Nonlinear Optical Properties of Zinc Oxide Nanoparticle Colloids Prepared by Pulsed Laser Ablation in Distilled Water
Abstract
:1. Introduction
2. Experimental Setup
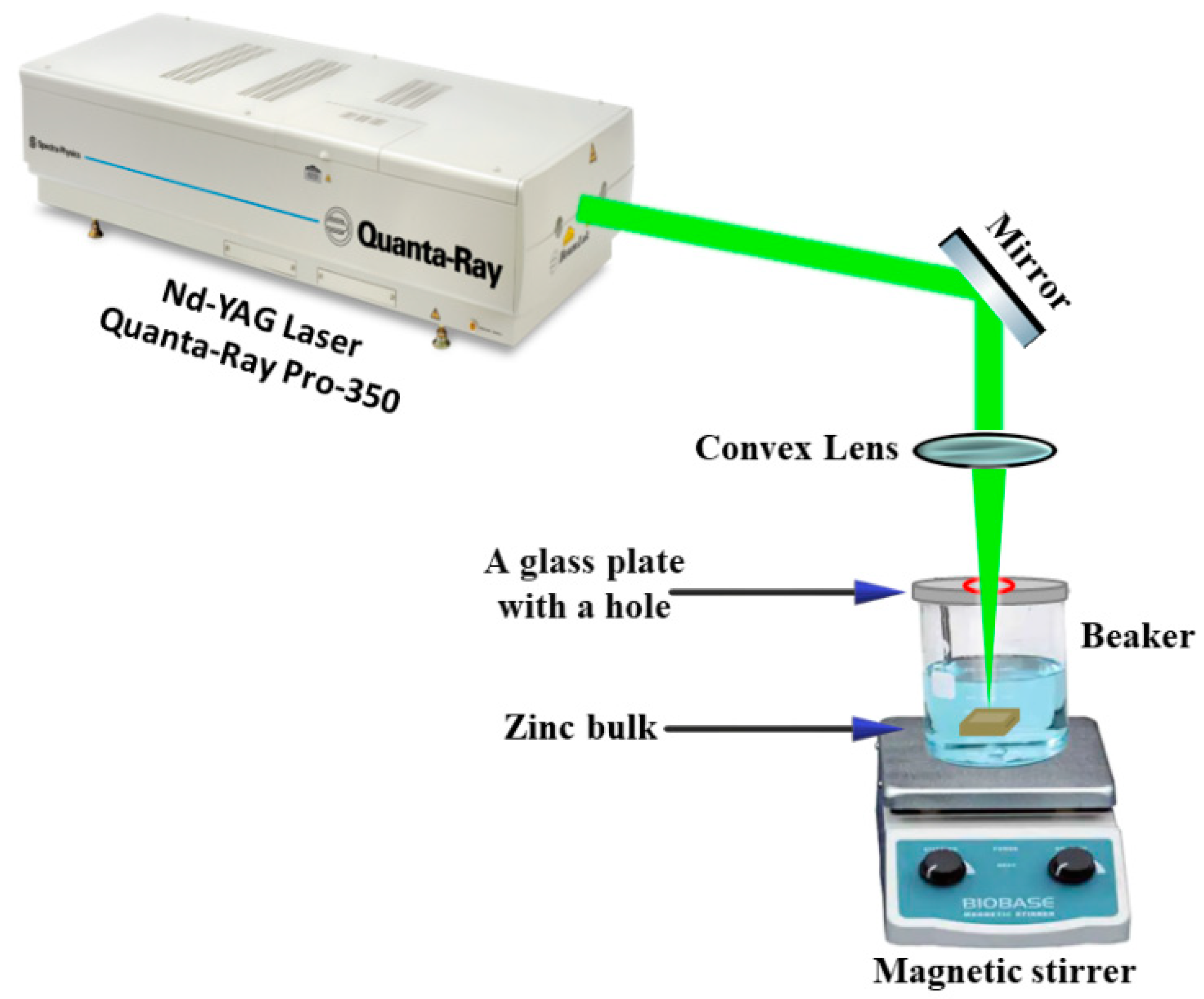
2.1. Sample Preparation
2.2. Z-Scan Setup
3. Results and Discussion
3.1. ZnONPs Colloids Analysis
3.2. OA Z-Scan Measurements of ZnONPs
3.3. CA Z-Scan Measurements of ZnONPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auston, D.H. Ultrafast Optoelectronics. In Ultrashort Laser Pulses and Applications; Kaiser, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 60, pp. 183–233. [Google Scholar] [CrossRef]
- Barbara, P.F.; Knox, W.H.; Mourou, G.A.; Zewail, A.H. Ultrafast Phenomena IX. In Proceedings of the 9th International Conference, Manchester, UK, 5–7 September 1994; Volume 60, ISBN 978-3-642-85178-0. [Google Scholar]
- Mukamel, S. Principles of Nonlinear Optical Spectroscopy; Oxford University Press on Demand: Oxford, UK, 1999; ISBN 0195092783. [Google Scholar]
- Shen, Y.R. A few selected applications of surface nonlinear optical spectroscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 12104–12111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Yin, L.; Agrawal, G.P.; Fauchet, P.M. Ultrafast optical switching based on nonlinear polarization rotation in silicon waveguides. Opt. Express 2010, 18, 11514–11523. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, S.; Geng, Y.; Wang, Y.; Li, X.; Wu, Y.; Dun, A. Nano-optical information storage induced by the nonlinear saturable absorption effect. Nanoscale 2011, 3, 3233–3237. [Google Scholar] [CrossRef]
- Adur, J.; Carvalho, H.F.; Cesar, C.L.; Casco, V.H. Nonlinear Microscopy Techniques: Principles and Biomedical Applications; Intech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef] [Green Version]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Chung, I.; Kanatzidis, M.G. Metal Chalcogenides: A Rich Source of Nonlinear Optical Materials. Chem. Mater. 2014, 26, 849–869. [Google Scholar] [CrossRef]
- Isaenko, L.I.; Yelisseyev, A.P. Recent studies of nonlinear chalcogenide crystals for the mid-IR. Semicond. Sci. Technol. 2016, 31, 123001. [Google Scholar] [CrossRef]
- Kowalevicz, A.M.; Sharma, V.; Ippen, E.P.; Fujimoto, J.G.; Minoshima, K. Three-dimensional photonic devices fabricated in glass by use of a femtosecond laser oscillator. Opt. Lett. 2005, 30, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Sandeep, C.S.S.; Philip, R.; Baesso, M.L. Study of optical properties and effective three-photon absorption in Bi-doped ZnO nanoparticles. J. Appl. Phys. 2009, 106, 114304. [Google Scholar] [CrossRef]
- Chopra, K.L.; Major, S.; Pandya, D.K. Transparent conductors—A status review. Thin Solid Film. 1983, 102, 1–46. [Google Scholar] [CrossRef]
- Hamberg, I.; Granqvist, C.G. Evaporated Sn-doped In2O3 films: Basic optical properties and applications to energy-efficient windows. J. Appl. Phys. 1986, 60, R123–R160. [Google Scholar] [CrossRef]
- Karaagac, H.; Yengel, E.; Islam, M.S. Physical properties and heterojunction device demonstration of aluminum-doped ZnO thin films synthesized at room ambient via sol–gel method. J. Alloy. Compd. 2012, 521, 155–162. [Google Scholar] [CrossRef]
- Li, C.; Shi, G.; Xu, H.; Guang, S.; Yin, R.; Song, Y. Nonlinear optical properties of the PbS nanorods synthesized via surfactant-assisted hydrolysis. Mater. Lett. 2007, 61, 1809–1811. [Google Scholar] [CrossRef]
- Zhong, X.; Xie, R.; Zhang, Y.; Basché, T.; Knoll, W. High-Quality Violet- to Red-Emitting ZnSe/CdSe Core/Shell Nanocrystals. Chem. Mater. 2005, 17, 4038–4042. [Google Scholar] [CrossRef]
- Mahmood, K.; Park, S.B. Atmospheric pressure based electrostatic spray deposition of transparent conductive ZnO and Al-doped ZnO (AZO) thin films: Effects of Al doping and annealing treatment. Electron. Mater. Lett. 2013, 9, 161–170. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Sivasubramanian, D.; Sreekanth, P.; Gandhiraj, V.; Philip, R.; Bhalerao, G.M. Nonlinear optical interactions of Co: ZnO nanoparticles in continuous and pulsed mode of operations. RSC Adv. 2015, 5, 80756–80765. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, Y.; Yang, D.; She, J.; Wang, Z. Improved piezoelectric performance of two-dimensional ZnO nanodisks-based flexible nanogengerators via ZnO/Spiro-MeOTAD PN junction. J. Mater. Sci. Mater. Electron. 2020, 31, 5584–5590. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-based solar photocatalysts for the efficient decontamination of persistent organic pollutants and hexavalent chromium in wastewater. Catalysts 2019, 9, 974. [Google Scholar] [CrossRef]
- Kaushik, V.; Rajput, S.; Kumar, M. Broadband optical modulation in a zinc-oxide-based heterojunction via optical lifting. Opt. Lett. 2020, 45, 363. [Google Scholar] [CrossRef]
- Torres-Torres, C.; García-Cruz, M.; Castañeda, L.; Rojo, R.R.; Tamayo-Rivera, L.; Maldonado, A.; Avendaño-Alejo, M.; Torres-Martínez, R. Photoconductivity, photoluminescence and optical Kerr nonlinear effects in zinc oxide films containing chromium nanoclusters. J. Lumin. 2012, 132, 1083–1088. [Google Scholar] [CrossRef]
- Torres-Torres, C.; Uc, B.A.C.; Rangel-Rojo, R.; Castañeda, L.; Torres-Martínez, R.; Gil, C.G.; Khomenko, A.V. Optical Kerr phase shift in a nanostructured nickel-doped zinc oxide thin solid film. Opt. Express 2013, 21, 21357–21364. [Google Scholar] [CrossRef] [PubMed]
- Kachynski, A.V.; Kuzmin, A.N.; Nyk, M.; Roy, I.; Prasad, P.N. Zinc Oxide Nanocrystals for Nonresonant Nonlinear Optical Microscopy in Biology and Medicine. J. Phys. Chem. C 2008, 112, 10721–10724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.W.; Barille, R.; Nunzi, J.M.; Tam, K.H.; Leung, Y.H.; Chan, W.K.; Djurišić, A. Second harmonic generation in zinc oxide nanorods. Appl. Phys. B 2006, 84, 351–355. [Google Scholar] [CrossRef]
- Johnson, J.C.; Yan, H.; Schaller, R.D.; Petersen, P.B.; Yang, A.P.; Saykally, R.J. Near-Field Imaging of Nonlinear Optical Mixing in Single Zinc Oxide Nanowires. Nano Lett. 2002, 2, 279–283. [Google Scholar] [CrossRef]
- Jurablu, S.; Farahmandjou, M.; Firoozabadi, T.P. Sol-Gel Synthesis of Zinc Oxide (ZnO) Nanoparticles: Study of Structural and Optical Properties. J. Sci. Islam. Repub. Iran 2015, 26, 281–285. [Google Scholar] [CrossRef]
- Ko, T.S.; Yang, S.; Hsu, H.C.; Chu, C.P.; Lin, H.F.; Liao, S.C.; Wang, S.C. ZnO nanopowders fabricated by dc thermal plasma synthesis. Mater. Sci. Eng. B 2006, 134, 54–58. [Google Scholar] [CrossRef]
- Aneesh, P.M.; Vanaja, K.A.; Jayaraj, M.K. Synthesis of ZnO nanoparticles by hydrothermal method. In Nanophotonic materials IV; SPIE: Bellingham, WA, USA, 2007; Volume 6639, pp. 47–55. [Google Scholar] [CrossRef]
- Huang, H.; Lai, J.; Lu, J.; Li, Z. Pulsed laser ablation of bulk target and particle products in liquid for nanomaterial fabrication. AIP Adv. 2019, 9, 015307. [Google Scholar] [CrossRef]
- Tri, P.N.; Ouellet-Plamondon, C.; Rtimi, S.; Assadi, A.A.; Nguyen, T.A. Methods for synthesis of hybrid nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Woodhead Publishing: Sawston, UK, 2019; pp. 51–63. [Google Scholar]
- Singh, S.; Gopal, R. Synthesis of colloidal zinc oxide nanoparticles by pulsed laser ablation in aqueous media. Phys. E Low-Dimens. Syst. Nanostructures 2008, 40, 724–730. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and processes of pulsed laser ablation in liquids during nanoparticle production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Tsuji, T.; Iryo, K.; Nishimura, Y.; Tsuji, M. Preparation of metal colloids by a laser ablation technique in solution: Influence of laser wavelength on the ablation efficiency (II). J. Photochem. Photobiol. A Chem. 2001, 145, 201–207. [Google Scholar] [CrossRef]
- Batani, D.; Vinci, T.; Bleiner, D. Laser-ablation and induced nanoparticle synthesis. Laser Part. Beams 2014, 32, 1–7. [Google Scholar] [CrossRef] [Green Version]
- de Araújo, C.B.; Gomes, A.S.L.; Boudebs, G. Techniques for nonlinear optical characterization of materials: A review. Rep. Prog. Phys. 2016, 79, 036401. [Google Scholar] [CrossRef] [Green Version]
- Antoine, R.; Bonačić-Koutecký, V. Measurement Techniques of Optical Nonlinearities—Two-Photon Absorption/Fluorescence and Hyper-Rayleigh Scattering. In Liganded Silver and Gold Quantum Clusters. Towards a New Class of Nonlinear Optical Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–62. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.; Wei, T.-H.; Hagan, D.; Van Stryland, E. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Gómez, S.L.; Cuppo, F.L.S.; Neto, A.M.F.; Kosa, T.; Muramatsu, M.; Horowicz, R.J. Z-scan measurement of the nonlinear refractive indices of micellar lyotropic liquid crystals with and without the ferrofluid doping. Phys. Rev. E 1999, 59, 3059–3063. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Hutchings, D.; Hagan, D.; Van Stryland, E. Dispersion of bound electron nonlinear refraction in solids. IEEE J. Quantum Electron. 1991, 27, 1296–1309. [Google Scholar] [CrossRef]
- DeSalvo, R.; Said, A.A.; Hagan, D.J.; Van Stryland, E.W.; Sheik-Bahae, M. Infrared to ultraviolet measurements of two-photon absorption and n2 in wide bandgap solids. IEEE J. Quantum Electron. 1996, 32, 1324–1333. [Google Scholar] [CrossRef]
- Bindra, K.; Oak, S.; Rustagi, K. Intensity dependence of Z-scan in semiconductor-doped glasses for separation of third and fifth order contributions in the below band gap region. Opt. Commun. 1999, 168, 219–225. [Google Scholar] [CrossRef]
- Nagaraja, K.; Pramodini, S.; Kumar, A.S.; Nagaraja, H.; Poornesh, P.; Kekuda, D. Third-order nonlinear optical properties of Mn doped ZnO thin films under cw laser illumination. Opt. Mater. 2013, 35, 431–439. [Google Scholar] [CrossRef]
- Sony, T.; Zaker, T.A.; Zakar, A.T.; Mohammed, H.N. Nonlinear Optical Properties of ZnO Thin Film at Low Laser Intensity Using Z-Scan Technique. Rafidain J. Sci. 2021, 30, 32–38. [Google Scholar] [CrossRef]
- Kepceoğlu, A.; Gezgin, S.Y.; Gündoğdu, Y.; Küçükçelebi, H.; Kılıç, H. Nonlinear Optical Properties of Zinc Oxide Thin Films Produced by Pulsed Laser Deposition. Mater. Today: Proc. 2019, 18, 1819–1825. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zuo, Y.; Xu, F.; Zuo, D. Z-scan measurements of nonlinear refraction and absorption for aluminum-doped zinc oxide thin film. Appl. Opt. 2019, 58, 6112–6117. [Google Scholar] [CrossRef]
- Wen, X.; Han, Y.; Yao, C.; Zhang, K.; Li, J.; Sun, W.; Wu, J.D. The photoluminescence, ultrafast nonlinear optical properties and carrier dynamics of 1D In-doped ZnO nanostructures: Experiment and mechanism. Opt. Mater. 2018, 77, 67–76. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Lee, S.; Koh, K.; Park, J.-Y.; Kim, K.; Rotermund, F. Ultrafast third-order optical nonlinearities of vertically-aligned ZnO nanorods. Chem. Phys. Lett. 2007, 447, 86–90. [Google Scholar] [CrossRef]
- Raied, K.J.; Mohammed, T.H.; Abdulla, M. Three-Photon Absorption in ZnO Film Using Ultra Short Pulse Laser. J. Mod. Phys. 2012, 3, 856–864. [Google Scholar]
- Chang, Q.; Zhang, D.; Gao, M.; Zhen, S. Effects of Laser Pulse Width and Solvent on the Optical Nonlinearities of ZnO Nanoparticles. In Machinery, Materials Science and Energy Engineering (ICMMSEE), Proceedings of the 3rd International Conference, Wuhan, China, 18 –19 July 2015; World Scientific Publishing Company: Singapore; pp. 512–520.
- Kavitha, M.K.; Haripadmam, P.; Gopinath, P.; Krishnan, B.; John, H. Effect of morphology and solvent on two-photon absorption of nano zinc oxide. Mater. Res. Bull. 2013, 48, 1967–1971. [Google Scholar] [CrossRef]
- Ahmed, H.; Mahmoud, A.; Mobarak, M.; El-Salam, H.M.A.; Mohamed, T. Using femtosecond laser pulses to study the nonlinear optical properties of rhodamine 6G dissolved in water. J. Mol. Liq. 2021, 340, 117199. [Google Scholar] [CrossRef]
- Farrukh, M.A. (Ed.) Atomic Absorption Spectroscopy; ACS Publications: Washington, DC, USA, 2012; ISBN 978-953-307-817-5. [Google Scholar]
- Coulter, J.B.; Birnie, D.P., III. Assessing Tauc plot slope quantification: ZnO thin films as a model system. Phys. Status Solidi B 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Barcikowski, S.; Devesa, F.; Moldenhauer, K. Impact and structure of literature on nanoparticle generation by laser ablation in liquids. J. Nanoparticle Res. 2009, 11, 1883–1893. [Google Scholar] [CrossRef]
- Van Overschelde, O.; Guisbiers, G. Photo-fragmentation of selenium powder by Excimer laser ablation in liquids. Opt. Laser Technol. 2015, 73, 156–161. [Google Scholar] [CrossRef]
- Mamdouh, S.; Mahmoud, A.; Samir, A.; Mobarak, M.; Mohamed, T. Using femtosecond laser pulses to investigate the nonlinear optical properties of silver nanoparticles colloids in distilled water synthesized by laser ablation. Phys. B Condens. Matter 2022, 631, 413727. [Google Scholar] [CrossRef]
- Ashour, M.; GFaris, H.; Ahmed, H.; Mamdouh, S.; Thambiratnam, K.; Mohamed, T. Using Femtosecond Laser Pulses to Explore the Nonlinear Optical Properties of Au NP Colloids That Were Synthesized by Laser Ablation. Nanomaterials 2022, 12, 2980. [Google Scholar] [CrossRef]
- Aghdam, H.D.; Azadi, H.; Esmaeilzadeh, M.; Bellah, S.M.; Malekfar, R. Ablation time and laser fluence impacts on the composition, morphology and optical properties of copper oxide nanoparticles. Opt. Mater. 2019, 91, 433–438. [Google Scholar] [CrossRef]
- Rao, A.S.; Sethuraman, G.; Ghosh, O.S.N.; Sharan, A.; Viswanath, A.K. Third order nonlinear study of ZnO nano particles under femto-second laser illumination. arXiv 2018, arXiv:1806.09404. [Google Scholar]
- Saad, N.A.; Dar, M.H.; Ramya, E.; Naraharisetty, S.R.G.; Rao, D.N. Saturable and reverse saturable absorption of a Cu2O–Ag nanoheterostructure. J. Mater. Sci. 2019, 54, 188–199. [Google Scholar] [CrossRef]
- Falconieri, M. Thermo-optical effects in Z -scan measurements using high-repetition-rate lasers. J. Opt. A: Pure Appl. Opt. 1999, 1, 662–667. [Google Scholar] [CrossRef]
- Shehata, A.; Mohamed, T. Method for an accurate measurement of nonlinear refractive index in the case of high-repetition-rate femtosecond laser pulses. JOSA B 2019, 36, 1246–1251. [Google Scholar] [CrossRef]
- Kwak, C.H.; Lee, Y.L.; Kim, S.G. Analysis of asymmetric Z-scan measurement for large optical nonlinearities in an amorphous As2S3 thin film. JOSA B 1999, 16, 600–604. [Google Scholar] [CrossRef]
- Severiano-Carrillo, I.; Alvarado-Méndez, E.; Trejo-Durán, M.; Méndez-Otero, M. Improved Z-scan adjustment to thermal nonlinearities by including nonlinear absorption. Opt. Commun. 2017, 397, 140–146. [Google Scholar] [CrossRef]
- Sheik-Bahae, M. Relation between N2 and two-photon absorption. In Laser-Induced Damage in Optical Materials 1989; SPIE: Bellingham, WA, USA, 1990; Volume 1438, pp. 613–619. [Google Scholar]
- Sheik-Bahae, M.; Hagan, D.J.; Said, A.A.; Young, J.; Wei, T.-H.; Van Stryland, E.W. Kramers-Kronig relation between n2 and two-photon absorption . In Proceedings of the Electro-Optical Materials for Switches, Coatings, Sensor Optics, and Detectors, Orlando, FL, USA, 16–20 April 1990; pp. 395–400. [Google Scholar] [CrossRef]
- Walden, S.L.; Fernando, J.F.S.; Shortell, M.P.; Waclawik, E.R.; Jaatinen, E.A. Nonlinear Absorption and Fluorescence in ZnO and ZnO–Au Nanostructures. Adv. Opt. Mater. 2016, 4, 2133–2138. [Google Scholar] [CrossRef] [Green Version]
- Abrinaei, F.; Molahasani, N. Effects of Mn doping on the structural, linear, and nonlinear optical properties of ZnO nanoparticles. J. Opt. Soc. Am. B 2018, 35, 2015–2022. [Google Scholar] [CrossRef]
- Solati, E.; Dorranian, D. Nonlinear optical properties of the mixture of ZnO nanoparticles and graphene nanosheets. Appl. Phys. A 2016, 122, 76. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, H.; Rui, G.; Lv, C.; He, J.; Cui, Y.; Gu, B. Preparation of Ag@ZnO core–shell nanostructures by liquid-phase laser ablation and investigation of their femtosecond nonlinear optical properties. Appl. Phys. B 2020, 126, 1–9. [Google Scholar] [CrossRef]
- Rout, A.; Boltaev, G.S.; Ganeev, R.A.; Rao, K.S.; Fu, D.; Rakhimov, R.Y.; Guo, C. Low-and high-order nonlinear optical studies of ZnO nanocrystals, nanoparticles, and nanorods. Eur. Phys. J. D 2019, 73, 1–8. [Google Scholar] [CrossRef]














| Laser Parameters. | ZnONPs Avg. Size (nm) | n2 | β | Ref. | ||
|---|---|---|---|---|---|---|
| Pulse Duration | ||||||
| 532 | 5 ns | 10 | 100 | ---- | [71] | |
| 532 | 10 ns | 200 | 41 | [72] | ||
| 532 | 7ns | 5 | 29.5 | [73] | ||
| 800 | 170 fs | 1000 | 65 | ---- | [74] | |
| 400 800 | 40 fs | 1000 | 80 40 | [75] | ||
| 800 | 100 fs | 19.5 16.2 | Current work | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, T.; Farhan, A.; Ahmed, H.; Ashour, M.; Mamdouh, S.; Schuch, R. Nonlinear Optical Properties of Zinc Oxide Nanoparticle Colloids Prepared by Pulsed Laser Ablation in Distilled Water. Nanomaterials 2022, 12, 4220. https://doi.org/10.3390/nano12234220
Mohamed T, Farhan A, Ahmed H, Ashour M, Mamdouh S, Schuch R. Nonlinear Optical Properties of Zinc Oxide Nanoparticle Colloids Prepared by Pulsed Laser Ablation in Distilled Water. Nanomaterials. 2022; 12(23):4220. https://doi.org/10.3390/nano12234220
Chicago/Turabian StyleMohamed, Tarek, Ali Farhan, Hanan Ahmed, Mohamed Ashour, Samar Mamdouh, and Reinhold Schuch. 2022. "Nonlinear Optical Properties of Zinc Oxide Nanoparticle Colloids Prepared by Pulsed Laser Ablation in Distilled Water" Nanomaterials 12, no. 23: 4220. https://doi.org/10.3390/nano12234220








