Exploring the Effect of Selenidation Time on the Ni-Doped Cu2ZnSn(S,Se)4 Solar Cell
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
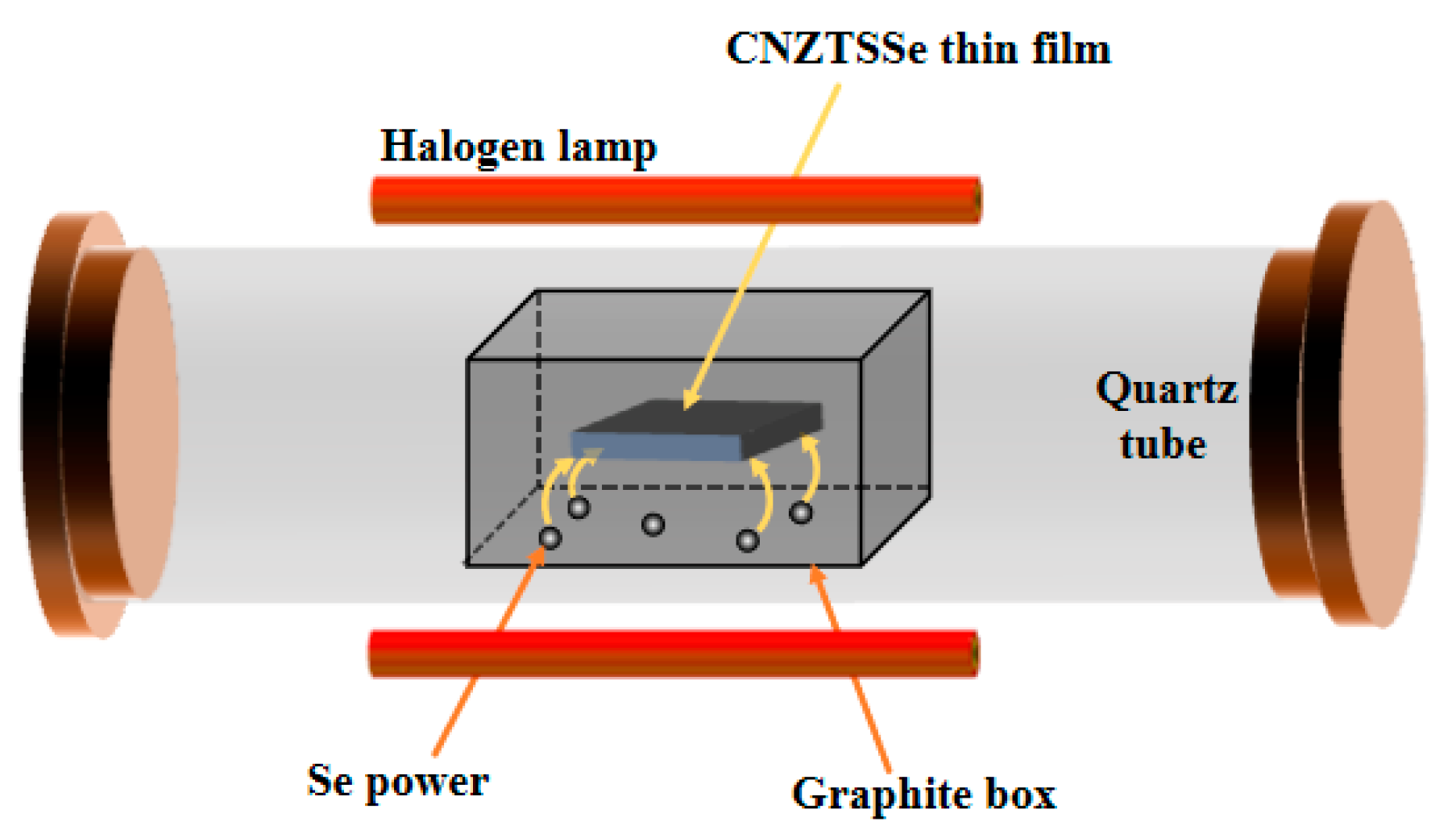
2.2. Fabrication
2.3. Characterization
3. Results and Discussion
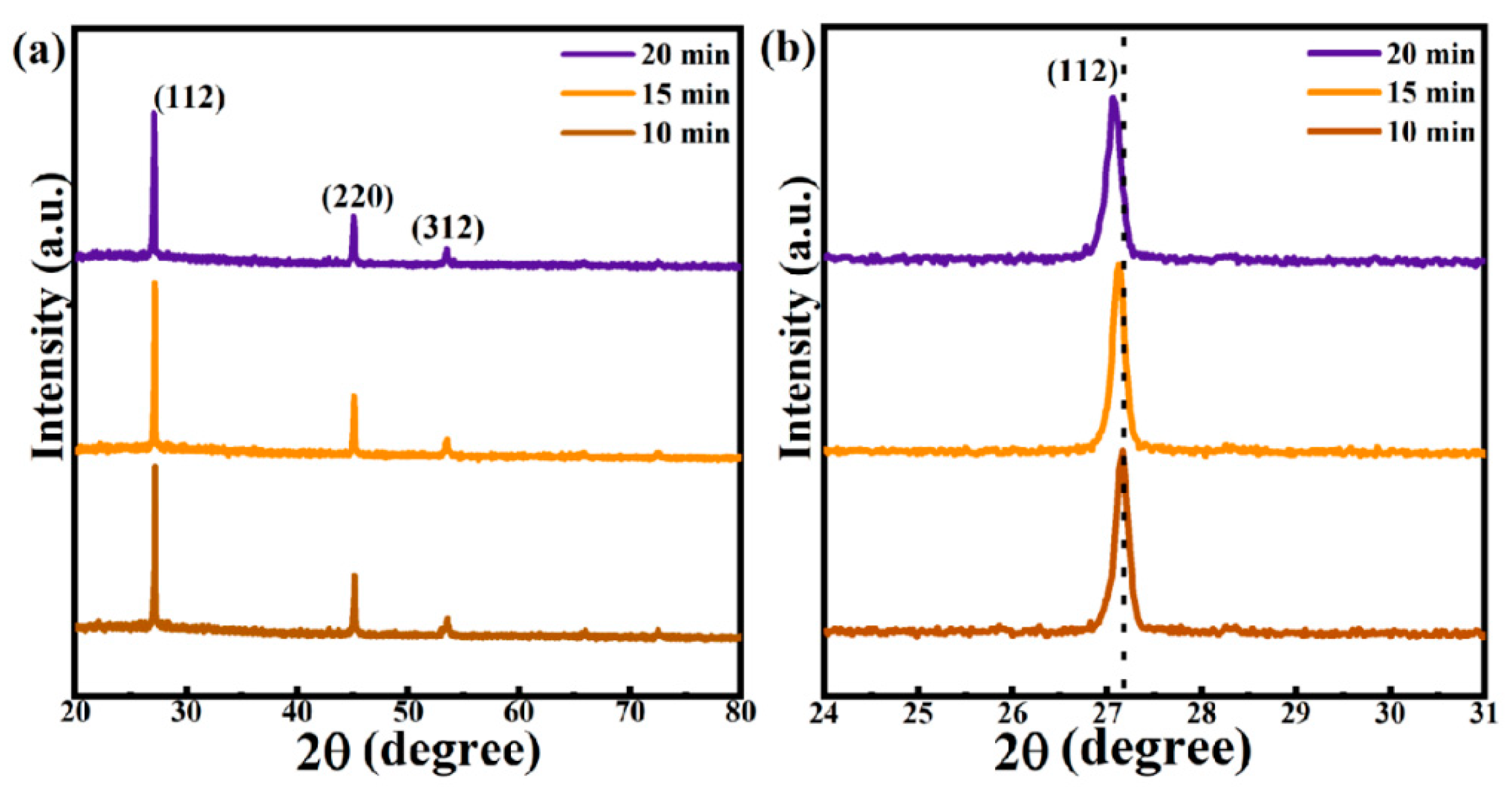
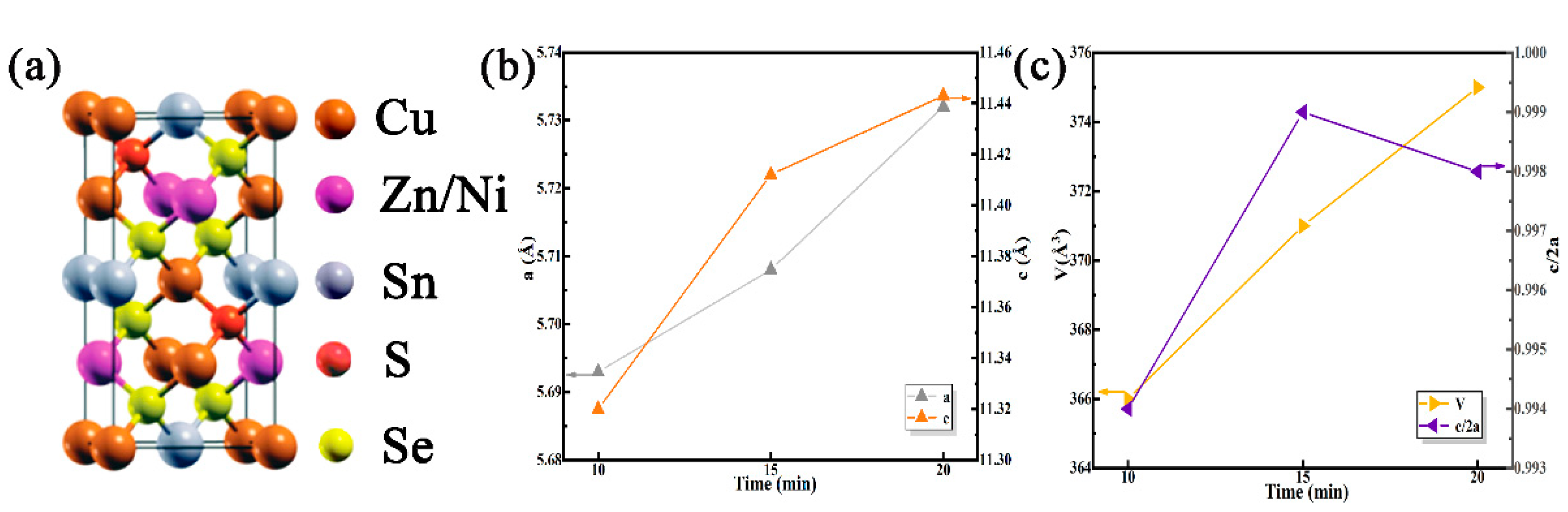
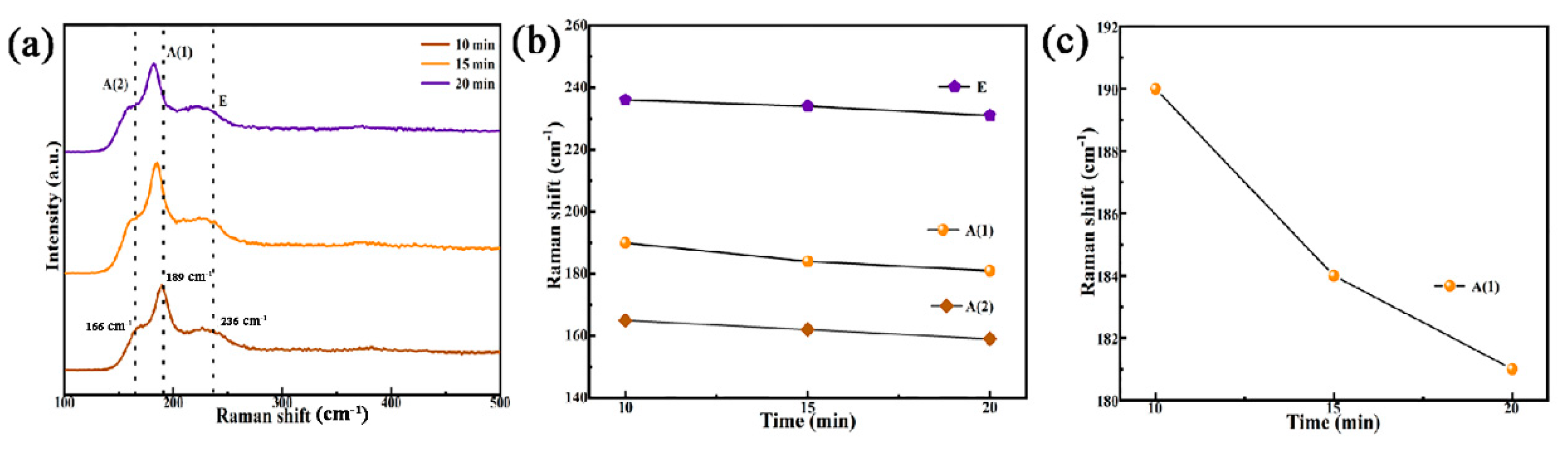
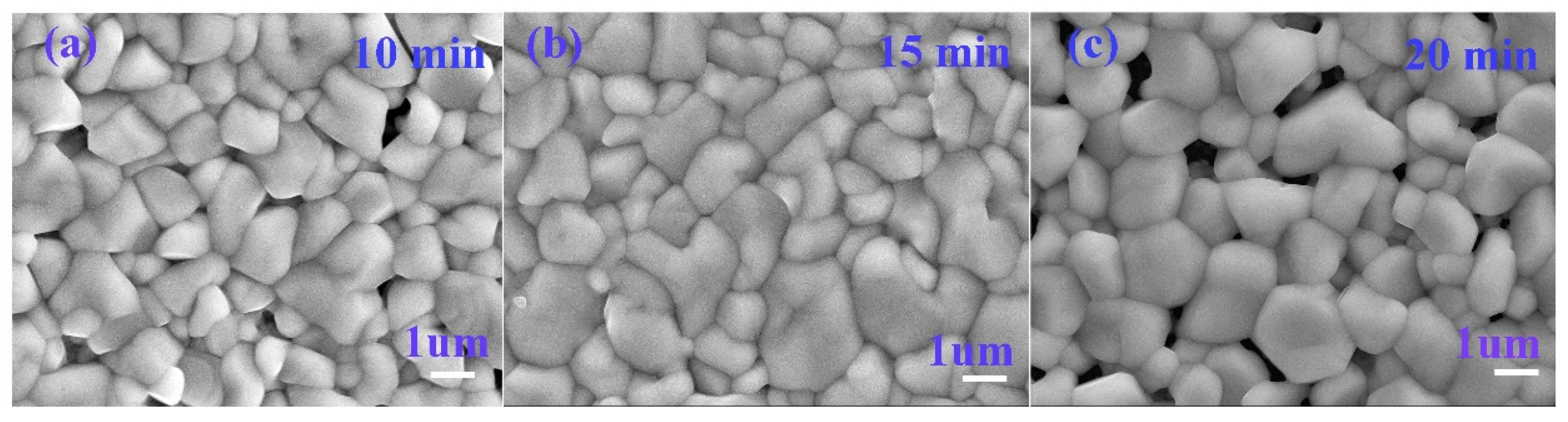
3.1. Effect of Selenidation Time on the Structure and Morphology of Thin Films
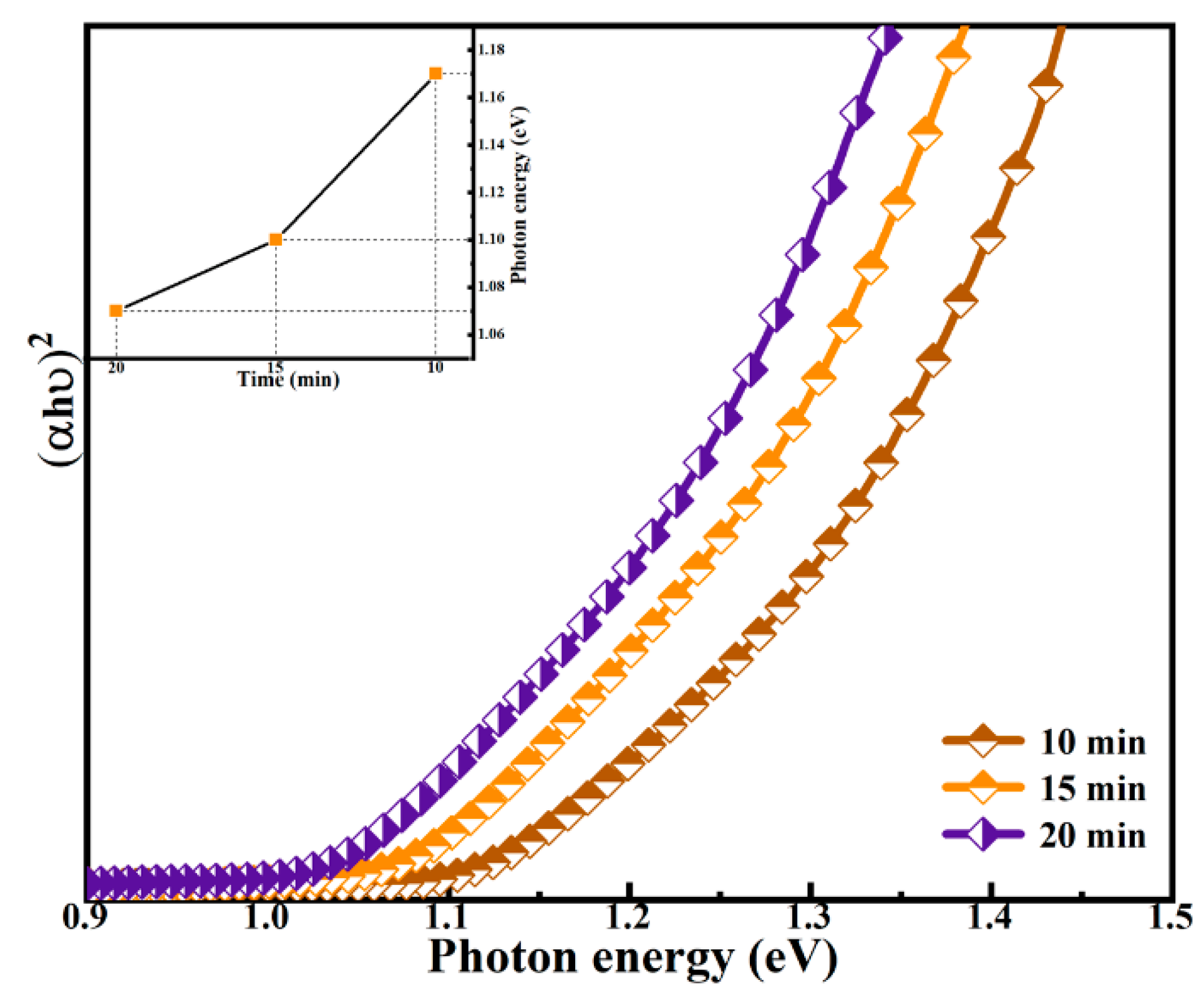
3.2. Effect of Selenidation Time on Optical and Electrical Properties of Thin Films
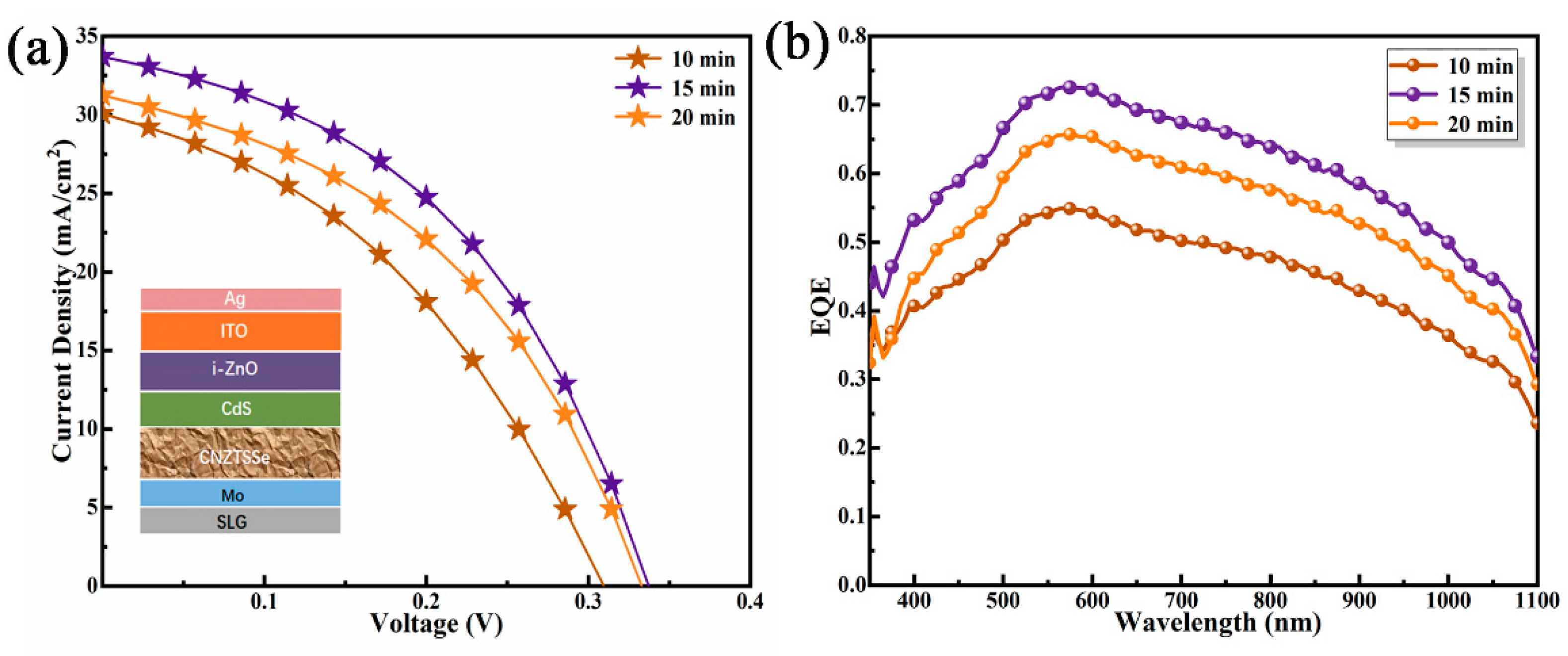
3.3. Effect of Selenidation Time on Device Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Su, Y.W.; Chang, C.H. Inkjet printed chalcopyrite CuInxGa1-xSe2 thin film solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 2616–2620. [Google Scholar] [CrossRef]
- Jackson, P.; Hariskos, D.; Wuerz, R.; Kiowski, O.; Bauer, A.; Friedlmeier, T.M.; Powalla, M. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Phys. Status Solidi (RRL) 2015, 9, 28–31. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Defect Engineering in Multinary Earth-Abundant Chalcogenide Photovoltaic Materials. J. Appl. Phys. 1961, 32, 510. [Google Scholar] [CrossRef]
- Shin, D.; Saparov, B.; Mitzi, D.B. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. Adv. Energy Mater. 2017, 7, 29. [Google Scholar]
- Fan, P.; He, Y.; Liang, G.; Xie, Z.; Yu, Z.; Lin, J.; Chen, S.; Zheng, Z.; Luo, J.; Su, Z. Enhancing Ag-alloyed Cu2ZnSnS4 solar cell performance by interfacial modification via In and Al. J. Mater. Chem. A 2021, 9, 25196–25207. [Google Scholar] [CrossRef]
- Bag, S.; Gunawan, O.; Gokmen, T.; Zhu, Y.; Todorov, T.K.; Mitzi, D.B. Low band gap liquid-processed CZTSe solar cell with 10.1% efficiency. Energy Environ. Sci. 2012, 5, 5. [Google Scholar] [CrossRef]
- Nitsche, R.; Sargent, D.F.; Wild, P. Crystal growth of quaternary 122464 chalcogenides by iodine vapor transport. J. Cryst. Growth 1967, 1, 52–53. [Google Scholar] [CrossRef]
- Ito, K.; Nakazawa, T. Electrical and Optical Properties of Stannite-Type Quaternary Semiconductor Thin Films. Jpn. J. Appl. Phys. 1988, 27, 2094–2097. [Google Scholar] [CrossRef]
- Son, D.H.; Kim, S.H.; Kim, S.Y.; Kim, Y.I.; Sim, J.H.; Park, S.N.; Kim, D.H. Effect of solid-H2S gas reactions on CZTSSe thin film growth and photovoltaic properties of a 12.62% efficiency device. J. Mater. Chem. A 2019, 7, 25279–25289. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.C.; Qiu, R.C.; Niu, C.Y.; Fu, J.J.; Jedlicka, E.; Giridharagopal, R.; Zhu, Q.; Zhou, Y.G.; Yan, W.B.; Yu, S.T.; et al. Ag Incorporation with Controlled Grain Growth Enables 12.5% Efficient Kesterite Solar Cell with Open Circuit Voltage Reached 64.2% Shockley-Queisser Limit. Adv. Funct. Mater. 2021, 31, 11. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamaguchi, K.; Kimoto, Y.; Yasaki, Y.; Kato, T.; Sugimoto, H. Cd-Free Cu(In,Ga)(Se,S)2 Thin-Film Solar Cell With Record Efficiency of 23.35 %. IEEE J. Photovolt. 2019, 9, 1863–1867. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X.J. Solar cell efficiency tables (version 57). Prog. Photovolt. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Chen, S.Y.; Walsh, A.; Gong, X.G.; Wei, S.H. Classification of Lattice Defects in the Kesterite Cu2ZnSnS4 and Cu2ZnSnSe4 Earth-Abundant Solar Cell Absorbers. Adv. Mater. 2013, 25, 1522–1539. [Google Scholar] [CrossRef]
- Han, D.; Sun, Y.Y.; Bang, J.; Zhang, Y.Y.; Sun, H.B.; Li, X.B.; Zhang, S.B. Deep electron traps and origin of p-type conductivity in the earth-abundant solar-cell material Cu2ZnSnS4. Phys. Rev. B 2013, 87, 155206. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.Z.; Xu, X.; Duan, B.W.; Wu, H.J.; Shi, J.J.; Luo, Y.H.; Li, D.M.; Meng, Q.B. Regulating crystal growth via organic lithium salt additive for efficient Kesterite solar cells. Nano Energy 2021, 89, 12. [Google Scholar] [CrossRef]
- Rey, G.; Redinger, A.; Ler, J.S.; Weiss, T.P.; Thevenin, M.; Guennou, M.; El Adib, B.; Siebentritt, S. The band gap of Cu2ZnSnSe4: Effect of order-disorder. Appl. Phys. Lett. 2014, 105, 4. [Google Scholar] [CrossRef]
- Scragg, J.J.S.; Choubrac, L.; Lafond, A.; Ericson, T.; Platzer-Bjorkman, C. A low-temperature order-disorder transition in Cu2ZnSnS4 thin films. Appl. Phys. Lett. 2014, 104, 4. [Google Scholar] [CrossRef] [Green Version]
- Lang, M.; Renz, T.; Opolka, A.; Zimmermann, C.; Krammer, C.; Neuwirth, M.; Kalt, H.; Hetterich, M. Impact of the degree of Cu-Zn order in Cu2ZnSn(S,Se)4 solar cell absorbers on defect states and band tails. Appl. Phys. Lett. 2018, 113, 5. [Google Scholar] [CrossRef]
- Cabas-Vidani, A.; Haass, S.G.; Andres, C.; Caballero, R.; Figurei, R.; Schreiner, C.; Marquez, J.A.; Hages, C.; Unold, T.; Bleiner, D.; et al. High-Efficiency (LixCu1-x)2ZnSn(S,Se)4 Kesterite Solar Cells with Lithium Alloying. Adv. Energy Mater. 2018, 8, 8. [Google Scholar] [CrossRef]
- Yuan, Z.K.; Chen, S.Y.; Xiang, H.J.; Gong, X.G.; Walsh, A.; Park, J.S.; Repins, I.; Wei, S.H. Engineering Solar Cell Absorbers by Exploring the Band Alignment and Defect Disparity: The Case of Cu- and Ag-Based Kesterite Compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Su, Z.H.; Tan, J.M.R.; Li, X.L.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation Substitution of Solution-Processed Cu2ZnSnS4 Thin Film Solar Cell with over 9% Efficiency. Adv. Energy Mater. 2015, 5, 7. [Google Scholar] [CrossRef]
- Zeng, F.; Sui, Y.; Ma, M.; Zhao, N.; Wang, T.; Yang, L.; Wang, Z.W.; Wang, F.; Yao, B. Strengthening the Properties of Earth-Abundant Cu2ZnSn(S,Se)4 Photovoltaic Materials via Cation Incorporation with Ni. ACS Appl. Energ. Mater. 2022, 5, 1271–1281. [Google Scholar] [CrossRef]
- Marquez-Prieto, J.; Yakushev, M.V.; Forbes, I.; Krustok, J.; Edwards, P.R.; Zhivulko, V.D.; Borodavchenko, O.M.; Mudryi, A.V.; Dimitrievska, M.; Izquerdo-Roca, V.; et al. Impact of the selenisation temperature on the structural and optical properties of CZTSe absorbers. Sol. Energy Mater. Sol. Cells 2016, 152, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Son, D.H.; Kim, Y.I.; Kim, S.H.; Nam, D.; Cheong, H.; Kang, J.K.; Yang, K.J.; Kim, D.H. Effects of S and Se contents on the physical and photovoltaic properties of Cu2ZnSn(Sx, Se1-x)4 thin films: Achieving a PCE of 9.47 %. J. Mater. Chem. A 2019, 7, 22986–22995. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Wu, M.; Liu, J.; Lai, F.; Zhu, C. Effect of post sulfurization temperature on the microstructure of Cu2ZnSn(S,Se)4 thin film. Mater. Lett. 2015, 159, 32–34. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Yao, B.; Li, Y.F.; Ding, Z.H.; Gao, Z.M.; Zhao, H.F.; Zhang, L.G.; Zhang, Z.Z.; Sui, Y.R.; Wang, G. Influencing Mechanism of the Selenization Temperature and Time on the Power Conversion Efficiency of Cu2ZnSn(S,Se)4-Based Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 17334–17342. [Google Scholar] [CrossRef]
- Huang, W.C.; Wei, S.Y.; Cai, C.H.; Ho, W.H.; Lai, C.H. The role of Ag in aqueous solution processed (Ag,Cu)2ZnSn(S,Se)4 kesterite solar cells: Antisite defect elimination and importance of Na passivation. J. Mater. Chem. A 2018, 6, 15170–15181. [Google Scholar] [CrossRef]
- Qin, H.M.; Li, L.S.; Guo, F.Q.; Su, S.J.; Peng, J.B.; Cao, Y.; Peng, X.B. Solution-processed bulk heterojunction solar cells based on a porphyrin small molecule with 7 % power conversion efficiency. Energy Environ. Sci. 2014, 7, 1397–1401. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Sun, X.D.; Zhang, P.H.; Yuan, X.; Huang, F.Q.; Zhang, W.Q. Structural properties and quasiparticle band structures of Cu-based quaternary semiconductors for photovoltaic applications. J. Appl. Phys. 2012, 111, 6. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, S.; Kannan, P.K.; Dey, S.R. Investigation of optimum annealing parameters for formation of dip coated Cu2ZnSnS4 thin film. Thin Solid Film. 2016, 612, 456–462. [Google Scholar] [CrossRef]
- Du, Y.C.; Wang, S.S.; Tian, Q.W.; Zhao, Y.C.; Chang, X.H.; Xiao, H.Q.; Deng, Y.Q.; Chen, S.Y.; Wu, S.X.; Liu, S.Z. Defect Engineering in Earth-Abundant Cu2ZnSn(S,Se)4 Photovoltaic Materials via Ga3+-Doping for over 12% Efficient Solar Cells. Adv. Funct. Mater. 2021, 31, 11. [Google Scholar] [CrossRef]
- Su, R.; Quan, Y.; Yang, S.; Hu, M.; Yang, J.; Gao, M. Destroying the symmetric structure to promote phase transition: Improving the SERS performance and catalytic activity of MoS2 nanoflowers. J. Alloys Compd. 2021, 886, 161268. [Google Scholar] [CrossRef]
- Llanos, J.; Buljan, A.; Mujica, C.; Ramirez, R. Electron transfer and electronic structure of KCuFeS2. J. Alloys Compd. 1996, 234, 40–42. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Shin, S.W.; Agawane, G.L.; Suryawanshi, M.P.; Gurav, K.V.; Patil, P.S.; Kim, J.H. Effect of post-annealing atmosphere on the grain-size and surface morphological properties of pulsed laser deposited CZTS thin films. Ceram. Int. 2014, 40, 15097–15103. [Google Scholar] [CrossRef]
- Qi, Y.F.; Liu, Y.; Kou, D.X.; Zhou, W.H.; Zhou, Z.J.; Tian, Q.W.; Yuan, S.J.; Meng, Y.N.; Wu, S.X. Enhancing Grain Growth for Efficient Solution-Processed (Cu,Ag)2ZnSn(S,Se)4 Solar Cells Based on Acetate Precursor. ACS Appl. Mater. Interfaces 2020, 12, 14213–14223. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, W.H.; Guo, J.; Zhou, Y.L.; Hou, Z.L.; Jiao, J.; Zhou, Z.J.; Du, Z.L.; Wu, S.X. Synthesis of Pure Metastable Wurtzite CZTS Nanocrystals by Facile One-Pot Method. J. Phys. Chem. C 2012, 116, 26507–26516. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, G.; Meng, R.T.; Sun, Y.L.; Wang, S.Y.; Zhang, S.L.; Wu, J.Y.; Wu, L.; Liang, G.X.; Li, H.; et al. An efficient Li+-doping strategy to optimize the band alignment of a Cu2ZnSn(S,Se)4/CdS interface by a Se & LiF co-selenization process. J. Mater. Chem. A 2020, 8, 22065–22074. [Google Scholar]
- Thompson, M.J.; Blakeney, K.J.; Cady, S.D.; Reichert, M.D.; Del Pilar-Albaladejo, J.; White, S.T.; Vela, J. Cu2ZnSnS4 Nanorods Doped with Tetrahedral, High Spin Transition Metal Ions: Mn2+, Co2+, and Ni2+. Chem. Mater. 2016, 28, 1668–1677. [Google Scholar] [CrossRef] [Green Version]
- Rondiya, S.; Wadnerkar, N.; Jadhav, Y.; Jadkar, S.; Haram, S.; Kabir, M. Structural, Electronic, and Optical Properties of Cu2NiSnS4: A Combined Experimental and Theoretical Study toward Photovoltaic Applications. Chem. Mater. 2017, 29, 3133–3142. [Google Scholar] [CrossRef]
- Gershon, T.; Lee, Y.S.; Antunez, P.; Mankad, R.; Singh, S.; Bishop, D.; Gunawan, O.; Hopstaken, M.; Haight, R. Photovoltaic Materials and Devices Based on the Alloyed Kesterite Absorber (AgxCu1-x)2ZnSnSe4. Adv. Energy Mater. 2016, 6, 1502468. [Google Scholar] [CrossRef]
- Khalate, S.A.; Kate, R.S.; Kim, J.H.; Pawar, S.M.; Deokate, R.J. Effect of deposition temperature on the properties of Cu2ZnSnS4 (CZTS) thin films. Superlattices Microstruct. 2017, 103, 335–342. [Google Scholar] [CrossRef]
- Walsh, A.; Chen, S.Y.; Wei, S.H.; Gong, X.G. Kesterite Thin-Film Solar Cells: Advances in Materials Modelling of Cu2ZnSnS4. Adv. Energy Mater. 2012, 2, 400–409. [Google Scholar] [CrossRef]
- Emrani, A.; Vasekar, P.; Westgate, C.R. Effects of sulfurization temperature on CZTS thin film solar cell performances. Sol. Energy 2013, 98, 335–340. [Google Scholar] [CrossRef]
- Katagiri, H.; Saitoh, K.; Washio, T.; Shinohara, H.; Kurumadani, T.; Miyajima, S. Development of thin film solar cell based on Cu2ZnSnS4 thin films. Sol. Energy Mater. Sol. Cells 2001, 65, 141–148. [Google Scholar] [CrossRef]
- Scragg, J.J.; Dale, P.J.; Peter, L.M.; Zoppi, G.; Forbes, I. New routes to sustainable photovoltaics: Evaluation of Cu2ZnSnS4 as an alternative absorber material. Phys. Status Solidi B-Basic Solid State Phys. 2008, 245, 1772–1778. [Google Scholar] [CrossRef]
- Chen, G.L.; Wang, W.H.; Zhang, B.Y.; Chen, S.Y.; Huang, Z.G.; Zhuang, B. Influence of selenization atmosphere on the Cu2ZnSn(S,Se)4 thin films and its correlation to the performance of solar cells. Mater. Res. Bull. 2017, 94, 164–169. [Google Scholar] [CrossRef]



| Sample | Cu(% at) | Ni(% at) | Zn(% at) | Sn(% at) | S(% at) | Se(% at) | Cu/(Zn + Ni + Sn) | (Ni + Zn)/Sn |
|---|---|---|---|---|---|---|---|---|
| 10 min | 23.65 | 0.41 | 17.28 | 10.41 | 1.78 | 46.47 | 0.84 | 1.69 |
| 15 min | 23.58 | 0.40 | 17.10 | 10.24 | 1.74 | 46.95 | 0.85 | 1.70 |
| 20 min | 22.67 | 0.42 | 17.32 | 10.60 | 1.71 | 47.29 | 0.80 | 1.67 |
| Samples | Resistivity (Ω·cm) | Carrier Concentration (cm−3) | Mobility (cm2V−1s−1) | Type |
|---|---|---|---|---|
| 10 min | 1.51 × 102 | 1.21 × 1016 | 3.39 | p |
| 15 min | 9.98 × 101 | 1.68 × 1016 | 3.78 | p |
| 20 min | 1.75 × 102 | 1.66 × 1016 | 2.12 | p |
| Device | Active Area | VOC (mV) | JSC (mA/cm2) | FF (%) | PCE (%) | Rs (Ω cm2) | Rsh (Ω cm2) |
|---|---|---|---|---|---|---|---|
| CNZTSSe (T = 10 min) | 0.19 cm2 | 309 | 29.94 | 39.46 | 3.6 | 2.58 | 186.01 |
| CNZTSSe (T = 15 min) | 0.19 cm2 | 337 | 33.61 | 44.15 | 5.0 | 1.86 | 253.05 |
| CNZTSSe (T = 20 min) | 0.19 cm2 | 333 | 31.15 | 42.86 | 4.4 | 2.01 | 212.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Wang, J.; Ma, M.; Zhao, N.; Wang, T.; Chen, G.; Yao, B.; Sui, Y. Exploring the Effect of Selenidation Time on the Ni-Doped Cu2ZnSn(S,Se)4 Solar Cell. Nanomaterials 2022, 12, 4311. https://doi.org/10.3390/nano12234311
Zeng F, Wang J, Ma M, Zhao N, Wang T, Chen G, Yao B, Sui Y. Exploring the Effect of Selenidation Time on the Ni-Doped Cu2ZnSn(S,Se)4 Solar Cell. Nanomaterials. 2022; 12(23):4311. https://doi.org/10.3390/nano12234311
Chicago/Turabian StyleZeng, Fancong, Jingshu Wang, Meiling Ma, Na Zhao, Tianyue Wang, Guangbo Chen, Bin Yao, and Yingrui Sui. 2022. "Exploring the Effect of Selenidation Time on the Ni-Doped Cu2ZnSn(S,Se)4 Solar Cell" Nanomaterials 12, no. 23: 4311. https://doi.org/10.3390/nano12234311




