Molecular Doping of CVD-Graphene Surfaces by Perfluoroalkyl-Substituted Perylene Diimides Derivatives
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Morphological Analysis
3.2. Photoelectron Spectroscopies
3.2.1. PDI8-CN2 Film Growth
3.2.2. PDIF-CN2 Film Growth
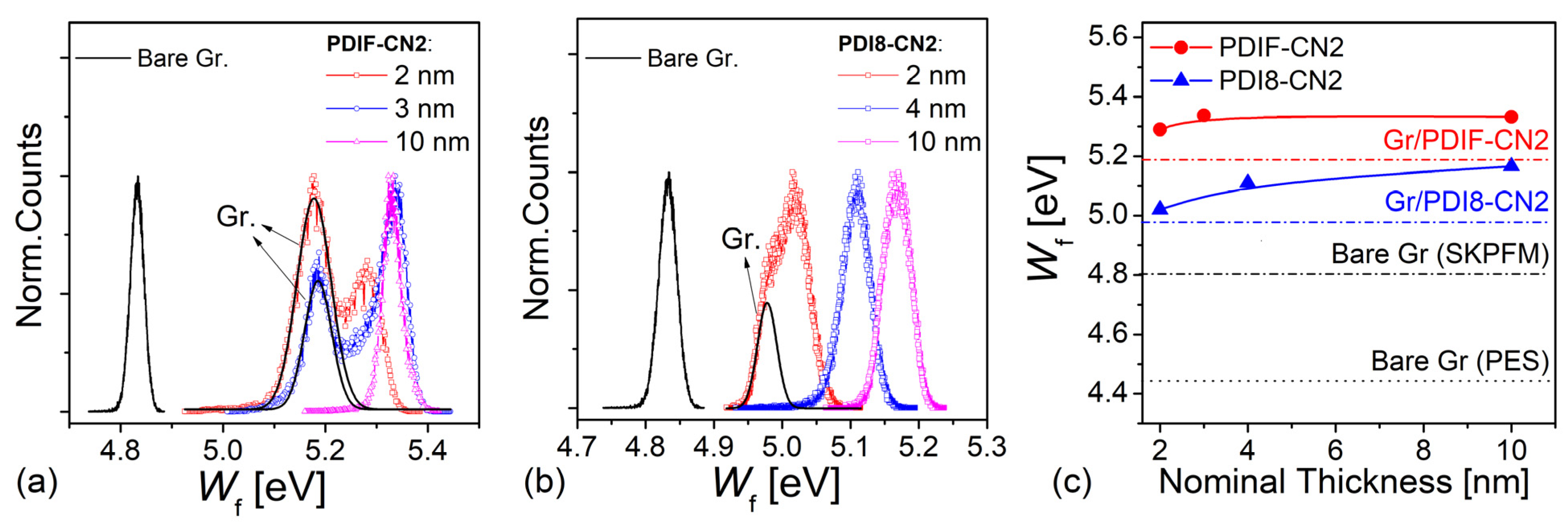
3.3. UPS
3.4. SKPFM Analysis
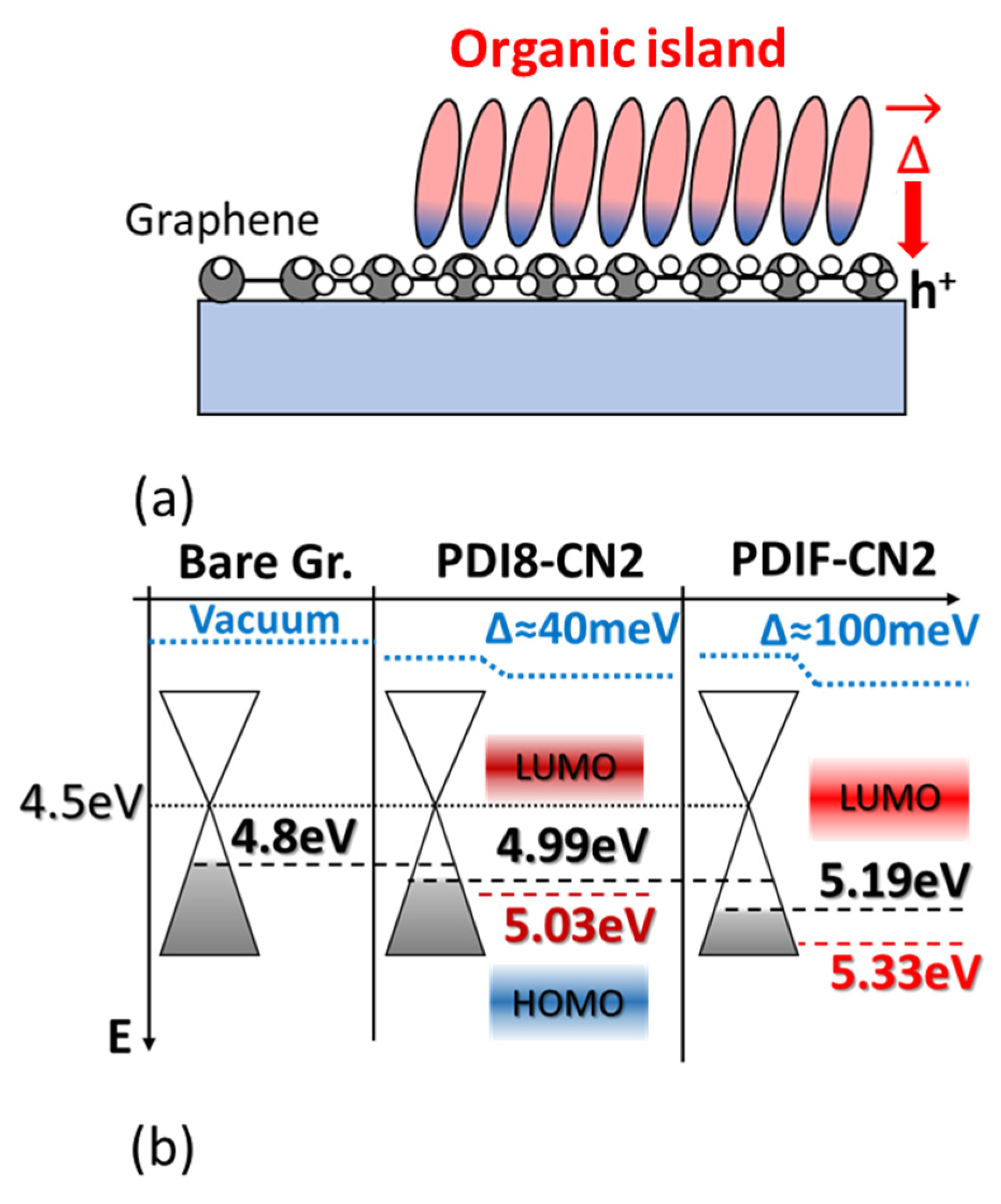
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Z.; Sun, Z.; Wu, T.; Gao, Y.; Li, H.; Li, F.; Niu, L. An advanced lithium ion battery based on a high quality graphitic graphene anode and a Li[Ni0.6Co0.2Mn0.2]O2 cathode. Electrochim. Acta 2018, 259, 48–55. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Jo, G.; Choe, M.; Lee, S.; Park, W.; Kahng, Y.H.; Lee, T. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer grapheme. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; De, S. Are there fundamental limitations on the sheet resistance and transmittance of thin graphene films? ACS Nano 2010, 4, 2713–2720. [Google Scholar]
- Pang, S.; Hernandez, Y.; Feng, X.; Müllen, K. Graphene as transparent electrode material for organic electronics. Adv. Mater. 2011, 23, 2779–2795. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.; Sim, S.H.; Lim, S.; Kim, K.S.; Hong, B.H.; Cho, K. Surface-directed molecular assembly of pentacene on monolayer graphene for high-performance organic transistors. J. Am. Chem. Soc. 2011, 133, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Chianese, F.; Chiarella, F.; Barra, M.; Candini, A.; Affronte, M.; Cassinese, A. Suppression of the morphology mismatch at graphene/n-type organic semiconductor interfaces: A scanning Kelvin probe force microscopy investigation. J. Mater. Chem. C 2020, 8, 8145–8154. [Google Scholar] [CrossRef]
- Chianese, F.; Candini, A.; Affronte, M.; Mishra, N.; Coletti, C.; Cassinese, A. Linear conduction in N-type organic field effect transistors with nanometric channel lengths and graphene as electrodes. Appl. Phys. Lett. 2018, 112, 213301. [Google Scholar] [CrossRef]
- Chianese, F.; Candini, A.; Lumetti, S.; Mishra, N.; Coletti, C.; Affronte, M.; Cassinese, A. Evaluating the use of graphene electrodes in sub-micrometric, high-frequency n-type organic transistors. Synth. Met. 2021, 273, 116683. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the graphene work function by electric field effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef]
- Parui, S.; Ribeiro, M.; Atxabal, A.; Bairagi, K.; Zuccatti, E.; Safeer, C.K.; Llopis, R.; Casanova, F.; Hueso, L.E. Gate-tunable graphene-organic interface barrier for vertical transistor and logic inverter. Appl. Phys. Lett. 2018, 113, 153301. [Google Scholar] [CrossRef]
- Parui, S.; Pietrobon, L.; Ciudad, D.; Vélez, S.; Sun, X.; Casanova, F.; Stoliar, P.; Hueso, L.E. Gate-controlled energy barrier at a graphene/molecular semiconductor junction. Adv. Funct. Mater. 2015, 25, 2972–2979. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Pumera, M. Will Any Crap We Put into Graphene Increase Its Electrocatalytic Effect? ACS Nano 2020, 14, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Jang, C.; Adam, S.; Fuhrer, M.S.; Williams, E.D.; Ishigami, M. Charged-impurity scattering in graphene. Nat. Phys. 2008, 4, 377–381. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of n-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, J.; Koch, R.J.; Zhao, W.; Höfert, O.; Gotterbarm, K.; Mammadov, S.; Papp, C.; Görling, A.; Steinrück, H.P.; Seyller, T. Growth and electronic structure of boron-doped graphene. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 87, 155437. [Google Scholar] [CrossRef]
- Criado, A.; Melchionna, M.; Marchesan, S.; Prato, M. The Covalent Functionalization of Graphene on Substrates. Angew. Chem. Int. Ed. 2015, 54, 10734–10750. [Google Scholar] [CrossRef]
- Dong, X.; Fu, D.; Fang, W.; Shi, Y.; Chen, P.; Li, L.-J. Doping Single-Layer Graphene with Aromatic Molecules. Small 2009, 5, 1422–1426. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.-B.; Xie, W.; Du, J. Quantitative analysis of graphene doping by organic molecular charge transfer. J. Phys. Chem. C 2011, 115, 7596–7602. [Google Scholar] [CrossRef]
- Zhang, F.; Klein, C.; Longhi, E.; Barlow, S.; Marder, S.R.; Sarusi, G.; Kahn, A. Molecular-Reductant-Induced Control of a Graphene-Organic Interface for Electron Injection. Chem. Mater. 2019, 31, 6624–6632. [Google Scholar] [CrossRef]
- Levesque, P.L.; Sabri, S.S.; Aguirre, C.M.; Guillemette, J.; Siaj, M.; Desjardins, P.; Szkopek, T.; Martel, R. Probing charge transfer at surfaces using graphene transistors. Nano Lett. 2011, 11, 132–137. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Dong, C.Q.; Xing, Y.G.; Wee, A.T.S. Surface transfer p-type doping of epitaxial graphene. J. Am. Chem. Soc. 2007, 129, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Lu, Y.H.; Chen, W.; Feng, Y.P.; Wee, A.T.S. Linear tuning of charge carriers in graphene by organic molecules and charge-transfer complexes. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 155403. [Google Scholar] [CrossRef]
- Coletti, C.; Riedl, C.; Lee, D.S.; Krauss, B.; Patthey, L.; Von Klitzing, K.; Smet, J.H.; Starke, U. Charge neutrality and band-gap tuning of epitaxial graphene on SiC by molecular doping. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 235401. [Google Scholar] [CrossRef]
- Barra, M.; Chiarella, F.; Chianese, F.; Vaglio, R.; Cassinese, A. Perylene-diimide molecules with cyano functionalization for electron-transporting transistors. Electronics 2019, 8, 249. [Google Scholar] [CrossRef]
- Tatti, R.; Aversa, L.; Verucchi, R.; Cavaliere, E.; Garberoglio, G.; Pugno, N.M.; Speranza, G.; Taioli, S. Synthesis of single layer graphene on Cu(111) by C60 supersonic molecular beam epitaxy. RSC Adv. 2016, 6, 37982–37993. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; O’Boyle, M.P.; Wickramasinghe, H.K. Kelvin probe force microscopy. Appl. Phys. Lett. 1991, 58, 2921–2923. [Google Scholar] [CrossRef]
- Salerno, M.; Dante, S. Scanning Kelvin probe microscopy: Challenges and perspectives towards increased application on biomaterials and biological samples. Materials 2018, 11, 951. [Google Scholar] [CrossRef]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Palermo, V.; Palma, M.; Samori, P. Electronic characterization of organic thin films by Kelvin probe force microscopy. Adv. Mater. 2006, 18, 145–164. [Google Scholar] [CrossRef]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar] [CrossRef]
- Liscio, F.; Milita, S.; Albonetti, C.; D’Angelo, P.; Guagliardi, A.; Masciocchi, N.; Della Valle, R.G.; Venuti, E.; Brillante, A.; Biscarini, F. Structure and morphology of PDI8-CN2 for n-type thin-film transistors. Adv. Funct. Mater. 2012, 22, 943–953. [Google Scholar] [CrossRef]
- Ciccullo, F.; Savu, S.A.; Gerbi, A.; Bauer, M.; Ovsyannikov, R.; Cassinese, A.; Chassé, T.; Casu, M.B. Chemisorption, morphology, and structure of a n-type perylene diimide derivative at the interface with gold: Influence on devices from thin films to single molecules. Chem.—A Eur. J. 2015, 21, 3766–3771. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wang, Q.; Ding, L. Self-assembling of cyano- and carboxyl-terminated monolayers using short-chain alkylsiloxane. Appl. Surf. Sci. 2009, 256, 1372–1376. [Google Scholar] [CrossRef]
- Salem, J.R.; Sequeda, F.O.; Duran, J.; Lee, W.Y.; Yang, R.M. Solventless polyimide films by vapor deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 369–374. [Google Scholar] [CrossRef]
- Buchwalter, L.P.; Silverman, B.D.; Witt, L.; Rossi, A.R. X-ray photoelectron spectroscopy analysis of hexafluorodianhydride–oxydianiline polyimide: Substantiation for substituent effects on aromatic carbon 1 s binding energies. J. Vac. Sci. Technol. A Vac. Surf. Film. 1987, 5, 226–230. [Google Scholar] [CrossRef]
- Nardi, M.; Verucchi, R.; Aversa, L.; Casarin, M.; Vittadini, A.; Mahne, N.; Giglia, A.; Nannarone, S.; Iannotta, S. Electronic properties of tetrakis(pentafluorophenyl)porphyrin. New J. Chem. 2013, 37, 1036–1045. [Google Scholar] [CrossRef]
- Huang, H.; Chen, S.; Gao, X.; Chen, W.; Wee, A.T.S. Structural and Electronic Properties of PTCDA Thin Films on Epitaxial Graphene. ACS Nano 2009, 3, 3431–3436. [Google Scholar] [CrossRef]
- Schöll, A.; Zou, Y.; Jung, M.; Schmidt, T.; Fink, R.; Umbach, E. Line shapes and satellites in high-resolution x-ray photoelectron spectra of large π-conjugated organic molecules. J. Chem. Phys. 2004, 121, 10260–10267. [Google Scholar] [CrossRef]
- Kassis, C.M.; Steehler, J.K.; Betts, D.E.; Guan, Z.; Romack, T.J.; DeSimone, J.M.; Linton, R.W. XPS studies of fluorinated acrylate polymers and block copolymers with polystyrene. Macromolecules 1996, 29, 3247–3254. [Google Scholar] [CrossRef]
- Longo, A.; Verucchi, R.; Aversa, L.; Tatti, R.; Ambrosio, A.; Orabona, E.; Coscia, U.; Carotenuto, G.; Maddalena, P. Graphene oxide prepared by graphene nanoplatelets and reduced by laser treatment. Nanotechnology 2017, 28, 224002. [Google Scholar] [CrossRef]
- Christodoulou, C.; Giannakopoulos, A.; Ligorio, G.; Oehzelt, M.; Timpel, M.; Niederhausen, J.; Pasquali, L.; Giglia, A.; Parvez, K.; Müllen, K.; et al. Tuning the Electronic Structure of Graphene by Molecular Dopants: Impact of the Substrate. ACS Appl. Mater. Interfaces 2015, 7, 19134–19144. [Google Scholar] [CrossRef] [PubMed]
- Frisenda, R.; Parlato, L.; Barra, M.; van der Zant, H.S.J.; Cassinese, A. Single-Molecule Break Junctions Based on a Perylene-Diimide Cyano-Functionalized (PDI8-CN2) Derivative. Nanoscale Res. Lett. 2015, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Aversa, L.; Verucchi, R.; Tatti, R.; Di Girolamo, F.V.; Barra, M.; Ciccullo, F.; Cassinese, A.; Iannotta, S. Surface doping in T6/PDI-8CN2 heterostructures investigated by transport and photoemission measurements. Appl. Phys. Lett. 2012, 101, 233504. [Google Scholar] [CrossRef]
- Stoeckel, M.; Olivier, Y.; Gobbi, M.; Dudenko, D.; Lemaur, V.; Zbiri, M.; Guilbert, A.A.Y.; D’Avino, G.; Liscio, F.; Migliori, A.; et al. Analysis of External and Internal Disorder to Understand Band-Like Transport in n-Type Organic Semiconductors. Adv. Mater. 2021, 33, e2007870. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, F.; Toccoli, T.; Barra, M.; Aversa, L.; Ciccullo, F.; Tatti, R.; Verucchi, R.; Iannotta, S.; Cassinese, A. High mobility n-type organic thin-film transistors deposited at room temperature by supersonic molecular beam deposition. Appl. Phys. Lett. 2014, 104, 143302. [Google Scholar] [CrossRef]
- Martin, J.; Akerman, N.; Ulbricht, G.; Lohmann, T.; Smet, J.H.; Von Klitzing, K.; Yacoby, A. Observation of electron-hole puddles in graphene using a scanning single-electron transistor. Nat. Phys. 2008, 4, 144–148. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
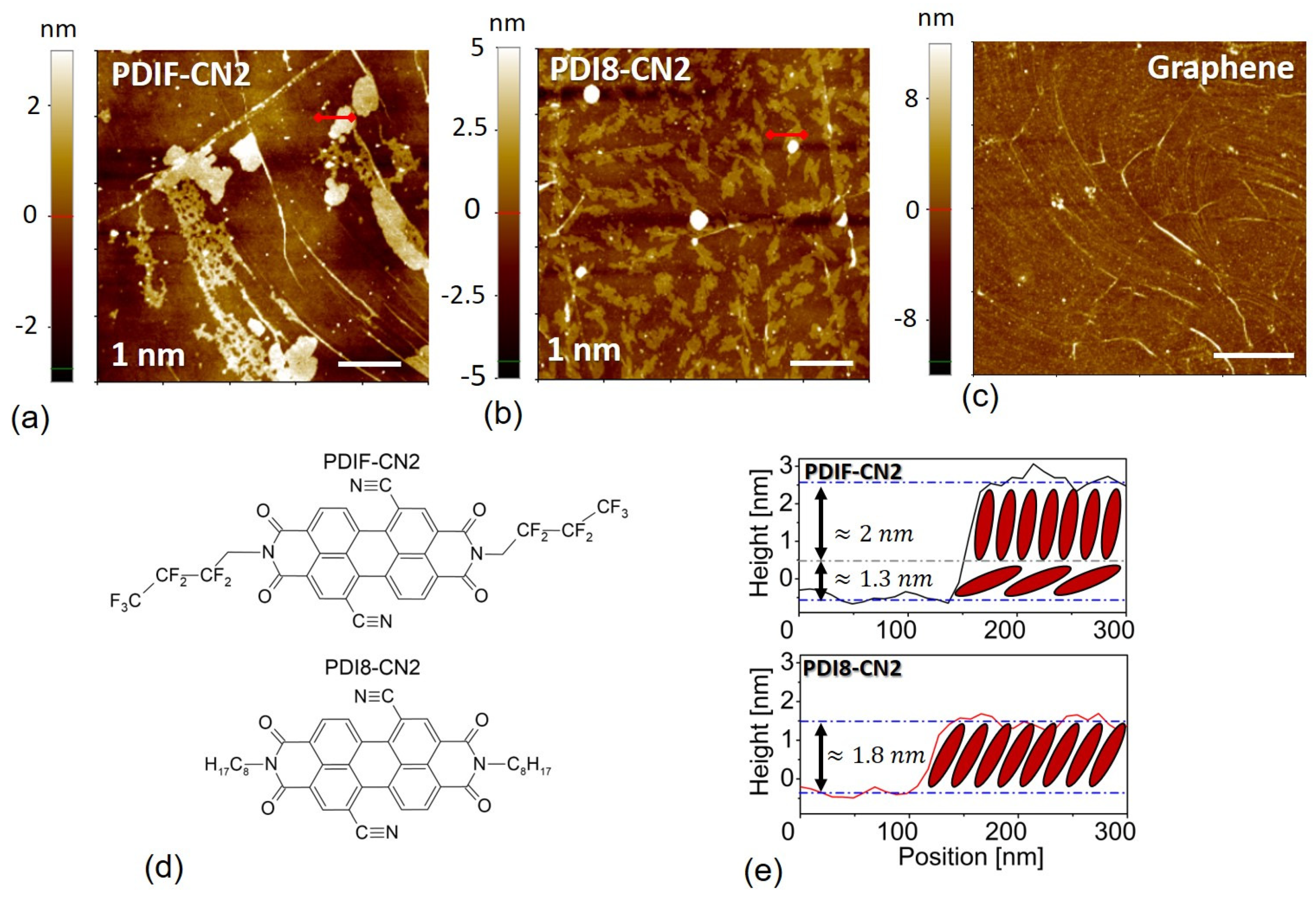

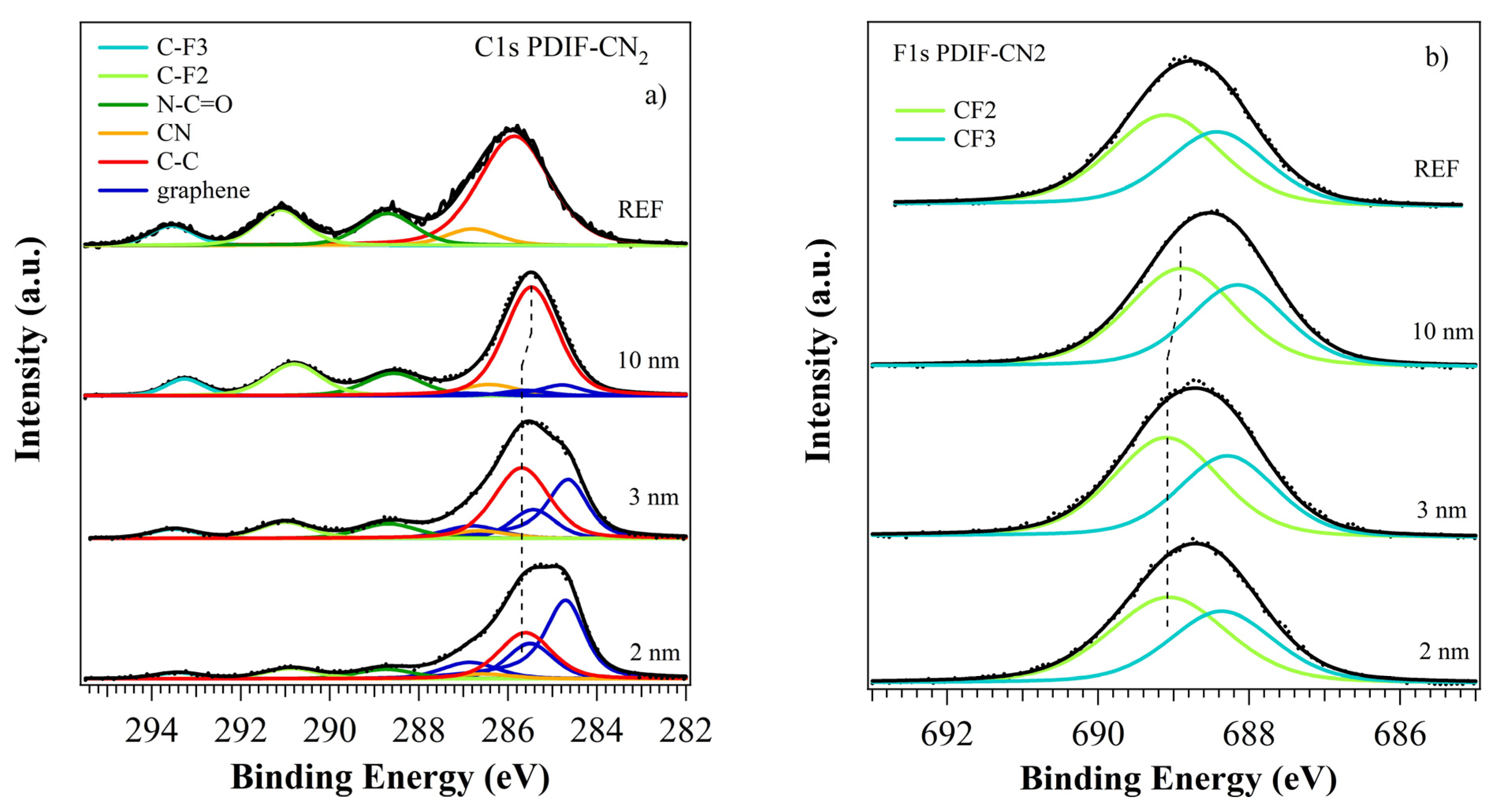
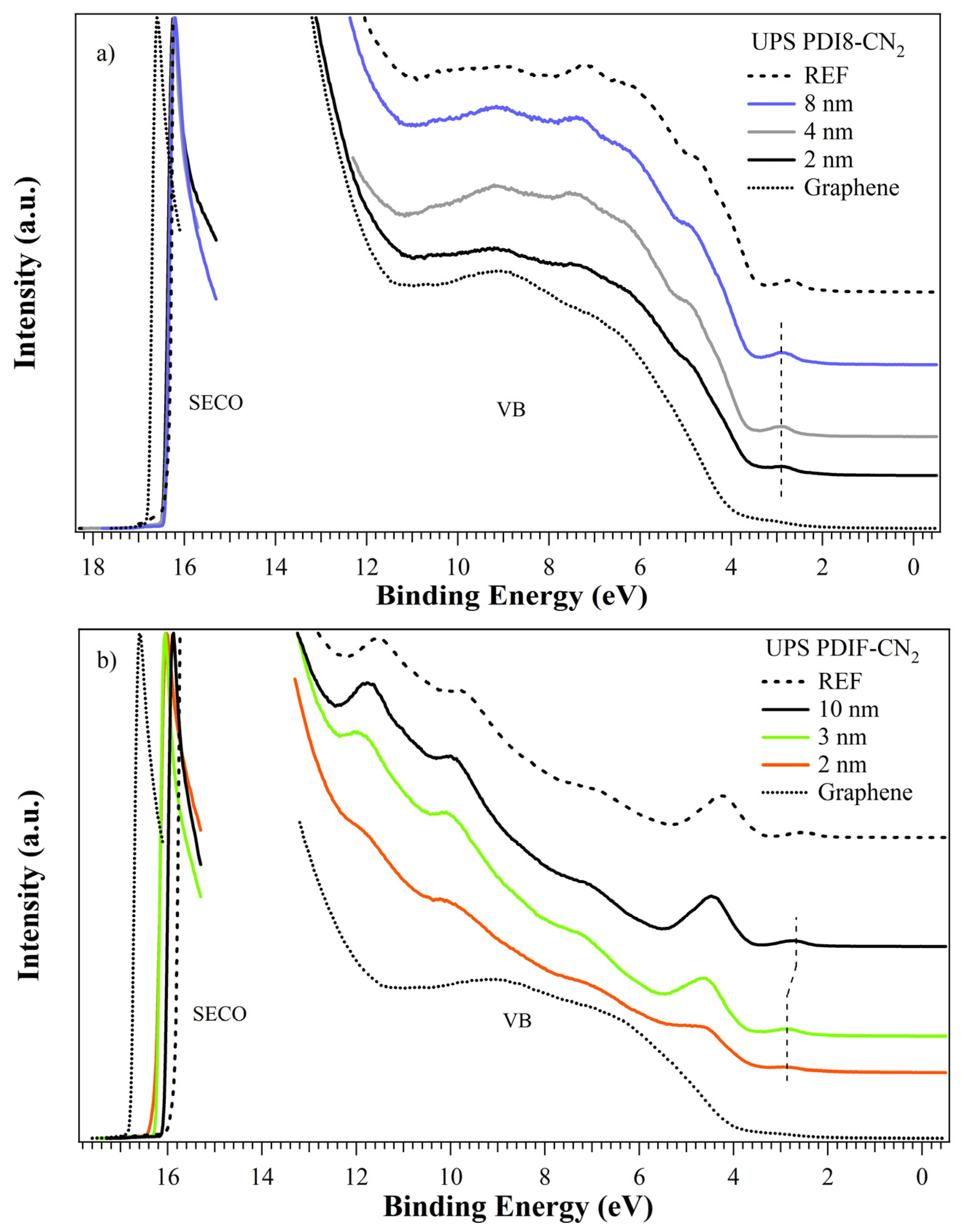
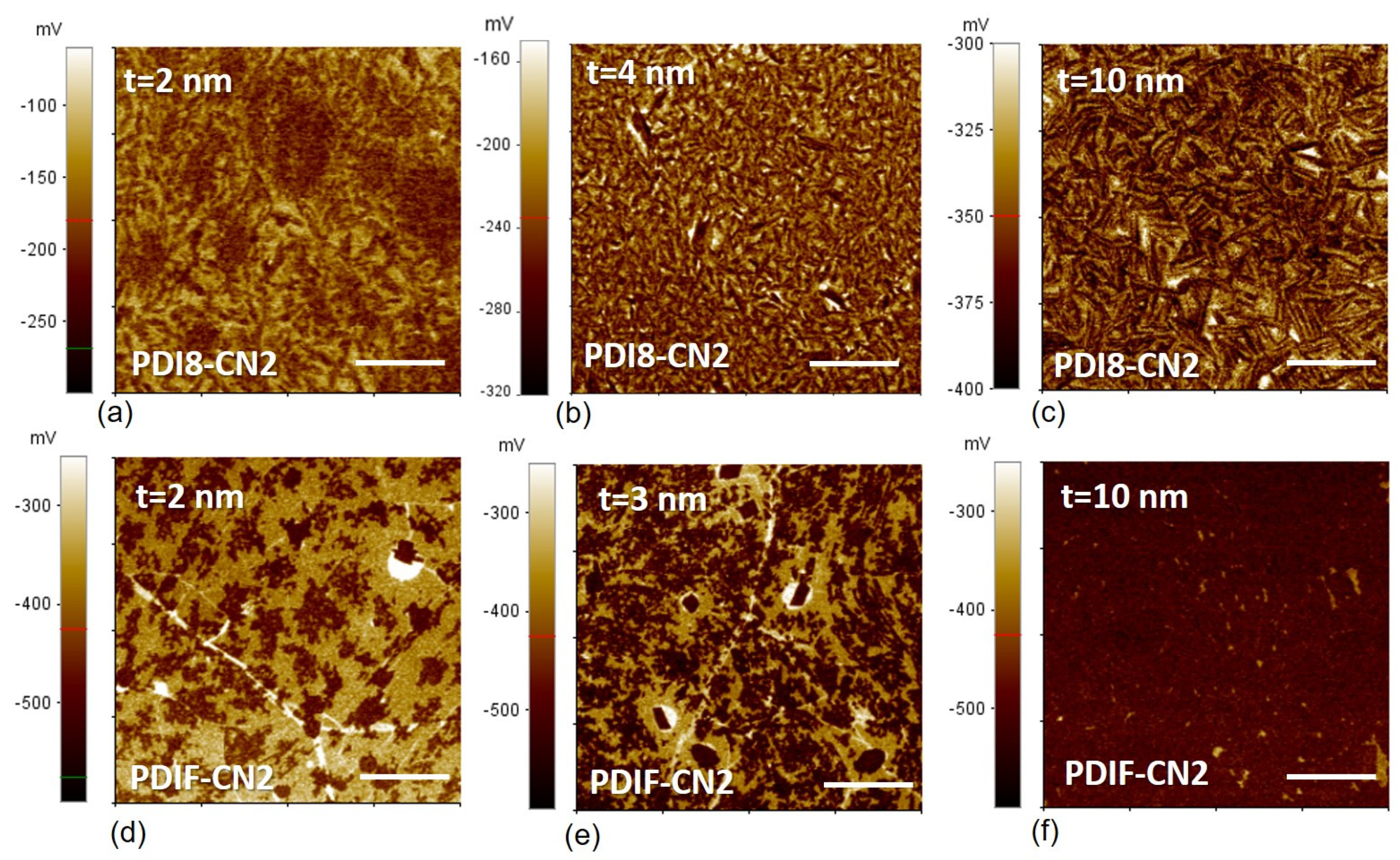
| PDI8-CN2 | PDIF-CN2 | |||||
|---|---|---|---|---|---|---|
| 2 nm | 4 nm | 8 nm | 2 nm | 3 nm | 10 nm | |
| Graphene on C1s total signal (%) | 59.8 | 49.9 | 21.1 | 54.3 | 38.2 | 6.2 |
| Sample | IP (eV) | WF (eV) | HOMO (eV) |
|---|---|---|---|
| Graphene | - | 4.41 | - |
| PDI8-CN2 2 nm | 7.04 | 4.79 | 2.85 |
| PDI8-CN2 4 nm | 7.02 | 4.77 | 2.87 |
| PDI8-CN2 8 nm | 7.04 | 4.79 | 2.85 |
| PDI8-CN2 thick | 7.10 | 4.92 | 2.71 |
| PDIF-CN2 2 nm | 7.09 | 4.93 | 2.87 |
| PDIF-CN2 3 nm | 7.12 | 4.96 | 2.87 |
| PDIF-CN2 10 nm | 7.38 | 5.12 | 2.75 |
| PDIF-CN2 thick | 7.33 | 5.38 | 2.54 |
| Doping Type | WF Shift [eV] | Ref. | |
|---|---|---|---|
| PDIF-CN2 | p-type | ≈0.5 (PES)/0.7 (SKPFM) | This work |
| PDI8-CN2 | ≈0.4 (PES)/0.5 (SKPFM) | ||
| F4-TCNQ | p-type | ≈1, 1.4 | [31,33] |
| TCNQ | ≈0.2 | [33] | |
| 6P | p-type | - | [30] |
| [RuCp*Mes]2 | n-type | 1.8 | [29] |
| VOPc | n-type | - | [28] |
| Na-NH2 | n-type | - | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chianese, F.; Aversa, L.; Verucchi, R.; Cassinese, A. Molecular Doping of CVD-Graphene Surfaces by Perfluoroalkyl-Substituted Perylene Diimides Derivatives. Nanomaterials 2022, 12, 4239. https://doi.org/10.3390/nano12234239
Chianese F, Aversa L, Verucchi R, Cassinese A. Molecular Doping of CVD-Graphene Surfaces by Perfluoroalkyl-Substituted Perylene Diimides Derivatives. Nanomaterials. 2022; 12(23):4239. https://doi.org/10.3390/nano12234239
Chicago/Turabian StyleChianese, Federico, Lucrezia Aversa, Roberto Verucchi, and Antonio Cassinese. 2022. "Molecular Doping of CVD-Graphene Surfaces by Perfluoroalkyl-Substituted Perylene Diimides Derivatives" Nanomaterials 12, no. 23: 4239. https://doi.org/10.3390/nano12234239
APA StyleChianese, F., Aversa, L., Verucchi, R., & Cassinese, A. (2022). Molecular Doping of CVD-Graphene Surfaces by Perfluoroalkyl-Substituted Perylene Diimides Derivatives. Nanomaterials, 12(23), 4239. https://doi.org/10.3390/nano12234239








