60, 4951. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D graphitic carbon nitride for energy conversion and storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Che, H.; Gao, X.; Chen, J.; Hou, J.; Ao, Y.; Wang, P. Iodide-induced fragmentation of polymerized hydrophilic carbon nitride for high-performance quasi-homogeneous photocatalytic H2O2 production. Angew. Chem. Int. Ed. 2021, 60, 25546–25550. [Google Scholar] [CrossRef]
- Hu, C.; Chen, F.; Wang, Y.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Exceptional cocatalyst-free photo-enhanced piezocatalytic hydrogen evolution of carbon nitride nanosheets from strong in-plane polarization. Adv. Mater. 2021, 33, 2101751. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Wang, F. Oxygen-controlled photo-reforming of biopolyols to CO over Z-scheme CdS@g-C3N4. Chem 2022, 8, 465–479. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, W.; Liao, G.; Li, X.; Wang, J.; Tang, Y.; Li, L. Strategy for improving photocatalytic ozonation activity of g-C3N4 by halogen doping for water purification. Appl. Catal. B Environ. 2022, 306, 121133. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/covalent organic framework van der Waals heterojunction toward highly efficient S-scheme CO2 photoreduction. Appl. Catal. B Environ. 2022, 301, 120814. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Q.; Zhao, Y.; Wen, H.; Jiang, Z. The exploration of metal-free catalyst g-C3N4 for NO degradation. J. Hazard. Mater. 2021, 404, 124153. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Yuan, X.; Qu, S.; Huang, X.; Xue, X.; Yuan, C.; Wang, S.; Wei, L.; Cai, P. Design of core-shelled g-C3N4@ZIF-8 photocatalyst with enhanced tetracycline adsorption for boosting photocatalytic degradation. Chem. Eng. J. 2021, 416, 129148. [Google Scholar] [CrossRef]
- Gong, S.; Teng, X.; Niu, Y.; Liu, X.; Xu, M.; Xu, C; Ji, L.; Chen, Z. Construction of S-Scheme 0D/2D heterostructures for enhanced visible-light-driven CO2 reduction. Appl. Catal. B Environ. 2021, 298, 120521. [Google Scholar] [CrossRef]
- Moradi, S.; Isari, A.A.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Co-implanting of TiO2 and liquid-phase-delaminated g-C3N4 on multi-functional graphene nanobridges for enhancing photocatalytic degradation of acetaminophen. Chem. Eng. J. 2021, 414, 128618. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Chen, Z.; Yu, Z.; Xue, J.; Luan, T.; Chen, S.; Zhou, S. Mechanisms of polystyrene microplastic degradation by the microbially driven Fenton reaction. Water Res. 2022, 223, 118979. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Mei, Y.; Li, J.; Yao, T.; Yang, Y.; Jia, W.; Tong, X.; Wu, J.; Xin, B. Highly efficient microwave-assisted Fenton degradation of metacycline using pine-needle-like CuCo2O4 nanocatalyst. Chem. Eng. J. 2019, 373, 1158–1167. [Google Scholar] [CrossRef]
- Sun, H.; Xie, G.; He, D.; Zhang, L. Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling. Appl. Catal. B Environ. 2020, 267, 118383. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Chen, N.; Ben, H.; Zhan, G.; Sun, H.; Li, Q.; Sun, J.; Zhang, L. Hydroxylamine enables rapid heterogeneous-homogeneous coupled Fenton sulfamethazine degradation on ferric phosphate. Appl. Catal. B Environ. 2022, 312, 121410. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Cui, J.; An, W.; Liu, L.; Liang, Y.; Cui, W. Surface oxygen vacancies enriched FeOOH/Bi2MoO6 photocatalysis-fenton synergy degradation of organic pollutants. J. Hazard. Mater. 2020, 384, 121399. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, B.F.; Tang, Z.Y.; Mai, X.Q.; Ouyang, T.; Liu, Z.Q. CH=OH selective oxidation to HCHO on Z-scheme Fe2O3/g-C3N4 hybrid: The rate-determining step of C-H bond scission. Chem. Eng. J. 2021, 422, 130086. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Liu, H.; Huang, F.; Shao, Q.; Liu, L.; Sun, J.; Sun, C.; Chen, D.; Dong, L. Single-Atom Ce-Modified α-Fe2O3 for Selective Catalytic Reduction of NO with NH3. Environ. Sci. Technol. 2022, 56, 10442–10453. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Banazadeh, A.; Warzywoda, J.; Zirak, M. Carbon quantum dots as nano-scaffolds for α-Fe=O3 growth: Preparation of Ti/CQD@α-Fe2O3 photoanode for water splitting under visible light irradiation. Appl. Catal. B Environ. 2018, 227, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Du, J.; Li, J.; Wu, Y.; Zhou, Y.; Yang, Y.; Yang, D.; He, H. Thermodynamic and kinetic influence of oxygen vacancies on the solar water oxidation reaction of α-Fe2O3 photoanodes. ACS Appl. Mater. Interfaces 2020, 12, 11625–11634. [Google Scholar] [CrossRef]
- He, B.C.; Zhang, C.; Luo, P.P.; Li, Y.; Lu, T.B. Integrating Z-scheme heterojunction of Co1-C3N4@α-Fe2O3 for efficient visible-light-driven photocatalytic CO2 reduction. Green Chem. 2020, 22, 7552–7559. [Google Scholar] [CrossRef]
- Li, F.; Huang, T.; Sun, F.; Chen, L.; Li, P.; Shao, F.; Yang, X.; Liu, W. Ferric oxide nanoclusters with low-spin FeIII anchored g-C3N4 rod for boosting photocatalytic activity and degradation of diclofenac in water under solar light. Appl. Catal. B Environ. 2022, 317, 121725. [Google Scholar] [CrossRef]
- Wang, R.; Che, G.; Wang, C.; Liu, C.; Liu, B.; Ohtani, B.; Liu, Y.; Zhang, X. Alcohol plasma processed surface amorphization for photocatalysis. ACS Catal. 2022, 12, 12206–12216. [Google Scholar] [CrossRef]
- Verma, R.; Belgamwar, R.; Polshettiwar, V. Plasmonic photocatalysis for CO2 conversion to chemicals and fuels. ACS Mater. Lett. 2021, 3, 574–598. [Google Scholar] [CrossRef]
- An, X.; Kays, J.C.; Lightcap, I.V.; Ouyang, T.; Dennis, A.M.; Reinhard, B.M. Wavelength-dependent bifunctional plasmonic photocatalysis in Au/chalcopyrite hybrid nanostructures. ACS Nano 2022, 16, 6813–6824. [Google Scholar] [CrossRef]
- Qiu, J.L.; Su, J.; Muhammad, N.; Zheng, W.T.; Yue, C.L.; Liu, F.Q.; Zuo, J.L.; Ding, Z.J. Facile encapsulating Ag nanoparticles into a tetrathiafulvalene-based Zr-MOF for enhanced photocatalysis. Chem. Eng. J. 2022, 427, 131970. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, B.; Zhai, J.; Hedin, N. Reactive oxygenated species generated on iodide-doped BiVO4/BaTiO3 heterostructures with Ag/Cu nanoparticles by coupled piezophototronic effect and plasmonic excitation. Adv. Funct. Mater. 2021, 31, 2009594. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, T.; Wang, Y.; Wu, M.; Jin, W.; Tian, Y.; Li, X. Decorating Ag/AgCl on UiO-66-NH2: Synergy between Ag plasmons and heterostructure for the realization of efficient visible light photocatalysis. Chin. J. Catal. 2019, 40, 1187–1197. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, S.; Ding, M.; Li, L.; Sun, Q.; Wang, Z.L. Enhanced photocatalysis by synergistic piezotronic effect and exciton-plasmon interaction based on (Ag-Ag2S)/BaTiO3 heterostructures. Adv. Funct. Mater. 2020, 30, 2005716. [Google Scholar] [CrossRef]
- Liu, M.; Jin, X.; Li, S.; Billeau, J.B.; Peng, T.; Li, H.; Zhao, L.; Zhang, Z.; Claverie, J.P.; Razzari, L.; et al. Enhancement of scattering and near field of TiO2-Au nanohybrids using a silver resonator for efficient plasmonic photocatalysis. ACS Appl. Mater. Interfaces 2021, 13, 34714–34723. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Biswas, S.; Ahmed, S.; Kalita, D.; Nath, P.; Choudhury, B. Plasmon activation versus plasmon quenching on the overall photocatalytic performance of Ag/Au bimetal decorated g-C3N4 nanosheets under selective photoexcitation: A mechanistic understanding with experiment and theory. Appl. Catal. B Environ. 2021, 298, 120614. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, H.; Lee, W.; Schebarchov, D.; Wy, Y.; Grand, J.; Auguié, B.; Wi, D.H.; Cortés, E.; Han, S.W. Core-shell bimetallic nanoparticle trimers for efficient light-to-chemical energy conversion. ACS Energy Lett. 2020, 5, 3881–3890. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Zaccaria, R.P; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef]
- Mo, J.; Barbosa, E.C.; Wu, S.; Li, Y.; Sun, Y.; Xiang, W.; Li, T.; Pu, S.; Robertson, A.; Wu, T.S.; et al. Atomic-precision tailoring of Au-Ag core-shell composite nanoparticles for direct electrochemical-plasmonic hydrogen evolution in water splitting. Adv. Funct. Mater. 2021, 31, 2102517. [Google Scholar] [CrossRef]
- Dutta, A.; Schürmann, R.; Kogikoski, S., Jr.; Mueller, N.S.; Reich, S.; Bald, I. Kinetics and mechanism of plasmon-driven dehalogenation reaction of brominated purine nucleobases on Ag and Au. ACS Catal. 2021, 11, 8370–8381. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, R.; Li, Z.; Zhao, J.; Huang, H.; Zhou, C.; Zhang, T. Photothermal-assisted photocatalytic nitrogen oxidation to nitric acid on palladium-decorated titanium oxide. Adv. Energy Mater. 2022, 12, 2103740. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, K.; Yang, Z.; Xing, Z.; Li, Z.; Pan, K.; Zhou, W. Plasma Cu-decorated TiO2-x/CoP particle-level hierarchical heterojunctions with enhanced photocatalytic-photothermal performance. J. Hazard. Mater. 2021, 414, 125487. [Google Scholar] [CrossRef]
- Guo, M.; Xing, Z.; Zhao, T.; Qiu, Y.; Tao, B.; Li, Z.; Zhou, W. Hollow flower-like polyhedral α-Fe2O3/Defective MoS2/Ag Z-scheme heterojunctions with enhanced photocatalytic-Fenton performance via surface plasmon resonance and photothermal effects. Appl. Catal. B Environ. 2020, 272, 118978. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, C.L.; Wang, B.; Chen, C.; Huang, Y.C.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of dopants and defects in graphitic carbon nitride with exceptionally modulated band structures for efficient photocatalytic oxygen evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Xing, Z.; Fang, B.; Cui, Y.; Li, Z.; Zhou, W. Plasmon Ag/Na-doped defective graphite carbon nitride/NiFe layered double hydroxides Z-scheme heterojunctions toward optimized photothermal-photocatalytic-Fenton performance. Appl. Catal. B Environ. 2022, 304, 120969. [Google Scholar] [CrossRef]
- Shin, J.; Eo, J.S.; Jeon, T.; Lee, T.; Wang, G. Advances of Various Heterogeneous Structure Types in Molecular Junction Systems and Their Charge Transport Properties. Adv. Sci. 2022, 9, 2202399. [Google Scholar] [CrossRef]
- She, X.J.; Wu, J.J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Yang, C.; et al. High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-scheme catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Mandal, S.; Nanavati, S.P.; Willock, D.J.; Ananthakrishnan, R. Band gap engineering of amine functionalized Ag(I)-based coordination polymers and their plasmonic Ag0 coupled novel visible light driven photo-redox system for selective oxidation of benzyl alcohol. Appl. Catal. B Environ. 2022, 303, 120821. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Li, Q.; Feng, Y.N.; Chen, F.F.; Yu, Y. Spatial distribution of ZnIn2S4 nanosheets on g-C3N4 microtubes promotes photocatalytic CO2 reduction. Chem. Eng. J. 2021, 418, 129476. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, C.; Tang, D.; Ding, L.; Zhang, Y.; Sheng, H.; Ji, H.; Song, W.; Ma, W.; Chen, C.; et al. α-Fe2O3 as a versatile and efficient oxygen atom transfer catalyst in combination with H2O as the oxygen source. Nat. Catal. 2021, 4, 684–691. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Han, D.; Liu, X.; Yu, X.; Xiong, J.; Li, Y.; Zhao, Z.; Liu, J.; Wei, Y. Activating well-defined α-Fe2O3 nanocatalysts by near-surface Mn atom functionality for auto-exhaust soot purification. Appl. Catal. B Environ. 2023, 321, 122077. [Google Scholar] [CrossRef]
- Zhang, H.C.; Kang, Z.X.; Han, J.J.; Wang, P.; Fan, J.T.; Sheng, G.P. Photothermal Nanoconfinement Reactor: Boosting Chemical Reactivity with Locally High Temperature in a Confined Space. Angew. Chem. Int. Ed. 2022, 61, e202200093. [Google Scholar]
- Wang, K.; Xing, Z.; Meng, D.; Zhang, S.; Li, Z.; Pan, K.; Zhou, W. Hollow MoSe2@Bi2S3/CdS core-shell nanostructure as dual Z-scheme heterojunctions with enhanced full spectrum photocatalytic-photothermal performance. Appl. Catal. B Environ. 2021, 281, 119482. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Bontempi, E.; Zafeiratos, S.; Delgado Jaén, J.J.; Fornasiero, P. Synthesis and photocatalytic application of visible-light active β-Fe2O3/g-C3N4 hybrid nanocomposites. Appl. Catal. B Environ. 2016, 187, 171–180. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wei, X.; Alothman, Z.; Albaqami, M.; Malgras, V.; Yamauchi, Y.; Kang, Y.; Wang, M.; Guan, W.; et al. Direct Z-scheme CuInS2/Bi2MoO6 heterostructure for enhanced photocatalytic degradation of tetracycline under visible light. J. Hazard. Mater. 2021, 415, 125591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, H.; Liu, H.; Niu, C.; Huang, D.; Yang, Y.; Liang, C.; Li, L.; Li, J. Construction of dual S-scheme Ag2CO3/Bi4O5I2/g-C3N4 heterostructure photocatalyst with enhanced visible-light photocatalytic degradation for tetracycline. Chem. Eng. J. 2022, 438, 135471. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Yin, B.; Li, D.; Zhang, Z.; Mao, B.; Fan, W.; Gu, W.; Shi, W. Promoting visible-light-induced photocatalytic degradation of tetracycline by an efficient and stable beta-Bi2O3@g-C3N4 core/shell nanocomposite. Chem. Eng. J. 2018, 338, 137–146. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2-x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Y.; Zhu, Z.; Zhang, H.; Yin, D. Novel recyclable Z-scheme g-C3N4/carbon nanotubes/Bi25FeO40 heterostructure with enhanced visible-light photocatalytic performance towards tetracycline degradation. Chem. Eng. J. 2022, 429, 132130. [Google Scholar] [CrossRef]
- Zhao, T.; Xing, Z.; Xiu, Z.; Li, Z.; Yang, S.; Zhou, W. Oxygen-doped MoS2 nanospheres/CdS quantum dots/g-C3N4 nanosheets super-architectures for prolonged charge lifetime and enhanced visible-light-driven photocatalytic performance. ACS Appl. Mater. Interfaces 2019, 11, 7104–7111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, Y.; Yan, D.; He, C.; Li, Y.; Ren, X.; Zhang, P.; Mi, H. Construction of k+ ion gradient in crystalline carbon nitride to accelerate exciton dissociation and charge separation for visible light H2 production. ACS Catal. 2021, 11, 6995–7005. [Google Scholar] [CrossRef]
- Yavuz, C.; Erten-Ela, S. Solar light-responsive α-Fe2O3/CdS/g-C3N4 ternary photocatalyst for photocatalytic hydrogen production and photodegradation of methylene blue. J. Alloys Compd. 2022, 908, 164584. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. Synthesis of α-Fe2O3/g-C3N4 photocatalyst for high-efficiency water splitting under full light. Mater. Des. 2020, 196, 109191. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Wu, C.; Zhang, H.; Sun, J.; Li, Q. Delaminating Ti3C2 MXene by blossom of ZnIn2S4 microflowers for noble-metal-free photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 120, 89–98. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, Y.; Yu, T.; Wang, J. Synthesis of g-C3N4/WO3-carbon microsphere composites for photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 10261–10276. [Google Scholar] [CrossRef]
- Pan, J.; Dong, Z.; Wang, B.; Jiang, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The enhancement of photocatalytic hydrogen production via Ti3+ self-doping black TiO2/g-C3N4 hollow core-shell nano-heterojunction. Appl. Catal. B Environ. 2019, 242, 92–99. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Yao, B.; Wang, Z.; Chen, T.; Xiao, X.; Wang, Y. Plasma Ag-Modified α-Fe2O3/g-C3N4 Self-Assembled S-Scheme Heterojunctions with Enhanced Photothermal-Photocatalytic-Fenton Performances. Nanomaterials 2022, 12, 4212. https://doi.org/10.3390/nano12234212
Xiao Y, Yao B, Wang Z, Chen T, Xiao X, Wang Y. Plasma Ag-Modified α-Fe2O3/g-C3N4 Self-Assembled S-Scheme Heterojunctions with Enhanced Photothermal-Photocatalytic-Fenton Performances. Nanomaterials. 2022; 12(23):4212. https://doi.org/10.3390/nano12234212
Chicago/Turabian StyleXiao, Yawei, Bo Yao, Zhezhe Wang, Ting Chen, Xuechun Xiao, and Yude Wang. 2022. "Plasma Ag-Modified α-Fe2O3/g-C3N4 Self-Assembled S-Scheme Heterojunctions with Enhanced Photothermal-Photocatalytic-Fenton Performances" Nanomaterials 12, no. 23: 4212. https://doi.org/10.3390/nano12234212




